Modulation of the Cardiac Myocyte Action Potential by the Magnesium-Sensitive TRPM6 and TRPM7-like Current
Abstract
:1. Introduction
2. Results
2.1. Expression of TRPM6 and TRPM7 in Cardiac Myocytes
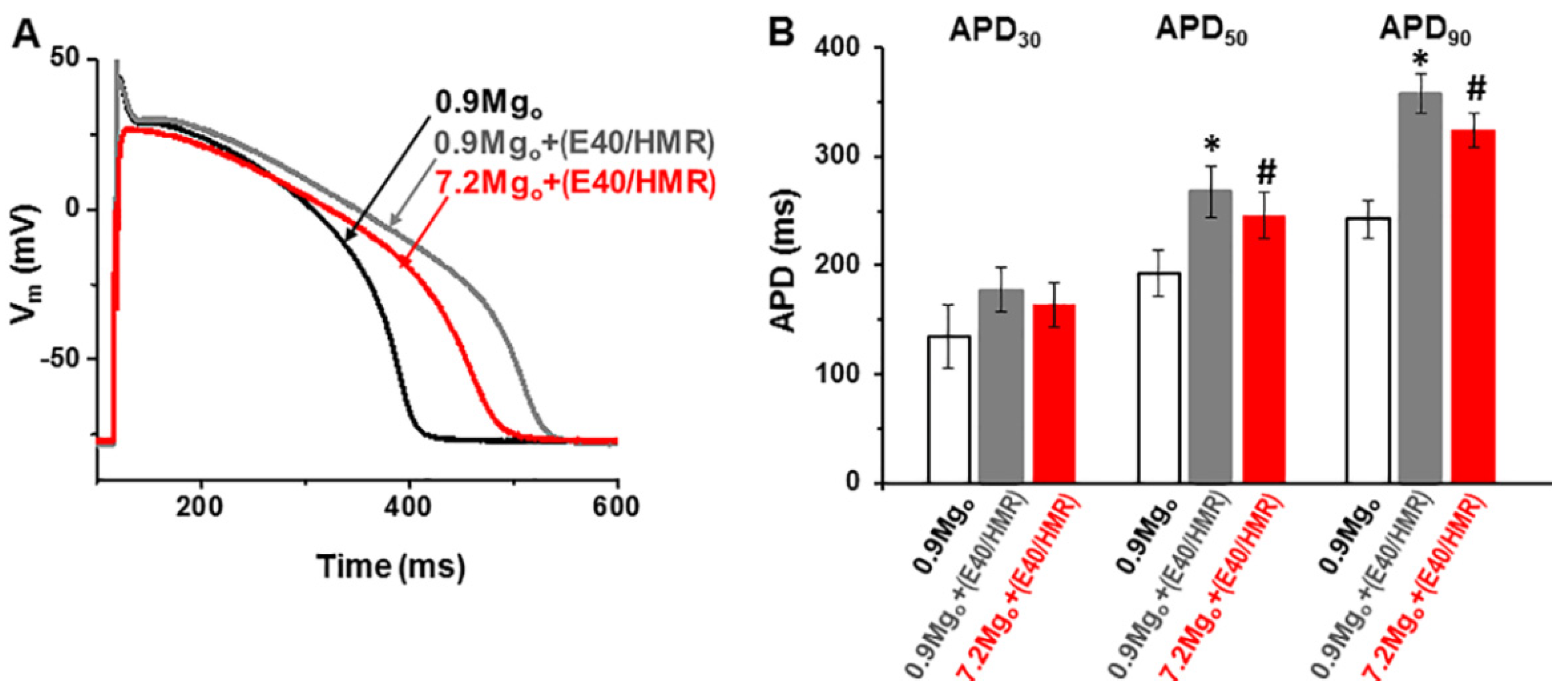
2.2. Impact of Mg2+-Sensitive Currents on the Action Potential
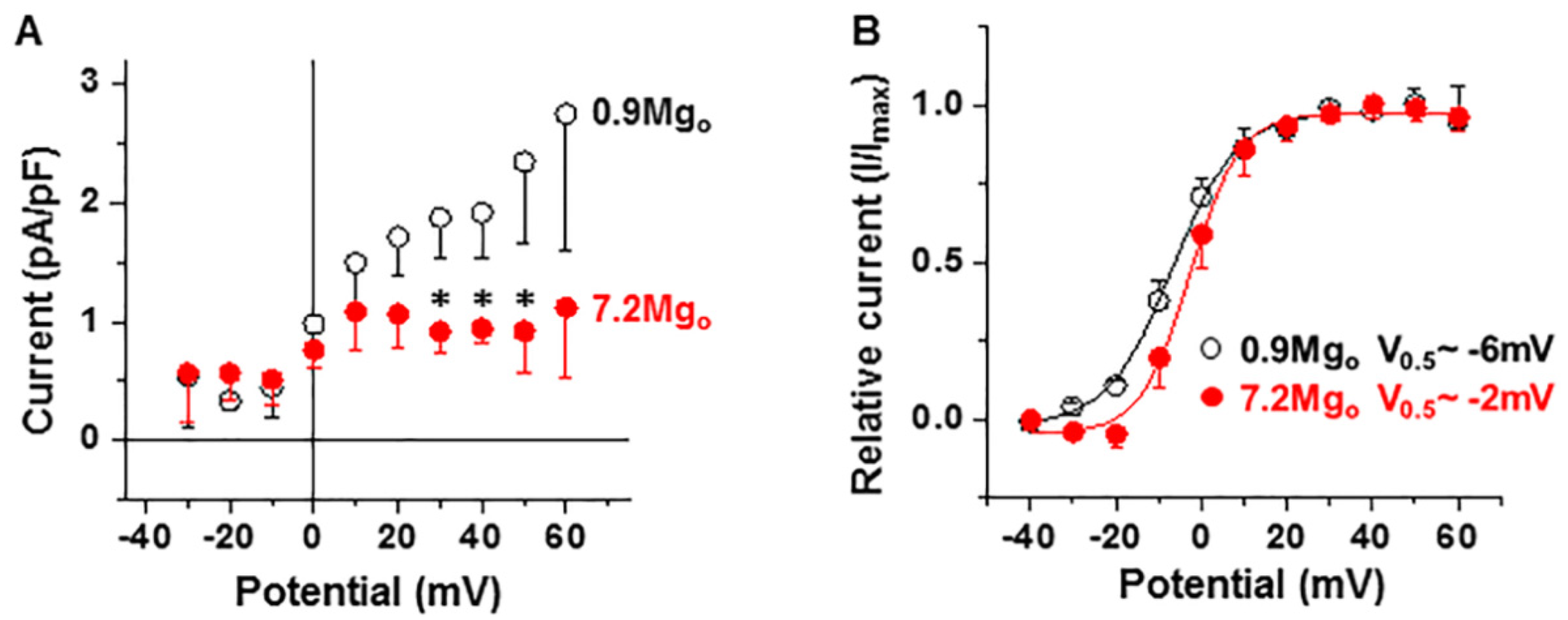
2.3. Effects of High Extracellular [Mg2+] on ICa-L and IK
3. Discussion
3.1. Molecular Candidate for Mg2+-Sensitive Channels
3.2. Modulation of Cardiac Electrical Activity
3.3. Clinical Implications
3.4. Limitations of the Study
3.5. Conclusion
4. Materials and Methods
4.1. Cell Isolation
4.2. Electrophysiology
4.3. Immunofluorescence
4.4. Solutions and Drugs
4.5. Data and Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- DiFrancesco, D. Serious workings of the funny current. Prog. Biophys. Mol. Biol. 2006, 90, 13–25. [Google Scholar] [CrossRef]
- Falcón, D.; Galeano-Otero, I.; Calderón-Sánchez, E.; Del Toro, R.; Martin-Bornez, M.; Rosado, J.A.; Hmadcha, A.; Smani, T. TRP channels: Current perspectives in the adverse cardiac remodeling. Front. Physiol. 2019, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Hof, T.; Chaigne, S.; Récalde, A.; Sallé, L.; Brette, F.; Guinamard, R. Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 2019, 16, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Q.; Kurahara, L.H.; Shioi, N.; Hiraishi, K.; Fujita, T.; Zhu, X.; Inoue, R. An Arrhythmic Mutation E7K Facilitates TRPM4 Channel Activation via Enhanced PIP2 Interaction. Cells 2021, 10, 983. [Google Scholar] [CrossRef]
- Feng, J.; Zong, P.; Yan, J.; Yue, Z.; Li, X.; Smith, C.; Ai, X.; Yue, L. Upregulation of transient receptor potential melastatin 4 (TRPM4) in ventricular fibroblasts from heart failure patients. Pflügers Arch. 2021, 473, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Seth, M.; Zhang, Z.S.; Mao, L.; Graham, V.; Burch, J.; Stiber, J.; Tsiokas, L.; Winn, M.; Abramowitz, J.; Rockman, H.A.; et al. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ. Res. 2009, 105, 1023–1030. [Google Scholar] [CrossRef]
- Alvarez, J.; Coulombe, A.; Cazorla, O.; Ugur, M.; Rauzier, J.M.; Magyar, J.; Mathieu, E.L.; Boulay, G.; Souto, R.; Bideaux, P.; et al. ATP/UTP activate cation-permeable channels with TRPC3/7 properties in rat cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H21–H28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onohara, N.; Nishida, M.; Inoue, R.; Kobayashi, H.; Sumimoto, H.; Sato, Y.; Mori, Y.; Nagao, T.; Kurose, H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006, 25, 5305–5316. [Google Scholar] [CrossRef]
- Chaigne, S.; Cardouat, G.; Louradour, J.; Vaillant, F.; Charron, S.; Sacher, F.; Ducret, T.; Guinamard, R.; Vigmond, E.; Hof, T. Transient receptor potential vanilloid 4 channel participates in mouse ventricular electrical activity. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1156–H1169. [Google Scholar] [CrossRef]
- Guinamard, R.; Chatelier, A.; Demion, M.; Potreau, D.; Patri, S.; Rahmati, M.; Bois, P. Functional characterization of a Ca2+-activated non-selective cation channel in human atrial cardiomyocytes. J. Physiol. 2004, 558, 75–83. [Google Scholar] [CrossRef]
- Sah, R.; Mesirca, P.; Van den Boogert, M.; Rosen, J.; Mably, J.; Mangoni, M.E.; Clapham, D.E. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc. Natl. Acad. Sci. USA 2013, 110, E3037–E3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sah, R.; Mesirca, P.; Mason, X.; Gibson, W.; Bates-Withers, C.; Van den Boogert, M.; Chaudhuri, D.; Pu, W.T.; Mangoni, M.E.; Clapham, D.E. Timing of myocardial Trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation 2013, 128, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volk, T.; Schwoerer, A.P.; Thiessen, S.; Schultz, J.H.; Ehmke, H. A polycystin-2-like large conductance cation channel in rat left ventricular myocytes. Cardiovasc. Res. 2003, 58, 76–88. [Google Scholar] [CrossRef]
- Gwanyanya, A.; Sipido, K.R.; Vereecke, J.; Mubagwa, K. ATP and PIP2 dependence of the magnesium-inhibited, TRPM7-like cation channel in cardiac myocytes. Am. J. Physiol. Cell Physiol. 2006, 291, C627–C635. [Google Scholar] [CrossRef] [Green Version]
- Gwanyanya, A.; Amuzescu, B.; Zakharov, S.I.; Macianskiene, R.; Sipido, K.R.; Bolotina, V.M.; Vereecke, J.; Mubagwa, K. Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: Permeation of divalent cations and pH-mediated regulation. J. Physiol. 2004, 559, 761–776. [Google Scholar] [CrossRef] [Green Version]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef]
- Schmitz, C.; Perraud, A.L.; Johnson, C.O.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Voets, T.; Nilius, B.; Hoefs, S.; van der Kemp, A.W.; Droogmans, G.; Bindels, R.J.; Hoenderop, J.G. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 2004, 279, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Fleig, A.; Chubanov, V. TRPM7. Handb. Exp. Pharmacol. 2014, 222, 521–546. [Google Scholar] [PubMed] [Green Version]
- Zhang, Y.J.; Ma, N.; Su, F.; Liu, H.; Mei, J. Increased TRPM6 expression in atrial fibrillation patients contribute to atrial fibrosis. Exp. Mol. Pathol. 2015, 98, 486–490. [Google Scholar] [CrossRef]
- Andriulė, I.; Pangonytė, D.; Almanaitytė, M.; Patamsytė, V.; Kuprytė, M.; Karčiauskas, D.; Mubagwa, K.; Mačianskienė, R. Evidence for the expression of TRPM6 and TRPM7 in cardiomyocytes from all four chamber walls of the human heart. Sci. Rep. 2021, 11, 1–14. [Google Scholar]
- Tashiro, M.; Inoue, H.; Konishi, M. Physiological pathway of magnesium influx in rat ventricular myocytes. Biophys. J. 2014, 107, 2049–2058. [Google Scholar] [CrossRef] [Green Version]
- Gräbner, W.; Pfitzer, P. Number of nuclei in isolated myocardial cells of pigs. Virchows Arch. B Cell Pathol. 1974, 15, 279–294. [Google Scholar]
- Macianskiene, R.; Moccia, F.; Sipido, K.R.; Flameng, W.; Mubagwa, K. Channels involved in transient currents unmasked by removal of extracellular calcium in cardiac cells. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1879–H1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.R.; Du, X.L.; Siow, Y.L.; O, K.; Tse, H.F.; Lau, C.P. Calcium-activated transient outward chloride current and phase 1 repolarization of swine ventricular action potential. Cardiovasc. Res. 2003, 58, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Hartzell, H.C.; White, R.E. Effects of magnesium on inactivation of the voltage-gated calcium current in cardiac myocytes. J. Gen. Physiol. 1989, 94, 745–767. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Obukhov, A.G.; Nowycky, M.C. A cytosolic residue mediates Mg2+ block and regulates inward current amplitude of a transient receptor potential channel. J. Neurosci. 2005, 25, 1234–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, M.; Plant, T.D.; Obukhov, A.G.; Hofmann, T.; Gudermann, T.; Schultz, G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 2000, 275, 17517–17526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharov, S.I.; Smani, T.; Leno, E.; Macianskiene, R.; Mubagwa, K.; Bolotina, V.M. Monovalent cation (MC) current in cardiac and smooth muscle cells: Regulation by intracellular Mg2+ and inhibition by polycations. Br. J. Pharmacol. 2003, 138, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Niemann-Nejsum, L.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Waldegger, S.; Mederos y Schnitzler, M.; Vitzthum, H.; Sassen, M.C.; Seyberth, H.W.; Konrad, M.; Gudermann, T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. USA 2004, 101, 2894–2899. [Google Scholar] [CrossRef] [Green Version]
- Isenberg, G. Nonselective cation channels in cardiac and smooth muscle cells. Nonselective Cation Channels 1993, 66, 247–260. [Google Scholar]
- Zakharov, S.I.; Mongayt, D.A.; Cohen, R.A.; Bolotina, V.M. Monovalent cation and L-type Ca2+ channels participate in calcium paradox-like phenomenon in rabbit aortic smooth muscle cells. J. Physiol. 1999, 514, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.M.; Park, M.K.; Lee, S.H.; Ho, W.K.; Earm, Y.E. Contribution of Ca2+-activated K+ channels and non-selective cation channels to membrane potential of pulmonary arterial smooth muscle cells of the rabbit. J. Physiol. 1999, 514, 747–758. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Youm, J.B.; Sung, H.K.; Lee, S.H.; Ryu, S.Y.; Ho, W.K.; Earm, Y.E. Stretch-activated and background non-selective cation channels in rat atrial myocytes. J. Physiol. 2000, 523, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, A.N.; Makhina, E.N.; Nichols, C.G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 1994, 372, 366–369. [Google Scholar] [CrossRef]
- Cartwright, J.H.; Aziz, Q.; Harmer, S.C.; Thayyil, S.; Tinker, A.; Munroe, P.B. Genetic variants in TRPM7 associated with unexplained stillbirth modify ion channel function. Hum. Mol. Genet. 2020, 29, 1797–1807. [Google Scholar] [CrossRef]
- Kozak, J.A.; Kerschbaum, H.H.; Cahalan, M.D. Distinct properties of CRAC and MIC channels in RBL cells. J. Gen. Physiol. 2002, 120, 221–235. [Google Scholar] [CrossRef] [Green Version]
- Macianskiene, R.; Gwanyanya, A.; Vereecke, J.; Mubagwa, K. Inhibition of the magnesium-sensitive TRPM7-like channel in cardiac myocytes by nonhydrolysable GTP analogs: Involvement of phosphoinositide metabolism. Cell Physiol. Biochem. 2008, 22, 109–118. [Google Scholar] [CrossRef]
- Aarts, M.; Iihara, K.; Wei, W.L.; Xiong, Z.G.; Arundine, M.; Cerwinski, W.; MacDonald, J.F.; Tymianski, M. A key role for TRPM7 channels in anoxic neuronal death. Cell 2003, 115, 863–877. [Google Scholar] [CrossRef] [Green Version]
- Touyz, R. Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: Implication in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1103–H1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Jackson, M.; Martin, L.; Jansen, K.; Teves, L.; Cui, H.; Kiyonaka, S.; Mori, Y.; Jones, M.; Forder, J.P.; et al. Suppression of hipocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat. Neurosci. 2009, 12, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Krapivinsky, G.; Mochita, S.; Krapivinsky, L.; Cibulsky, S.; Clapham, D. The TRPM7 ion channel function in cholinergic synaptic vesicles and affects transmitter release. Neuron 2006, 52, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Xie, J.; Zhang, Z.; Tsujikawa, H.; Fusco, D.; Silverman, D.; Liang, B.; Yue, L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 2010, 106, 992–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.H.; Sun, H.Y.; Chen, K.H.; Du, X.L.; Liu, B.; Cheng, L.C.; Li, X.; Jin, M.W.; Li, G.R. Evidence for functional expression of TRPM7 channels in human atrial myocytes. Basic Res. Cardiol. 2012, 107, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macianskiene, R.; Almanaityte, M.; Jekabsone, A.; Mubagwa, K. Modulation of human cardiac TRPM7 current by extracellular acidic pH depends upon extracellular concentrations of divalent cations. PLoS ONE 2017, 12, e0170923. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Flockerzi, V. What do we really know and what do we need to know: Some controversies, perspectives, and surprises. Handb. Exp. Phalmacol. 2014, 223, 1239–1280. [Google Scholar]
- Scalia, C.R.; Gendusa, R.; Basciu, M.; Riva, L.; Tusa, L.; Musarò, A.; Veronese, S.; Formenti, A.; D’Angelo, D.; Ronzio, A.G.; et al. Epitope recognition in the Human–Pig comparison model on fixed and embedded material. J. Histochem. Cytochem. 2015, 63, 805–822. [Google Scholar] [CrossRef] [Green Version]
- Po, S.S.; Wang, D.W.; Johnson, J.P., Jr.; Nie, L.; Bennett, P.B. Modulation of HERG potassium channels by extracellular magnesium and quinidine. J. Cardiovasc. Pharmacol. 1999, 33, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sawanobori, T.; Adaniya, H.; Hirano, Y.; Hiraoka, M. Dual effects of external magnesium on action potential duration in guinea pig ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 1995, 268, H2321–H2328. [Google Scholar] [CrossRef] [PubMed]
- Macianskiene, R.; Martisiene, I.; Zablockaite, D.; Gendviliene, V. Characterization of Mg2+-regulated TRPM7-like current in human atrial myocytes. J. Biomed. Sci. 2012, 19, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
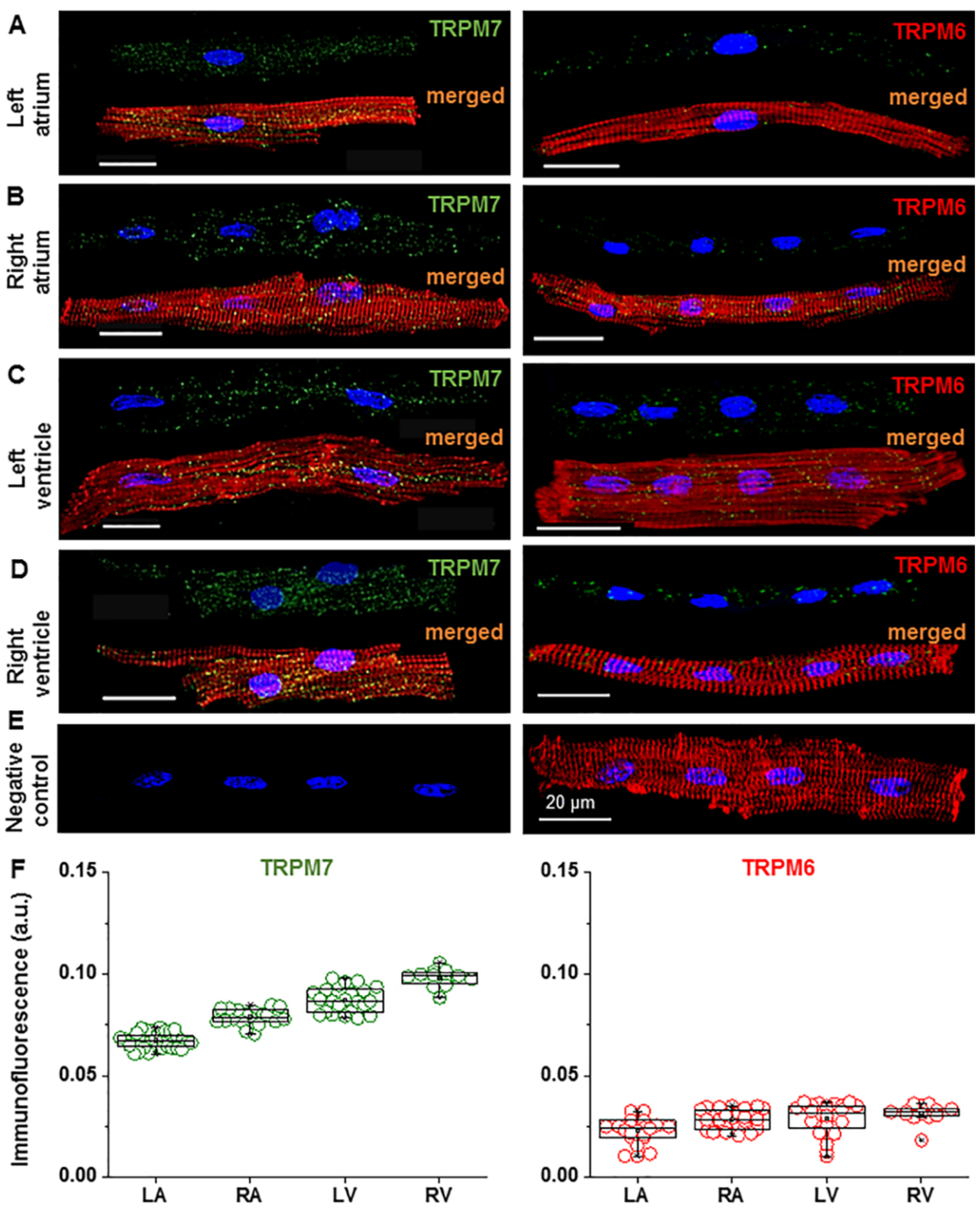
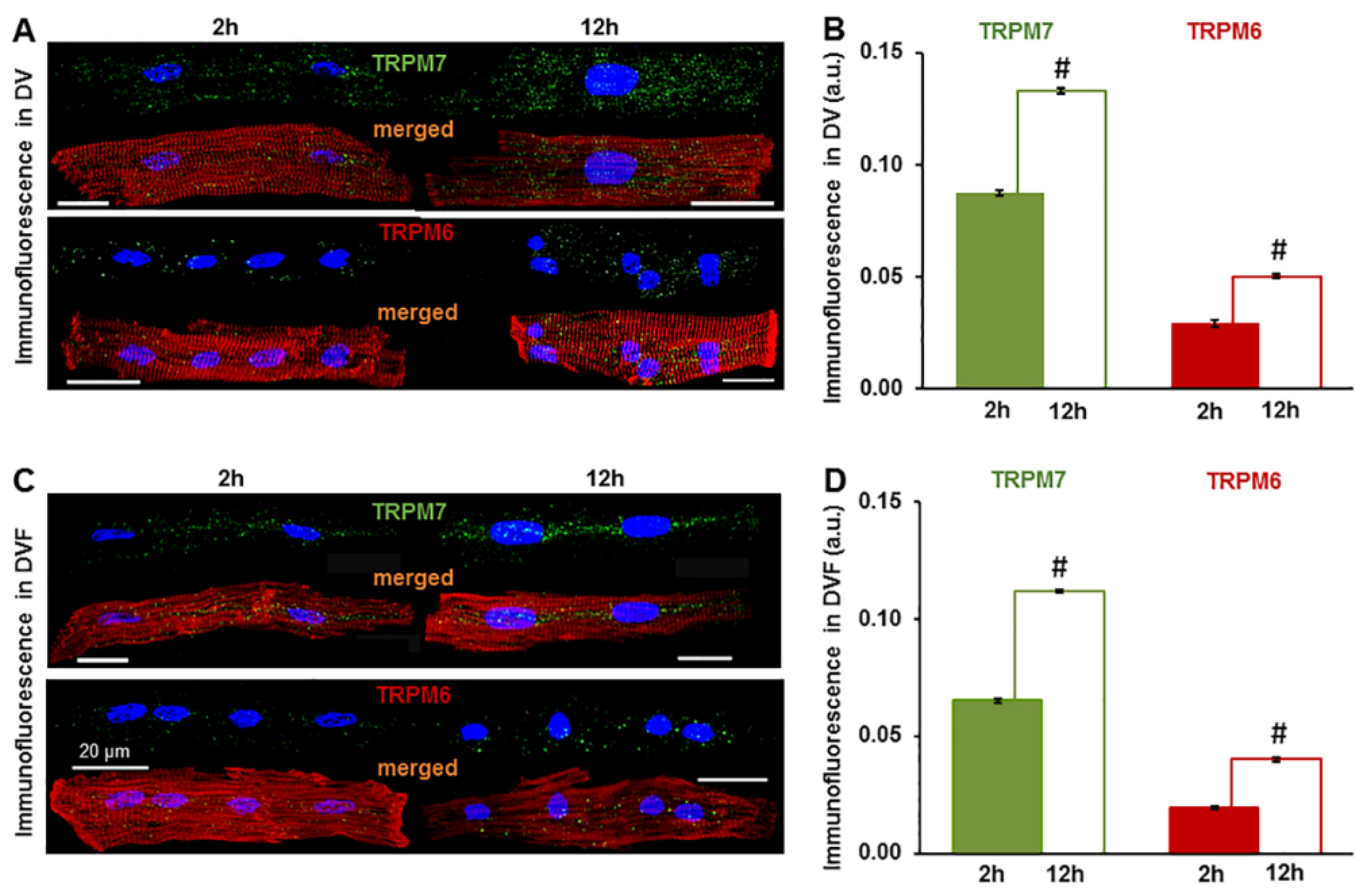


| Heart Chamber | TRPM7 Signal (a.u.) | TRPM6 Signal (a.u.) | ||
|---|---|---|---|---|
| DV | DVF | DV | DVF | |
| LA | 0.067 ± 0.0006 n = 37 | 0.053 ± 0.0009 # n = 13 | 0.023 ± 0.0013 n = 18 | 0.016 ± 0.0014 # n = 20 |
| RA | 0.079 ± 0.0009 n = 21 | 0.059 ± 0.0010 # n = 5 | 0.029 ± 0.0008 n = 32 | 0.018 ± 0.0011 # n = 4 |
| LV | 0.087 ± 0.0013 n = 23 | 0.065 ± 0.0009 # n = 25 | 0.029 ± 0.0015 n = 24 | 0.020 ± 0.0006 # n = 21 |
| RV | 0.098 ± 0.0013 n = 12 | 0.076 ± 0.0016 # n = 5 | 0.032 ± 0.0012 n = 14 | 0.025 ± 0.0020 # n = 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwanyanya, A.; Andriulė, I.; Istrate, B.M.; Easmin, F.; Mubagwa, K.; Mačianskienė, R. Modulation of the Cardiac Myocyte Action Potential by the Magnesium-Sensitive TRPM6 and TRPM7-like Current. Int. J. Mol. Sci. 2021, 22, 8744. https://doi.org/10.3390/ijms22168744
Gwanyanya A, Andriulė I, Istrate BM, Easmin F, Mubagwa K, Mačianskienė R. Modulation of the Cardiac Myocyte Action Potential by the Magnesium-Sensitive TRPM6 and TRPM7-like Current. International Journal of Molecular Sciences. 2021; 22(16):8744. https://doi.org/10.3390/ijms22168744
Chicago/Turabian StyleGwanyanya, Asfree, Inga Andriulė, Bogdan M. Istrate, Farjana Easmin, Kanigula Mubagwa, and Regina Mačianskienė. 2021. "Modulation of the Cardiac Myocyte Action Potential by the Magnesium-Sensitive TRPM6 and TRPM7-like Current" International Journal of Molecular Sciences 22, no. 16: 8744. https://doi.org/10.3390/ijms22168744
APA StyleGwanyanya, A., Andriulė, I., Istrate, B. M., Easmin, F., Mubagwa, K., & Mačianskienė, R. (2021). Modulation of the Cardiac Myocyte Action Potential by the Magnesium-Sensitive TRPM6 and TRPM7-like Current. International Journal of Molecular Sciences, 22(16), 8744. https://doi.org/10.3390/ijms22168744






