Acute Inflammatory Response in Osteoporotic Fracture Healing Augmented with Mechanical Stimulation is Regulated In Vivo through the p38-MAPK Pathway
Abstract
1. Introduction
2. Results
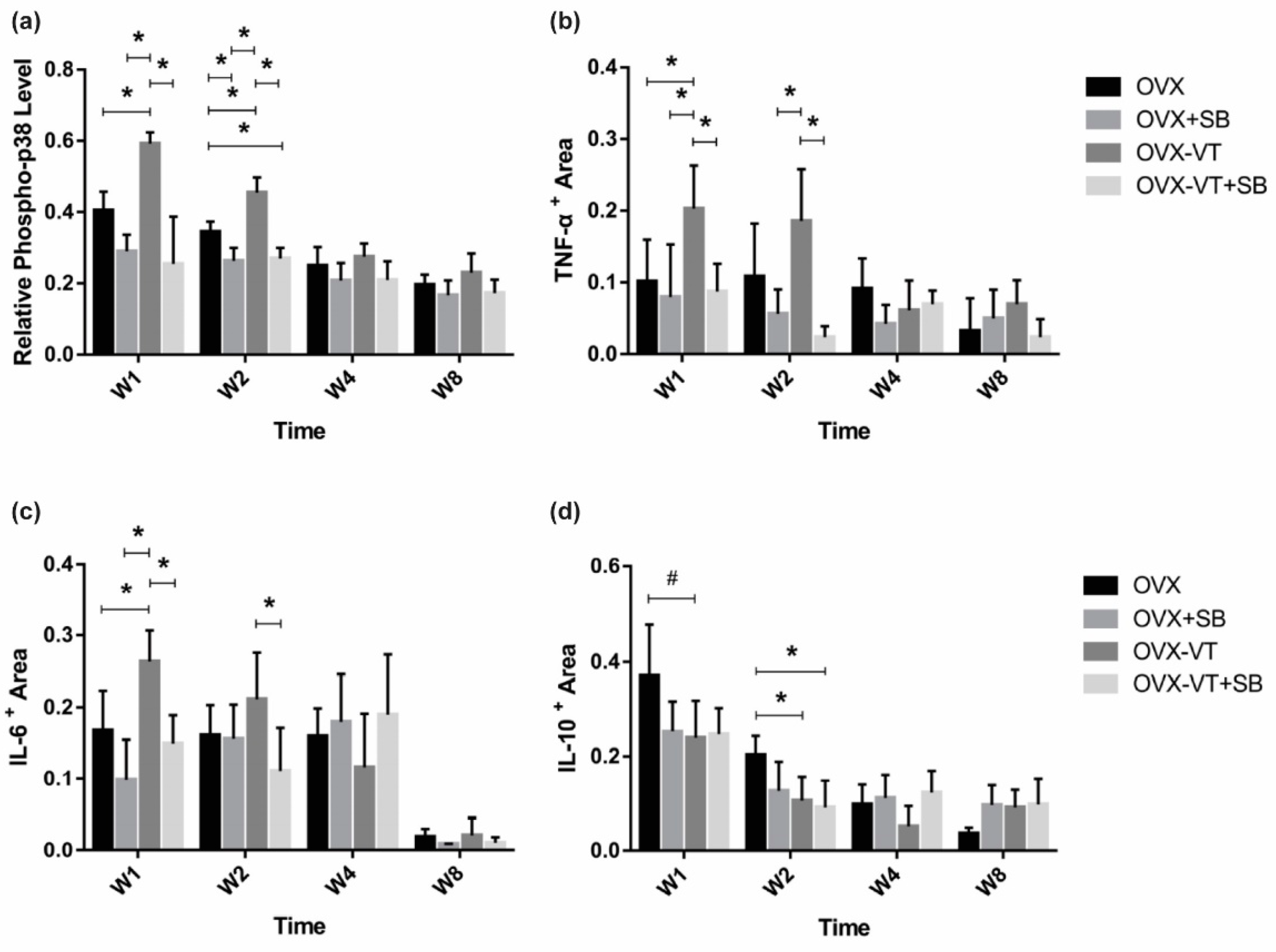
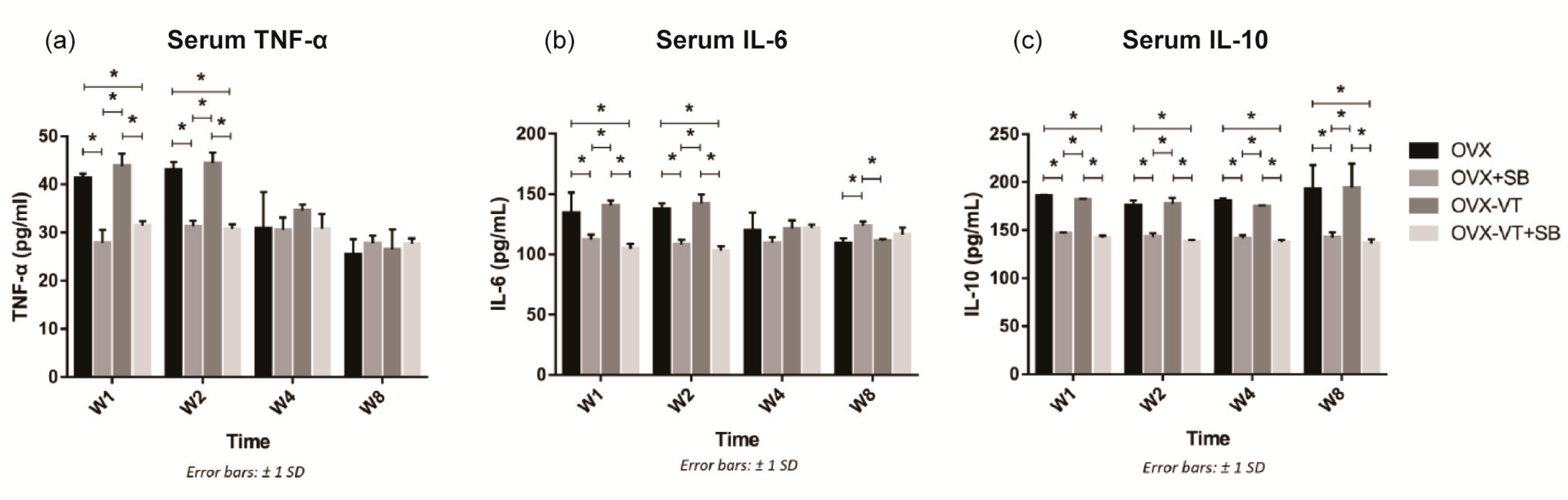
2.1. LMHFV Enhanced Activation of p38 MAPK and the Inflammatory Response of Osteoporotic Fracture Healing
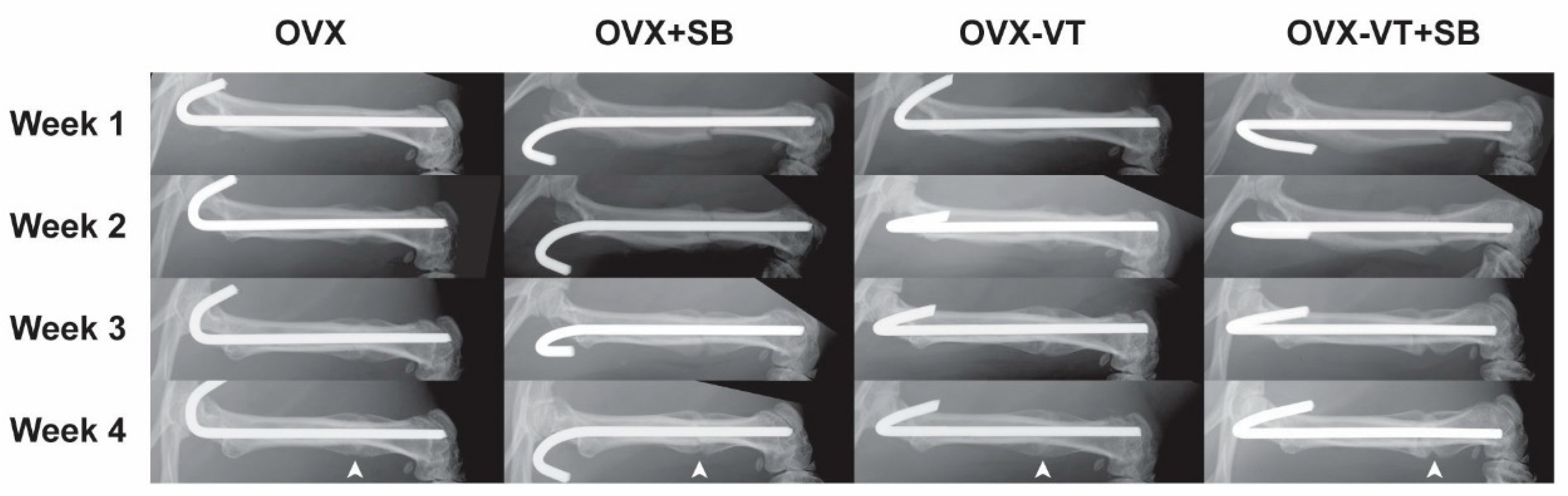
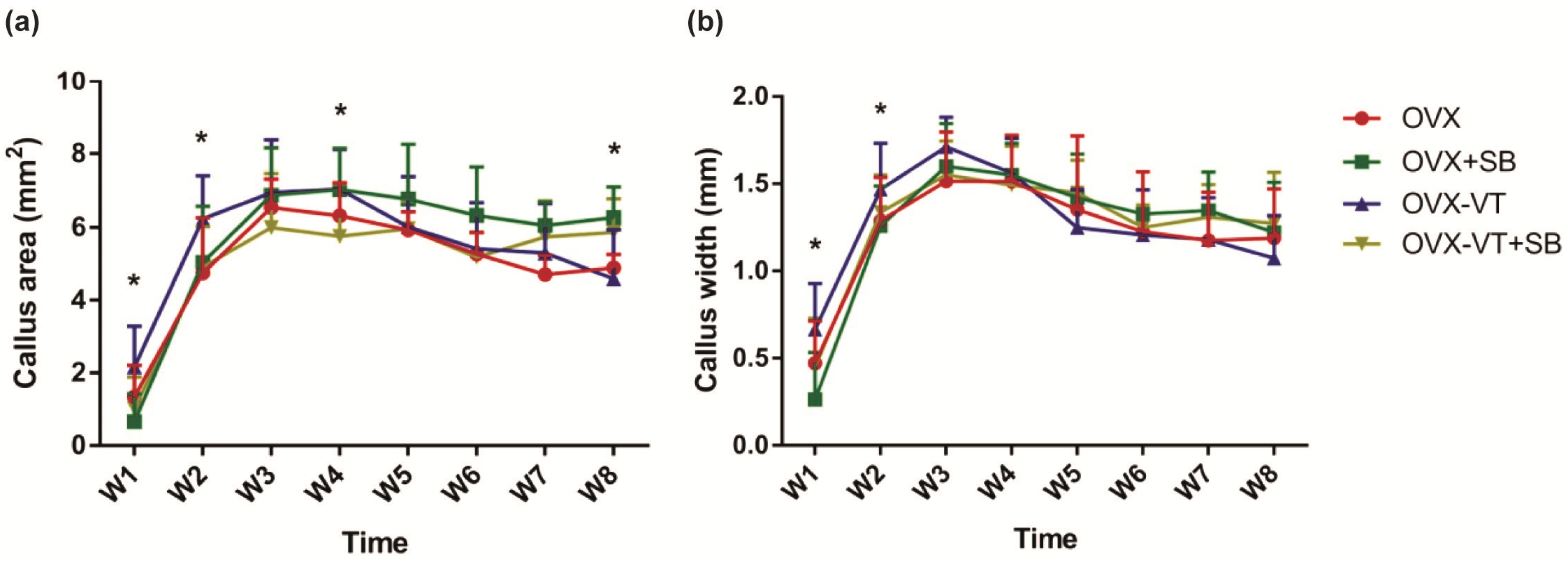
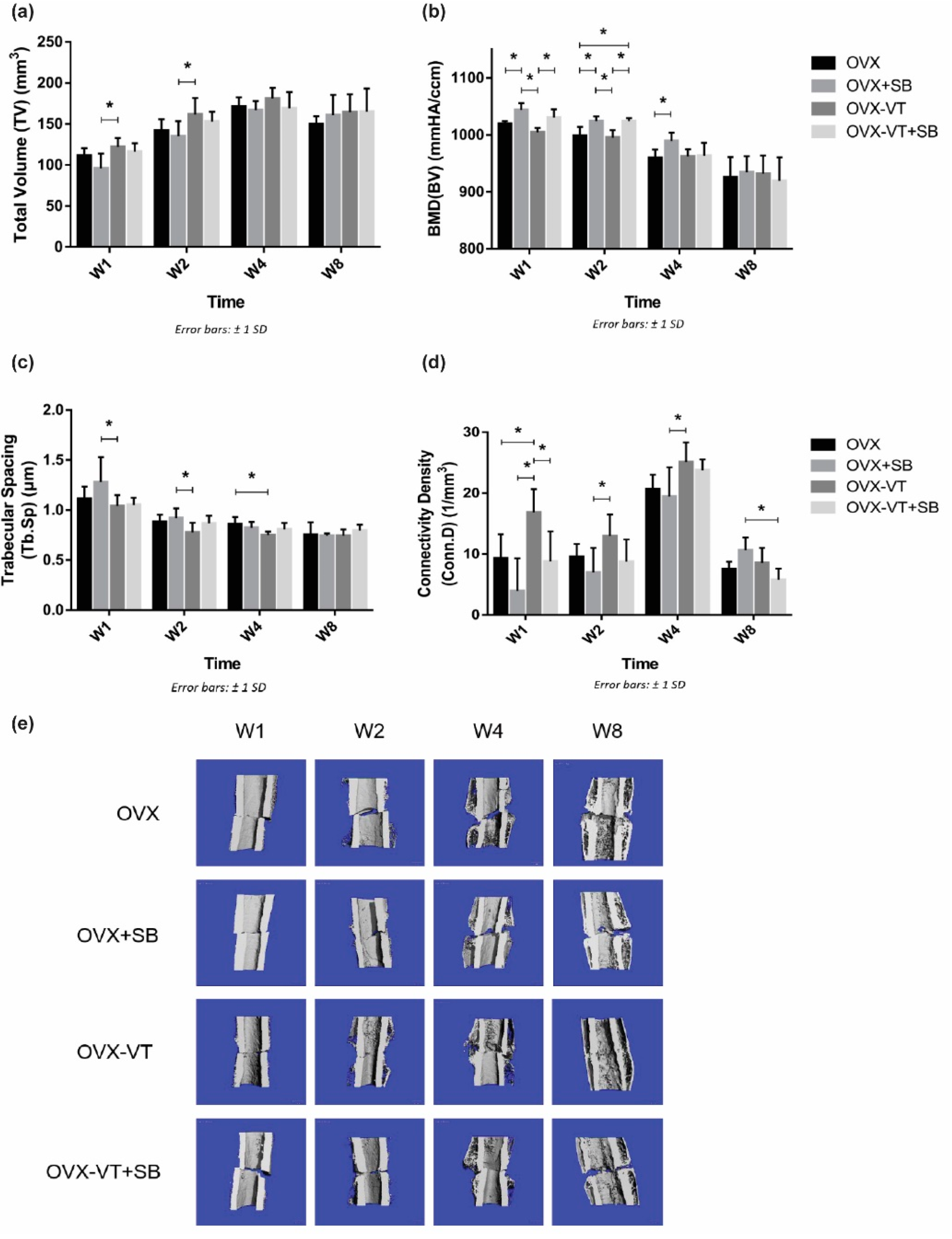
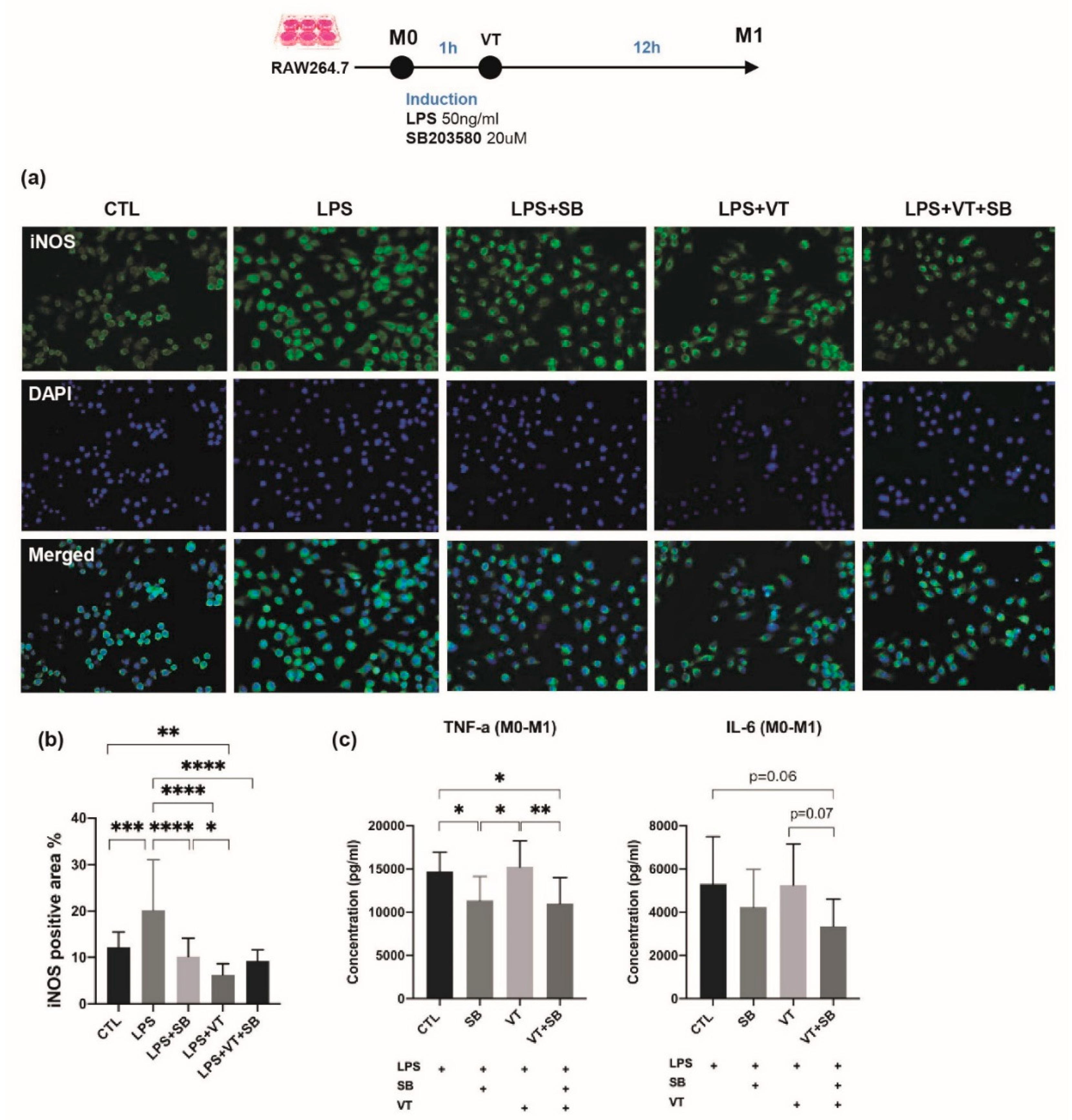
2.2. Inhibition of p38 MAPK Led to Impaired Inflammatory Response and Poorer Callus Formation
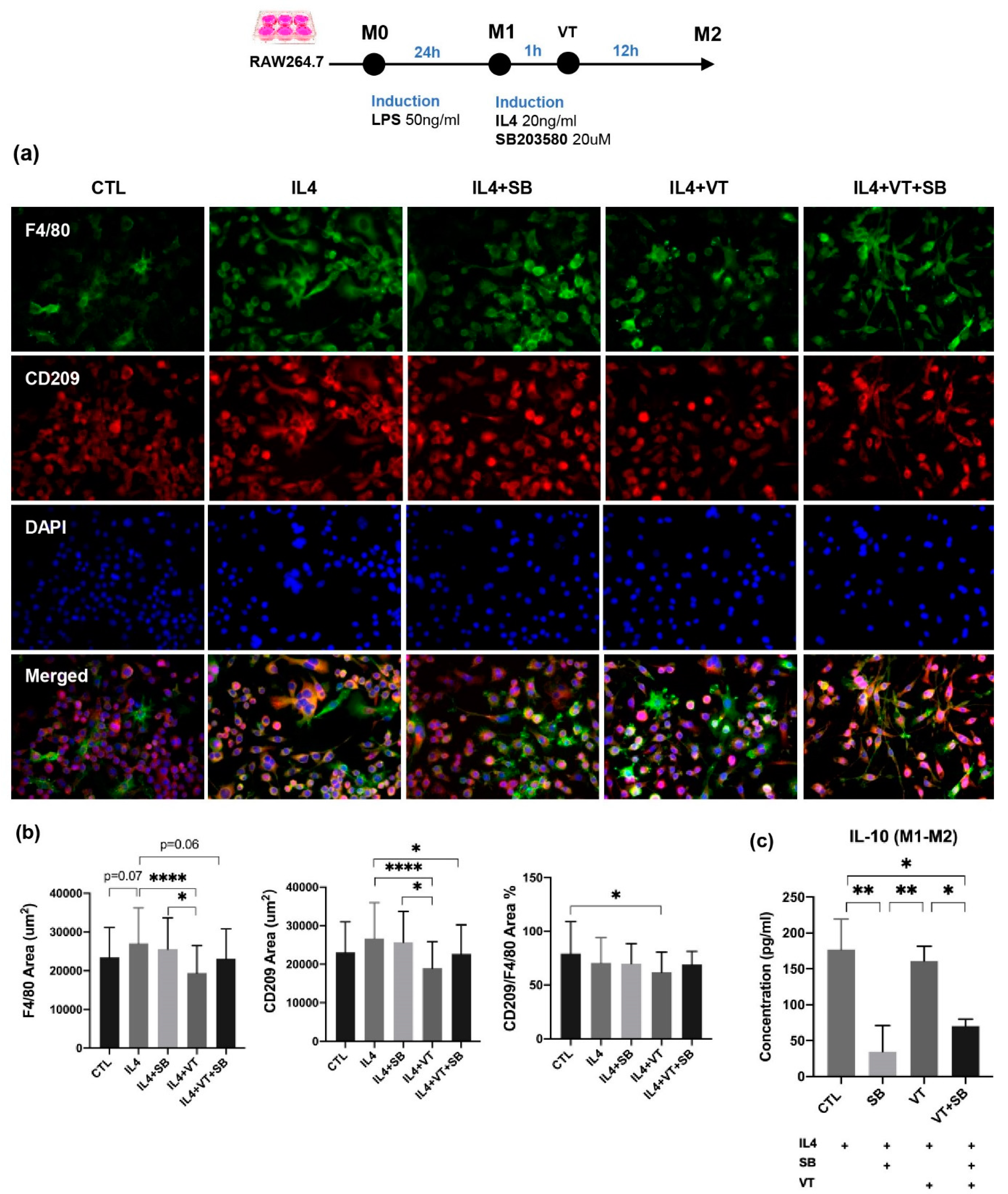
2.3. LMHFV Did Not Directly Enhance Macrophage Polarization In Vitro
3. Discussion
4. Materials and Methods
4.1. Animal Model and Interventions
4.2. Radiographic Analysis
4.3. 3-D Bone Morphometry and Micro-Finite Element Analysis
4.4. Systemic Inflammatory Cytokine Levels
4.5. Bone Histomorphometry
4.6. Immunohistochemistry (IHC)
4.7. Macrophage RAW 264.7 Culture
4.8. In Vitro Inflammatory Cytokine Levels
4.9. Immunofluorescence Assay for M1/M2 Markers
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, F.Y.; Leung, K.S.; Li, G.; Qin, J.; Chow, S.K.; Huang, S.; Sun, M.H.; Qin, L.; Cheung, W.H. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PLoS ONE 2014, 9, e106722. [Google Scholar] [CrossRef]
- Cheung, W.H.; Sun, M.H.; Zheng, Y.P.; Chu, W.C.; Leung, A.H.; Qin, L.; Wei, F.Y.; Leung, K.S. Stimulated angiogenesis for fracture healing augmented by low-magnitude, high-frequency vibration in a rat model-evaluation of pulsed-wave doppler, 3-D power Doppler ultrasonography and micro-CT microangiography. Ultrasound Med. Biol. 2012, 38, 2120–2129. [Google Scholar] [CrossRef]
- Shi, H.F.; Cheung, W.H.; Qin, L.; Leung, A.H.; Leung, K.S. Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone 2010, 46, 1299–1305. [Google Scholar] [CrossRef]
- Chow, D.H.; Leung, K.S.; Qin, L.; Leung, A.H.; Cheung, W.H. Low-magnitude high-frequency vibration (LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing. J. Orthop. Res. 2011, 29, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.K.; Leung, K.S.; Qin, L.; Wei, F.; Cheung, W.H. Callus formation is related to the expression ratios of estrogen receptors-alpha and -beta in ovariectomy-induced osteoporotic fracture healing. Arch. Orthop. Trauma Surg. 2014, 134, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Zimmerman, G.; Marcucio, R. Biological perspectives of delayed fracture healing. Injury 2014, 45 (Suppl. 2), S8–S15. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef]
- Kon, T.; Cho, T.J.; Aizawa, T.; Yamazaki, M.; Nooh, N.; Graves, D.; Gerstenfeld, L.C.; Einhorn, T.A. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 2001, 16, 1004–1014. [Google Scholar] [CrossRef]
- Cheung, W.H.; Miclau, T.; Chow, S.K.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47 (Suppl. 2), S21–S26. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef]
- Dishowitz, M.I.; Mutyaba, P.L.; Takacs, J.D.; Barr, A.M.; Engiles, J.B.; Ahn, J.; Hankenson, K.D. Systemic inhibition of canonical Notch signaling results in sustained callus inflammation and alters multiple phases of fracture healing. PLoS ONE 2013, 8, e68726. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.C.; Ko, K.I.; Mattos, M.; Fang, M.; Zhang, C.; Feinberg, D.; Sindi, H.; Li, S.; Alblowi, J.; Kayal, R.A.; et al. TNFalpha contributes to diabetes impaired angiogenesis in fracture healing. Bone 2017, 99, 26–38. [Google Scholar] [CrossRef]
- Chow, S.K.; Chim, Y.N.; Wang, J.; Zhang, N.; Wong, R.M.; Tang, N.; Leung, K.S.; Cheung, W.H. Vibration treatment modulates macrophage polarisation and enhances early inflammatory response in oestrogen-deficient osteoporotic-fracture healing. Eur. Cell Mater. 2019, 38, 228–245. [Google Scholar] [CrossRef]
- Ono, K.; Han, J. The p38 signal transduction pathway: Activation and function. Cell. Signal. 2000, 12, 1–13. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, Y.C.; Kim, J.H.; Kim, H.R.; Yu, T.; Byeon, S.E.; Unsworth, L.D.; Lee, J.; Cho, J.Y. Nanostructured, self-assembling peptide K5 blocks TNF-alpha and PGE(2) production by suppression of the AP-1/p38 pathway. Mediat. Inflamm. 2012, 2012, 489810. [Google Scholar] [CrossRef]
- Byeon, S.E.; Lee, J.; Yoo, B.C.; Sung, G.H.; Kim, T.W.; Park, H.J.; Cho, J.Y. p38-targeted inhibition of interleukin-12 expression by ethanol extract from Cordyceps bassiana in lipopolysaccharide-activated macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 90–96. [Google Scholar] [CrossRef]
- Garcia, J.; Lemercier, B.; Roman-Roman, S.; Rawadi, G. A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-kappaB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways. J. Biol. Chem. 1998, 273, 34391–34398. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef]
- Campbell, J.; Ciesielski, C.J.; Hunt, A.E.; Horwood, N.J.; Beech, J.T.; Hayes, L.A.; Denys, A.; Feldmann, M.; Brennan, F.M.; Foxwell, B.M. A novel mechanism for TNF-alpha regulation by p38 MAPK: Involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J. Immunol. 2004, 173, 6928–6937. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.; Jia, H.; Biswas, S.; Li, P.; Qiu, S.; Deng, Q.; Guo, X.; Ma, G.; Ling Chau, J.F.; Wang, Y.; et al. p38alpha MAPK Regulates Lineage Commitment and OPG Synthesis of Bone Marrow Stromal Cells to Prevent Bone Loss under Physiological and Pathological Conditions. Stem Cell Rep. 2016, 6, 566–578. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Shim, J.H.; Zou, W.; Sitara, D.; Schweitzer, M.; Hu, D.; Lotinun, S.; Sano, Y.; Baron, R.; Park, J.M.; et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J. Clin. Investig. 2010, 120, 2457–2473. [Google Scholar] [CrossRef] [PubMed]
- Thouverey, C.; Caverzasio, J. The p38alpha MAPK positively regulates osteoblast function and postnatal bone acquisition. Cell. Mol. Life Sci. CMLS 2012, 69, 3115–3125. [Google Scholar] [CrossRef]
- Rodriguez-Carballo, E.; Gamez, B.; Ventura, F. p38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef]
- Hu, Y.; Chan, E.; Wang, S.X.; Li, B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology 2003, 144, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carballo, E.; Gamez, B.; Sedo-Cabezon, L.; Sanchez-Feutrie, M.; Zorzano, A.; Manzanares-Cespedes, C.; Rosa, J.L.; Ventura, F. The p38alpha MAPK function in osteoprecursors is required for bone formation and bone homeostasis in adult mice. PLoS ONE 2014, 9, e102032. [Google Scholar] [CrossRef]
- Xiao, W.L.; Zhang, D.Z.; Fan, C.H.; Yu, B.J. Intermittent Stretching and Osteogenic Differentiation of Bone Marrow Derived Mesenchymal Stem Cells via the p38MAPK-Osterix Signaling Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Suzuki, N.; Mitsui, N.; Koyama, Y.; Otsuka, K.; Shimizu, N. Effects of compressive force on the differentiation of pluripotent mesenchymal cells. Life Sci. 2007, 81, 405–412. [Google Scholar] [CrossRef]
- Tang, M.; Peng, Z.; Mai, Z.; Chen, L.; Mao, Q.; Chen, Z.; Chen, Q.; Liu, L.; Wang, Y.; Ai, H. Fluid shear stress stimulates osteogenic differentiation of human periodontal ligament cells via the extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling pathways. J. Periodontol. 2014, 85, 1806–1813. [Google Scholar] [CrossRef]
- Chow, S.K.; Leung, K.S.; Qin, J.; Guo, A.; Sun, M.; Qin, L.; Cheung, W.H. Mechanical stimulation enhanced estrogen receptor expression and callus formation in diaphyseal long bone fracture healing in ovariectomy-induced osteoporotic rats. Osteoporos Int. 2016, 27, 2989–3000. [Google Scholar] [CrossRef]
- Wong, R.M.Y.; Choy, V.M.H.; Li, J.; Li, T.K.; Chim, Y.N.; Li, M.C.M.; Cheng, J.C.Y.; Leung, K.S.; Chow, S.K.; Cheung, W.H. Fibrinolysis as a target to enhance osteoporotic fracture healing by vibration therapy in a metaphyseal fracture model. Bone Jt. Res. 2021, 10, 41–50. [Google Scholar] [CrossRef]
- Kazi, A.A.; Koos, R.D. Estrogen-induced activation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology 2007, 148, 2363–2374. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Lu, Y.; Zhao, B. Inhibitory effects of 17beta-estradiol or a resveratrol dimer on hypoxia-inducible factor-1alpha in genioglossus myoblasts: Involvement of ERalpha and its downstream p38 MAPK pathways. Int. J. Mol. Med. 2017, 40, 1347–1356. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, Y.; Gan, X.; Yu, H. Low magnitude high frequency vibration promotes adipogenic differentiation of bone marrow stem cells via P38 MAPK signal. PLoS ONE 2017, 12, e0172954. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, Q.; Liu, Y.; Zhang, L.; Li, D.; Zhu, Z.; Gan, X.; Yu, H. Vibration loading promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells via p38 MAPK signaling pathway. J. Biomech. 2018, 71, 67–75. [Google Scholar] [CrossRef]
- Kumar, S.; Votta, B.J.; Rieman, D.J.; Badger, A.M.; Gowen, M.; Lee, J.C. IL-1-and TNF-induced bone resorption is mediated by p38 mitogen activated protein kinase. J. Cell. Physiol. 2001, 187, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Valbracht, J.; Lotz, M. Selective activation of the mitogen-activated protein kinase subgroups c-Jun NH2 terminal kinase and p38 by IL-1 and TNF in human articular chondrocytes. J. Clin. Investig. 1996, 98, 2425–2430. [Google Scholar] [CrossRef][Green Version]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Sonoyama, W.; Wang, Z.; Jin, Q.; Zhang, C.; Krebsbach, P.H.; Giannobile, W.; Shi, S.; Wang, C.Y. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J. Biol. Chem. 2007, 282, 30938–30948. [Google Scholar] [CrossRef]
- Kawaki, H.; Kubota, S.; Suzuki, A.; Suzuki, M.; Kohsaka, K.; Hoshi, K.; Fujii, T.; Lazar, N.; Ohgawara, T.; Maeda, T.; et al. Differential roles of CCN family proteins during osteoblast differentiation: Involvement of Smad and MAPK signaling pathways. Bone 2011, 49, 975–989. [Google Scholar] [CrossRef]
- Wang, X.Y.; Tang, Q.Q.; Zhang, J.L.; Fang, M.Y.; Li, Y.X. Effect of SB203580 on pathologic change of pancreatic tissue and expression of TNF-alpha and IL-1beta in rats with severe acute pancreatitis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 338–343. [Google Scholar]
- Xu, D.J.; Zhao, Y.Z.; Wang, J.; He, J.W.; Weng, Y.G.; Luo, J.Y. Smads, p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB Rep. 2012, 45, 247–252. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Davis, C.T.; Liu, W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. CMLS 2015, 72, 1303–1316. [Google Scholar] [CrossRef]
- Chu, S.Y.; Chou, C.H.; Huang, H.D.; Yen, M.H.; Hong, H.C.; Chao, P.H.; Wang, Y.H.; Chen, P.Y.; Nian, S.X.; Chen, Y.R.; et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat. Commun. 2019, 10, 1524. [Google Scholar] [CrossRef]
- Cheong, C.; Matos, I.; Choi, J.H.; Dandamudi, D.B.; Shrestha, E.; Longhi, M.P.; Jeffrey, K.L.; Anthony, R.M.; Kluger, C.; Nchinda, G.; et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 2010, 143, 416–429. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Pajarinen, J.; Takakubo, Y.; Gallo, J.; Nich, C.; Takagi, M.; Goodman, S.B. Macrophage polarization and activation in response to implant debris: Influence by “particle disease” and “ion disease”. J. Long-Term Eff. Med. Implants 2014, 24, 267–281. [Google Scholar] [CrossRef] [PubMed]
- El Khassawna, T.; Serra, A.; Bucher, C.H.; Petersen, A.; Schlundt, C.; Konnecke, I.; Malhan, D.; Wendler, S.; Schell, H.; Volk, H.D.; et al. T Lymphocytes Influence the Mineralization Process of Bone. Front. Immunol. 2017, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.M.Y.; Choy, M.H.V.; Li, M.C.M.; Leung, K.S.; Chow, S.K.H.; Cheung, W.H.; Cheng, J.C.Y. A systematic review of current osteoporotic metaphyseal fracture animal models. Bone Jt. Res. 2018, 7, 6–11. [Google Scholar] [CrossRef]
- Wong, R.M.; Thormann, U.; Choy, M.H.; Chim, Y.N.; Li, M.C.; Wang, J.Y.; Leung, K.S.; Cheng, J.C.; Alt, V.; Chow, S.K.; et al. A metaphyseal fracture rat model for mechanistic studies of osteoporotic bone healing. Eur. Cell Mater. 2019, 37, 420–430. [Google Scholar] [CrossRef]
- Cheung, W.H.; Chin, W.C.; Qin, L.; Leung, K.S. Low intensity pulsed ultrasound enhances fracture healing in both ovariectomy-induced osteoporotic and age-matched normal bones. J. Orthop. Res. 2012, 30, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, B.; Lin, A. Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol. Sci. 2007, 28, 286–295. [Google Scholar] [CrossRef]
- Kumar, S.; Boehm, J.; Lee, J.C. p38 MAP kinases: Key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003, 2, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef]
- Yan, W.; Xiaoli, L.; Guoliang, A.; Zhonghui, Z.; Di, L.; Ximeng, L.; Piye, N.; Li, C.; Lin, T. SB203580 inhibits epithelial-mesenchymal transition and pulmonary fibrosis in a rat silicosis model. Toxicol. Lett. 2016, 259, 28–34. [Google Scholar] [CrossRef]
- Wei, F.Y.; Chow, S.K.; Leung, K.S.; Qin, J.; Guo, A.; Yu, O.L.; Li, G.; Cheung, W.H. Low-Magnitude High-Frequency Vibration Enhanced Mesenchymal Stem Cell Recruitment in Osteoporotic Fracture Healing through the Sdf-1/Cxcr4 Pathway. Eur. Cells Mater. 2016, 31, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, K.Y.; Zhu, T.Y.; Yu, F.W.; Hung, V.W.; Lee, K.M.; Qin, L.; Cheng, J.C.; Lam, T.P. Abnormal Bone Mechanical and Structural Properties in Adolescent Idiopathic Scoliosis: A Study with Finite Element Analysis and Structural Model Index. Calcif. Tissue Int. 2015, 97, 343–352. [Google Scholar] [CrossRef]
- Rao, A.J.; Gibon, E.; Ma, T.; Yao, Z.; Smith, R.L.; Goodman, S.B. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012, 8, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Pajarinen, J.; Tamaki, Y.; Antonios, J.K.; Lin, T.H.; Sato, T.; Yao, Z.; Takagi, M.; Konttinen, Y.T.; Goodman, S.B. Modulation of mouse macrophage polarization in vitro using IL-4 delivery by osmotic pumps. J. Biomed. Mater. Res. A 2015, 103, 1339–1345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loi, F.; Cordova, L.A.; Zhang, R.; Pajarinen, J.; Lin, T.H.; Goodman, S.B.; Yao, Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res. Ther. 2016, 7, 15. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, S.K.H.; Cui, C.; Cheng, K.Y.K.; Chim, Y.N.; Wang, J.; Wong, C.H.W.; Ng, K.W.; Wong, R.M.Y.; Cheung, W.H. Acute Inflammatory Response in Osteoporotic Fracture Healing Augmented with Mechanical Stimulation is Regulated In Vivo through the p38-MAPK Pathway. Int. J. Mol. Sci. 2021, 22, 8720. https://doi.org/10.3390/ijms22168720
Chow SKH, Cui C, Cheng KYK, Chim YN, Wang J, Wong CHW, Ng KW, Wong RMY, Cheung WH. Acute Inflammatory Response in Osteoporotic Fracture Healing Augmented with Mechanical Stimulation is Regulated In Vivo through the p38-MAPK Pathway. International Journal of Molecular Sciences. 2021; 22(16):8720. https://doi.org/10.3390/ijms22168720
Chicago/Turabian StyleChow, Simon Kwoon Ho, Can Cui, Keith Yu Kin Cheng, Yu Ning Chim, Jinyu Wang, Carissa Hing Wai Wong, Ka Wai Ng, Ronald Man Yeung Wong, and Wing Hoi Cheung. 2021. "Acute Inflammatory Response in Osteoporotic Fracture Healing Augmented with Mechanical Stimulation is Regulated In Vivo through the p38-MAPK Pathway" International Journal of Molecular Sciences 22, no. 16: 8720. https://doi.org/10.3390/ijms22168720
APA StyleChow, S. K. H., Cui, C., Cheng, K. Y. K., Chim, Y. N., Wang, J., Wong, C. H. W., Ng, K. W., Wong, R. M. Y., & Cheung, W. H. (2021). Acute Inflammatory Response in Osteoporotic Fracture Healing Augmented with Mechanical Stimulation is Regulated In Vivo through the p38-MAPK Pathway. International Journal of Molecular Sciences, 22(16), 8720. https://doi.org/10.3390/ijms22168720






