Hypomethylated RRBP1 Potentiates Tumor Malignancy and Chemoresistance in Upper Tract Urothelial Carcinoma
Abstract
:1. Introduction
2. Results
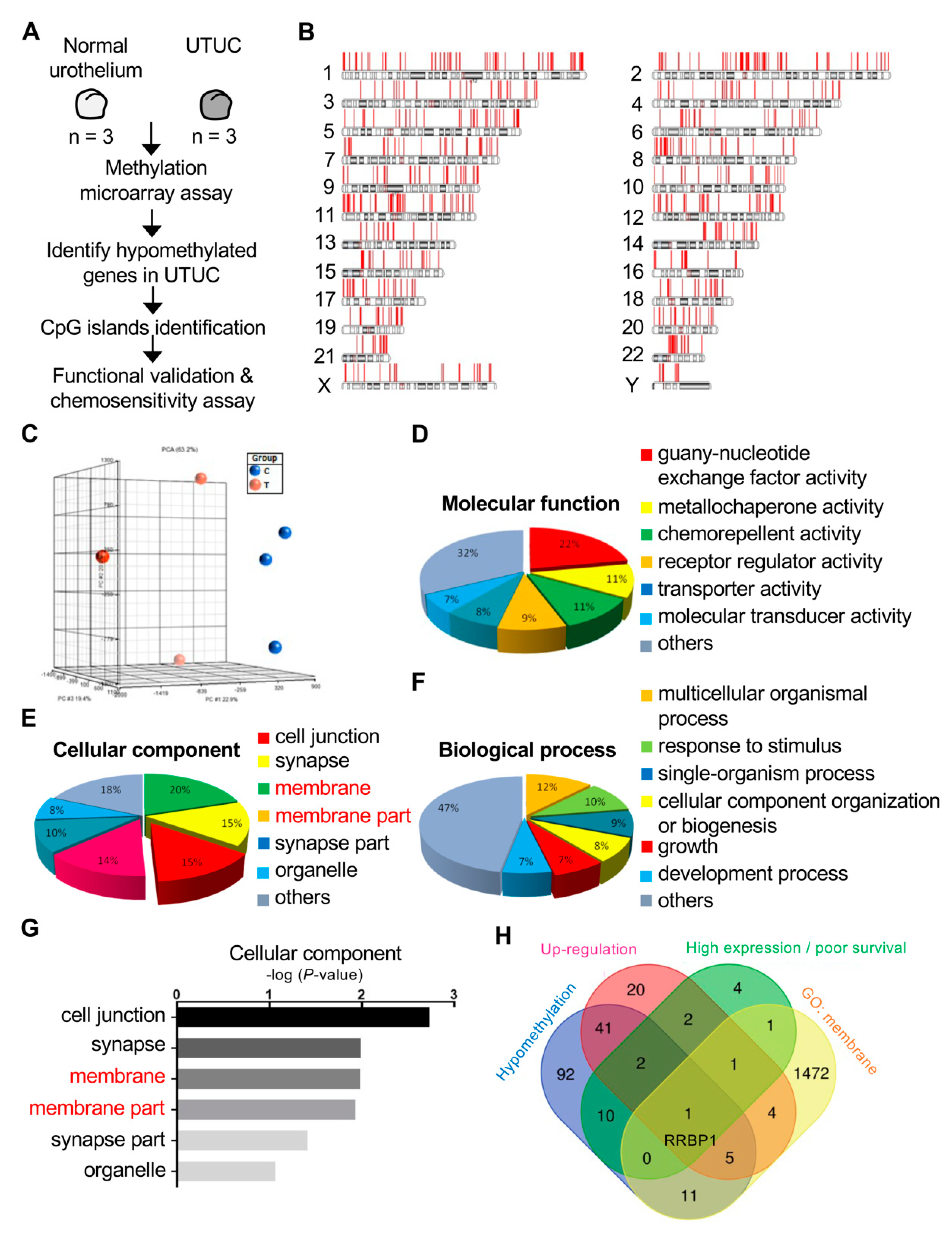
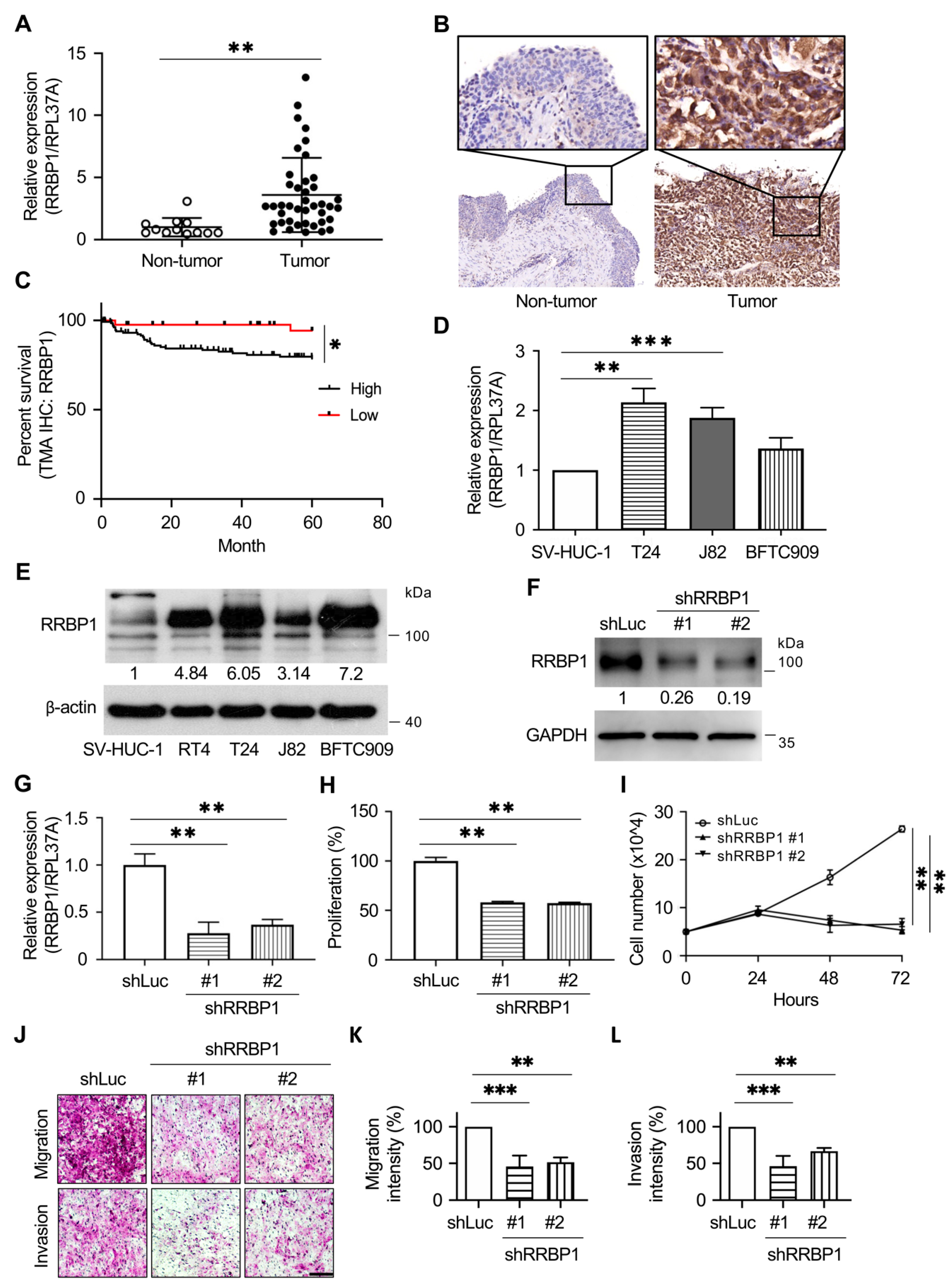
2.1. RRBP1 Is a Hypomethylated and Highly Expressed Oncogene in UTUC
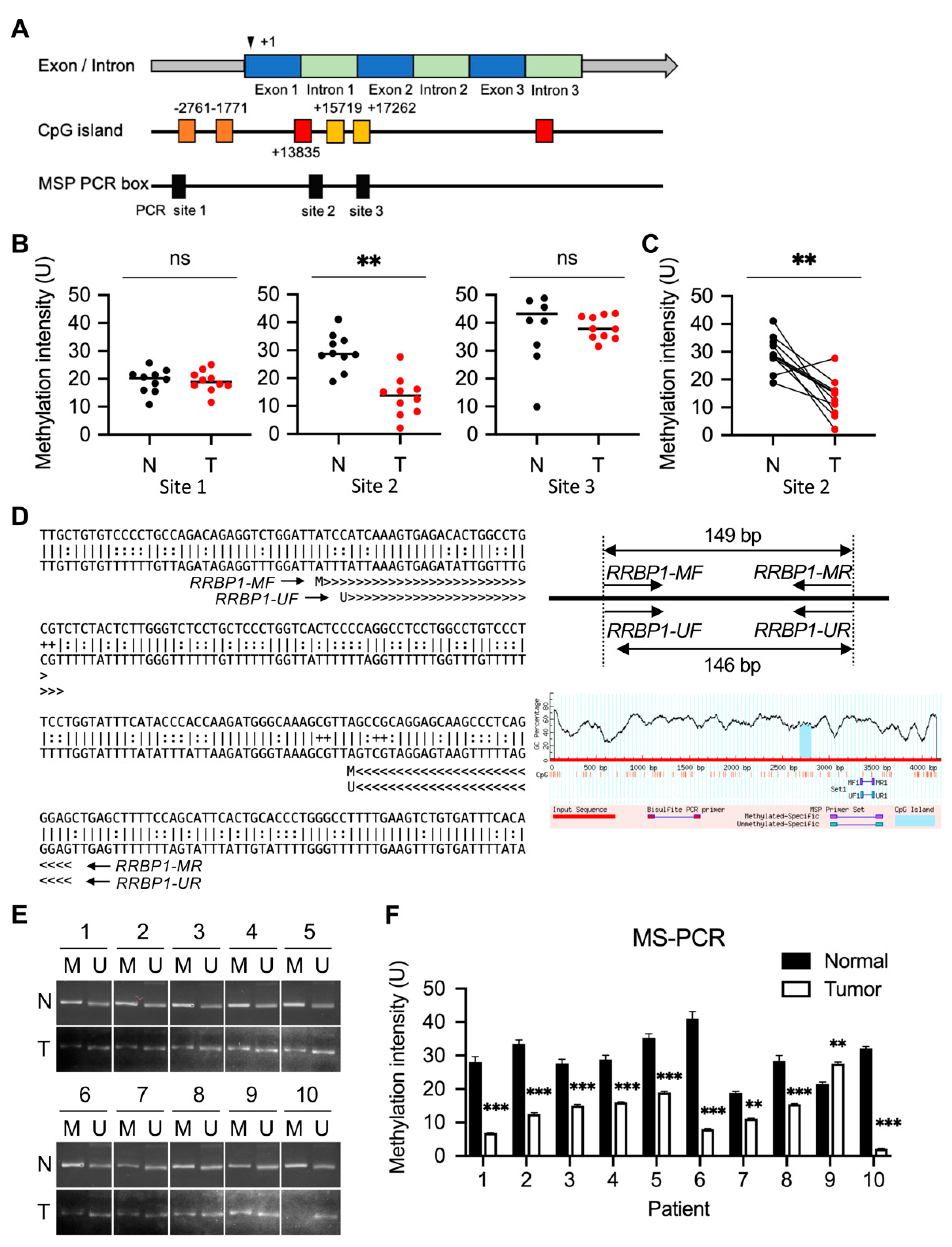
2.2. Methylation of RRBP1 in Bladder Cancer
2.3. Low Methylation of RRBP1 Gene on Chromosome 20: 17,613,678-17,682,283 in UTUC Patients
2.4. RRBP1 Is Highly Expressed in UTUC Tumor Tissues and Cancer Cell Lines of Urothelial Tumors
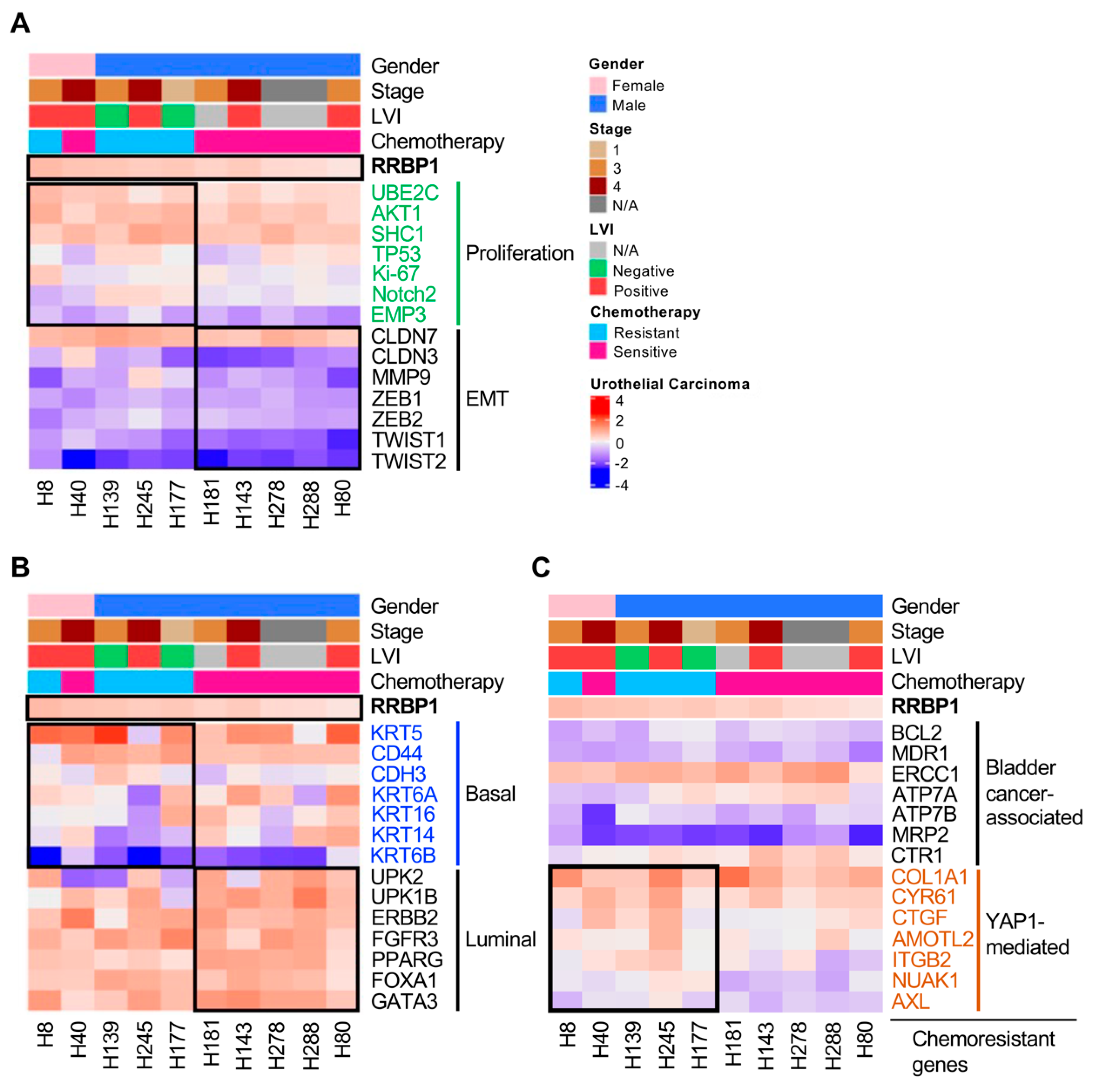
2.5. Analysis of Molecular Subtypes of RRBP1-Correlated Gene Expression in UTUC
2.6. Tumors with Low Expression of RRBP1 Are Sensitive to Cisplatin, Gemcitabine, and Epirubicin
3. Discussion
4. Materials and Methods
4.1. Tissue Samples and Cell Lines
4.2. Methylation-Specific PCR
4.3. Analysis of Clinical Characteristic and Methylation Status of RRBP1 in the BLCA Database
4.4. Immunohistochemistry and Patient Grouping
4.5. shRNA, PCR, and Quantitative Real-Time PCR
4.6. Western Blotting
4.7. In Vitro Migration and Invasion Assay
4.8. Drug Sensitivity Assay in Organoids
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Babjuk, M.; Compérat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Böhle, A.; van Rhijn, B.W.; Kaasinen, E.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur. Urol. 2015, 68, 868–879. [Google Scholar] [CrossRef]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef]
- Cha, E.K.; Shariat, S.F.; Kormaksson, M.; Novara, G.; Rink, M.; Lotan, Y.; Scherr, D.; Raman, J.D.; Kassouf, W.; Zigeuner, R.; et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. J. Clin. Oncol. 2012, 30, 267. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Compérat, E.; Zigeuner, R.; Sylvester, R.; Burger, M.; Cowan, N.; Böhle, A.; van Rhijn, B.W.; Kaasinen, E.; et al. European Guidelines on Upper Tract Urothelial Carcinomas: 2013 Update. Eur. Urol. 2013, 63, 1059–1071. [Google Scholar] [CrossRef]
- Kaag, M.G.; O’Malley, R.L.; O’Malley, P.; Godoy, G.; Chen, M.; Smaldone, M.C.; Hrebinko, R.L.; Raman, J.D.; Bochner, B.; Dalbagni, G.; et al. Changes in Renal Function Following Nephroureterectomy May Affect the Use of Perioperative Chemotherapy. Eur. Urol. 2010, 58, 581–587. [Google Scholar] [CrossRef]
- Xylinas, E.; Rink, M.; Margulis, V.; Clozel, T.; Lee, R.K.; Comploj, E.; Novara, G.; Raman, J.D.; Lotan, Y.; Weizer, A.; et al. Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int. 2013, 112, 453–461. [Google Scholar] [CrossRef]
- Vale, C. Neoadjuvant Chemotherapy in Invasive Bladder Cancer: Update of a Systematic Review and Meta-Analysis of Individual Patient Data. Eur. Urol. 2005, 48, 202–206. [Google Scholar] [CrossRef]
- Leow, J.J.; Martin-Doyle, W.; Rajagopal, P.S.; Patel, C.G.; Anderson, E.M.; Rothman, A.T.; Cote, R.J.; Urun, Y.; Chang, S.L.; Choueiri, T.K.; et al. Adjuvant Chemotherapy for Invasive Bladder Cancer: A 2013 Updated Systematic Review and Meta-Analysis of Randomized Trials. Eur. Urol. 2014, 66, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Oosterlinck, W. EAU Guidelines on Diagnosis and Treatment of Upper Urinary Tract Transitional Cell Carcinoma. Eur. Urol. 2004, 46, 147–154. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.H.; Stadler, W.M. The Role of Chemotherapy in Upper Tract Urothelial Carcinoma. Adv. Urol. 2009, 2009, 1–4. [Google Scholar] [CrossRef]
- Kang, M.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. The Characteristics of Recurrent Upper Tract Urothelial Carcinoma after Radical Nephroureterectomy without Bladder Cuff Excision. Yonsei Med, J. 2015, 56, 375–381. [Google Scholar] [CrossRef]
- Raman, J.D.; Scherr, D.S. Management of patients with upper urinary tract transitional cell carcinoma. Nat. Clin. Pract. Urol. 2007, 4, 432–443. [Google Scholar] [CrossRef]
- Bellmunt, J.; Petrylak, D.P. New Therapeutic Challenges in Advanced Bladder Cancer. Semin. Oncol. 2012, 39, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Van Oers, J.M.; Zwarthoff, E.C.; Rehman, I.; Azzouzi, A.R.; Cussenot, O.; Meuth, M.; Hamdy, F.C.; Catto, J.W. FGFR3 Mutations Indicate Better Survival in Invasive Upper Urinary Tract and Bladder Tumours. Eur. Urol. 2009, 55, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Martin-Doyle, W.; Fay, A.P.; Choueiri, T.K.; Chang, S.L.; Bellmunt, J. A Systematic Review and Meta-analysis of Adjuvant and Neoadjuvant Chemotherapy for Upper Tract Urothelial Carcinoma. Eur. Urol. 2014, 66, 529–541. [Google Scholar] [CrossRef]
- Godfrey, M.S.; Badalato, G.M.; Hruby, G.W.; Razmjoo, M.; McKiernan, J.M. Prognostic indicators for upper tract urothelial carcinoma after radical nephroureterectomy: The impact of lymphovascular invasion. BJU Int. 2012, 110, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, E.; Horiguchi, Y.; Nakashima, J.; Hatakeyama, N.; Matsumoto, M.; Nishiyama, T.; Murai, M. Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J. Urol. 2005, 174, 2120–2124. [Google Scholar] [CrossRef]
- Ku, J.H.; Byun, S.S.; Jeong, H.; Kwak, C.; Kim, H.H.; Lee, S.E. Lymphovascular invasion as a prognostic factor in the upper urinary tract urothelial carcinoma: A systematic review and meta-analysis. Eur. J. Cancer 2013, 49, 2665–2680. [Google Scholar] [CrossRef]
- Savitz, A.J.; Meyer, D.I. 180-kD ribosome receptor is essential for both ribosome binding and protein translocation. J. Cell Biol. 1993, 120, 853–863. [Google Scholar] [CrossRef]
- Liu, G.H.; Mao, C.Z.; Wu, H.Y.; Zhou, D.C.; Xia, J.B.; Kim, S.K.; Cai, D.Q.; Zhao, H.; Qi, X.F. Expression profile of rrbp1 genes during embryonic development and in adult tissues of Xenopus laevis. Gene Expr. Patterns 2017, 23–24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, M.; Zhang, M.; Li, X.; Zhu, Z.; Wang, H. Expression and significance of RRBP1 in esophageal carcinoma. Cancer Manag. Res. 2018, 10, 1243–1249. [Google Scholar] [CrossRef]
- Wanker, E.E.; Sun, Y.; Savitz, A.J.; Meyer, D.I. Functional characterization of the 180-kD ribosome receptor in vivo. J. Cell Biol. 1995, 130, 29–39. [Google Scholar] [CrossRef]
- Benyamini, P.; Webster, P.; Meyer, D.I. Knockdown of p180 Eliminates the Terminal Differentiation of a Secretory Cell Line. Mol. Biol. Cell 2009, 20, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, In Vivo, and Site-Specific Phosphorylation Dynamics in Signaling Networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Yang, Y.F.; Wu, A.T.; Yang, C.J.; Liu, Y.P.; Jan, Y.H.; Lee, C.H.; Hsiao, Y.W.; Yeh, C.T.; Shen, C.N.; et al. Endoplasmic reticulum ribosome-binding protein 1 (RRBP1) overexpression is frequently found in lung cancer patients and alleviates intracellular stress-induced apoptosis through the enhancement of GRP78. Oncogene 2013, 32, 4921–4931. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ren, S.; Ding, J.; Liu, S.; Zhu, J.; Ma, R.; Meng, F. Expression of RRBP1 in epithelial ovarian cancer and its clinical significance. Biosci. Rep. 2019, 39, BSR20190656. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Q.; Hong, X.; Li, H.; Yang, K.; Li, J.; Lei, B. RRBP1 is highly expressed in prostate cancer and correlates with prognosis. Cancer Manag. Res. 2019, 11, 3021–3027. [Google Scholar] [CrossRef]
- Pan, Y.; Cao, F.; Guo, A.; Chang, W.; Chen, X.; Ma, W.; Gao, X.; Guo, S.; Fu, C.; Zhu, J. Endoplasmic reticulum ribosome-binding protein 1, RRBP1, promotes progression of colorectal cancer and predicts an unfavourable prognosis. Br. J. Cancer 2015, 113, 763–772. [Google Scholar] [CrossRef]
- Telikicherla, D.; Marimuthu, A.; Kashyap, M.; Ramachandra, Y.L.; Mohan, S.; Roa, J.; Maharudraiah, J.; Pandey, A. Overexpression of ribosome binding protein 1 (RRBP1) in breast cancer. Clin. Proteom. 2012, 9, 7. [Google Scholar] [CrossRef]
- Liang, X.; Sun, S.; Zhang, X.; Wu, H.; Tao, W.; Liu, T.; Wei, W.; Geng, J.; Pang, D. Expression of ribosome-binding protein 1 correlates with shorter survival in Her-2 positive breast cancer. Cancer Sci. 2015, 106, 740–746. [Google Scholar] [CrossRef]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e17. [Google Scholar] [CrossRef]
- Kampen, K.R. Membrane Proteins: The Key Players of a Cancer Cell. J. Membr. Biol. 2011, 242, 69–74. [Google Scholar] [CrossRef]
- Luo, H.L.; Chiang, P.H.; Huang, C.C.; Su, Y.L.; Sung, M.T.; Tsai, E.M.; Lin, C.S.; Chiang, P.H. Methylation of SPARCL1 Is Associated with Oncologic Outcome of Advanced Upper Urinary Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2019, 20, 1653. [Google Scholar] [CrossRef]
- Lv, S.W.; Shi, Z.G.; Wang, X.H.; Zheng, P.Y.; Li, H.B.; Han, Q.J.; Li, Z.J. Ribosome Binding Protein 1 Correlates with Prognosis and Cell Proliferation in Bladder Cancer. OncoTargets Ther. 2020, 13, 6699–6707. [Google Scholar] [CrossRef]
- Wan, M.; Liu, S.; Zhou, B.; Wang, J.; Ping, H.; Xing, N. RRBP1 is highly expressed in bladder cancer and is associated with migration and invasion. Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef]
- Mari, A.; D’Andrea, D.; Abufaraj, M.; Foerster, B.; Kimura, S.; Shariat, S.F. Genetic determinants for chemo- and radiotherapy resistance in bladder cancer. Transl. Androl. Urol. 2017, 6, 1081–1089. [Google Scholar] [CrossRef]
- Shriwas, O.; Arya, R.; Mohanty, S.; Mohapatra, P.; Kumar, S.; Rath, R.; Kaushik, S.R.; Pahwa, F.; Murmu, K.C.; Majumdar, S.K.D.; et al. RRBP1 rewires cisplatin resistance in oral squamous cell carcinoma by regulating Hippo pathway. Br. J. Cancer 2021, 124, 2004–2016. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2020, 21, 71–88. [Google Scholar] [CrossRef]
- Andrews, A.M.; Tennant, M.D.; Thaxton, J.E. Stress relief for cancer immunotherapy: Implications for the ER stress response in tumor immunity. Cancer Immunol. Immunother. 2020, 70, 1165–1175. [Google Scholar] [CrossRef]
- Park, D.; Goh, C.; Kim, H.; Lee, J.; Hahn, Y. Loss of conserved ubiquitylation sites in conserved proteins during human evolution. Int. J. Mol. Med. 2018, 42, 2203–2212. [Google Scholar] [CrossRef]
- He, Y.; Huang, S.; Cheng, T.; Wang, Y.; Zhou, S.J.; Zhang, Y.M.; Yu, P. High glucose may promote the proliferation and metastasis of hepatocellular carcinoma via E2F1/RRBP1 pathway. Life Sci. 2020, 252, 117656. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Kang, X.; Zhang, G.; Fang, F.; Du, Y.; Lv, H. High expression of UBE2C is associated with the aggressive progression and poor outcome of malignant glioma. Oncol. Lett. 2016, 11, 2300–2304. [Google Scholar] [CrossRef]
- Mo, C.H.; Gao, L.; Zhu, X.F.; Wei, K.L.; Zeng, J.J.; Chen, G.; Feng, Z.B. The clinicopathological significance of UBE2C in breast cancer: A study based on immunohistochemistry, microarray and RNA-sequencing data. Cancer Cell Int. 2017, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Zhao, G.; Ke, B.; Liu, G.L.; Liang, H.; Liu, L.R.; Hao, X.S. Overexpression of UBE2C correlates with poor prognosis in gastric cancer patients. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 1665–1671. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, J.; Pan, B.; Ma, G.; Liu, L. UBE2C overexpression in melanoma and its essential role in G2/M transition. J. Cancer 2019, 10, 2176–2184. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, R.; Chi, S.; Zhang, W.; Xiao, C.; Zhou, X.; Zhao, Y.; Wang, H. UBE2C Is Upregulated by Estrogen and Promotes Epithelial–Mesenchymal Transition via p53 in Endometrial Cancer. Mol. Cancer Res. 2019, 18, 204–215. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, X.; Cui, L.; Xu, X.; Zhao, Y.; Younai, F.; Messadi, D.; Hu, S. UBE2C promotes the progression of head and neck squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2020, 523, 389–397. [Google Scholar] [CrossRef]
- Liu, P.F.; Chen, C.F.; Shu, C.W.; Chang, H.M.; Lee, C.H.; Liou, H.H.; Ger, L.P.; Chen, C.L.; Kang, B.H. UBE2C is a Potential Biomarker for Tumorigenesis and Prognosis in Tongue Squamous Cell Carcinoma. Diagnostics 2020, 10, 674. [Google Scholar] [CrossRef]
- Wright, K.D.; Miller, B.S.; El-Meanawy, S.; Tsaih, S.W.; Banerjee, A.; Geurts, A.M.; Sheinin, Y.; Sun, Y.; Kalyanaraman, B.; Rui, H.; et al. The p52 isoform of SHC1 is a key driver of breast cancer initiation. Breast Cancer Res. 2019, 21, 74. [Google Scholar] [CrossRef]
- Bhat, A.A.; Pope, J.L.; Smith, J.J.; Ahmad, R.; Chen, X.; Washington, M.K.; Beauchamp, R.D.; Singh, A.B.; Dhawan, P. Claudin-7 expression induces mesenchymal to epithelial transformation (MET) to inhibit colon tumorigenesis. Oncogene 2014, 34, 4570–4580. [Google Scholar] [CrossRef]
- Dahiya, N.; Becker, K.G.; Wood, W.H.; Zhang, Y.; Morin, P.J. Claudin-7 Is Frequently Overexpressed in Ovarian Cancer and Promotes Invasion. PLoS ONE 2011, 6, e22119. [Google Scholar] [CrossRef]
- Huang, Y.H.; Bao, Y.; Peng, W.; Goldberg, M.; Love, K.; Bumcrot, D.A.; Cole, G.; Langer, R.; Anderson, D.G.; Sawicki, J.A. Claudin-3 gene silencing with siRNA suppresses ovarian tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2009, 106, 3426–3430. [Google Scholar] [CrossRef]
- Pecoraro, A.; Pagano, M.; Russo, G.; Russo, A. Role of Autophagy in Cancer Cell Response to Nucleolar and Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2020, 21, 7334. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2015, 73, 79–94. [Google Scholar] [CrossRef]
- Corazzari, M.; Gagliardi, M.; Fimia, G.M.; Piacentini, M. Endoplasmic Reticulum Stress, Unfolded Protein Response, and Cancer Cell Fate. Front. Oncol. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Gong, J.; Wang, X.Z.; Wang, T.; Chen, J.J.; Xie, X.Y.; Hu, H.; Yu, F.; Liu, H.L.; Jiang, X.Y.; Fan, H.D. Molecular signal networks and regulating mechanisms of the unfolded protein response. J. Zhejiang Univ. Sci. B 2017, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Kurtoglu, M.; Liu, H.; Wangpaichitr, M.; You, M.; Liu, X.; Savaraj, N.; Lampidis, T.J. 2-Deoxy-d-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother. Pharmacol. 2010, 67, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.J.; Hendershot, L.M. UPR activation alters chemosensitivity of tumor cells. Cancer Biol. Ther. 2006, 5, 736–740. [Google Scholar] [CrossRef]
- Lee, W.; Yoo, W.; Chae, H. ER Stress and Autophagy. Curr. Mol. Med. 2015, 15, 735–745. [Google Scholar] [CrossRef]
- Van de Wetering, M.; Francies, H.; Francis, J.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Lamy, P.; Nordentoft, I.; Birkenkamp-Demtröder, K.; Thomsen, M.B.H.; Villesen, P.; Vang, S.; Hedegaard, J.; Borre, M.; Jensen, J.B.; Høyer, S.; et al. Paired Exome Analysis Reveals Clonal Evolution and Potential Therapeutic Targets in Urothelial Carcinoma. Cancer Res. 2016, 76, 5894–5906. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer–derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Pasch, C.A.; Favreau, P.F.; Yueh, A.E.; Babiarz, C.P.; Gillette, A.A.; Sharick, J.T.; Karim, M.R.; Nickel, K.P.; DeZeeuw, A.K.; Sprackling, C.M.; et al. Patient-Derived Cancer Organoid Cultures to Predict Sensitivity to Chemotherapy and Radiation. Clin. Cancer Res. 2019, 25, 5376–5387. [Google Scholar] [CrossRef]
- Driehuis, E.; Kolders, S.; Spelier, S.; Lõhmussaar, K.; Willems, S.M.; Devriese, L.A.; de Bree, R.; de Ruiter, E.J.; Korving, J.; Begthel, H.; et al. Oral Mucosal Organoids as a Potential Platform for Personalized Cancer Therapy. Cancer Discov. 2019, 9, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, G.; de Chiara, A.; Parafioriti, A.; Armiraglio, E.; Fazioli, F.; Gallo, M.; Aversa, L.; Camerlingo, R.; Cacciatore, F.; Colella, G.; et al. Patient-derived organoids as a potential model to predict response to PD-1/PD-L1 checkpoint inhibitors. Br. J. Cancer 2019, 121, 979–982. [Google Scholar] [CrossRef]
- Peng, J.M.; Tseng, R.H.; Shih, T.C.; Hsieh, S.Y. CAMK2N1 suppresses hepatoma growth through inhibiting E2F1-mediated cell-cycle signaling. Cancer Lett. 2021, 497, 66–76. [Google Scholar] [CrossRef]
- Cvetanova, B.; Li, M.Y.; Yang, C.C.; Hsiao, P.W.; Yang, Y.C.; Feng, J.H.; Shen, Y.C.; Nakagawa-Goto, K.; Lee, K.H.; Shyur, L.F. Sesquiterpene Lactone Deoxyelephantopin Isolated from Elephantopus scaber and Its Derivative DETD-35 Suppress BRAFV600E Mutant Melanoma Lung Metastasis in Mice. Int. J. Mol. Sci. 2021, 22, 3226. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.-L.; Liu, H.-Y.; Chang, Y.-L.; Sung, M.-T.; Chen, P.-Y.; Su, Y.-L.; Huang, C.-C.; Peng, J.-M. Hypomethylated RRBP1 Potentiates Tumor Malignancy and Chemoresistance in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2021, 22, 8761. https://doi.org/10.3390/ijms22168761
Luo H-L, Liu H-Y, Chang Y-L, Sung M-T, Chen P-Y, Su Y-L, Huang C-C, Peng J-M. Hypomethylated RRBP1 Potentiates Tumor Malignancy and Chemoresistance in Upper Tract Urothelial Carcinoma. International Journal of Molecular Sciences. 2021; 22(16):8761. https://doi.org/10.3390/ijms22168761
Chicago/Turabian StyleLuo, Hao-Lun, Hui-Ying Liu, Yin-Lun Chang, Ming-Tse Sung, Po-Yen Chen, Yu-Li Su, Chun-Chieh Huang, and Jei-Ming Peng. 2021. "Hypomethylated RRBP1 Potentiates Tumor Malignancy and Chemoresistance in Upper Tract Urothelial Carcinoma" International Journal of Molecular Sciences 22, no. 16: 8761. https://doi.org/10.3390/ijms22168761





