1. Introduction
Glial cells are non-neuronal cells in the central and peripheral nervous systems. The glial cells in the central nervous system (CNS) include oligodendrocytes, astrocytes, ependymal cells, and microglia [
1]. In this study, we focused on microglia and astrocytes. Microglia are resident immune cells of the CNS such as brain-specific macrophages. In a healthy brain, microglia show a ramified morphology in their resting state and patrol the brain to regulate brain homeostasis [
2]. Microglia become activated after brain injury, either classically activated or alternatively activated, and are referred to as M1 and M2 types. The activated microglia exhibit a morphological change from a ramified shape to rounded or elongated spindle shape and migrate to the injury site [
3]. At the injury site, two types of activated microglia play opposite roles. M1 microglia play a pro-inflammatory role under brain injury by releasing harmful cytokines, which induce neuronal death, while M2 microglia release anti-inflammatory cytokines, clean up the debris at the injury site by phagocytosis, and induce tissue repair by the secretion of neuroprotective growth factors [
4,
5].
In the case of stroke, M2 microglia/macrophages increase within five days after injury. However, after M2 microglia reach the peak, they decrease, while M1 microglia/macrophages show steady enhancement until 14 days after injury [
6]. Although it is unclear whether an increase in M1 microglia means an increasing M1 polarization of resting microglia or the skewing of M2 microglia to M1 microglia, an increase in M2 polarization or inhibition of M1 skewing are key for impeding the aggravation of injury and inducing tissue repair. Since the microglial population in the brain is low, it is important to increase microglial proliferation and reversibly convert M1 and M2 phenotypes in vitro for adoptive cell therapy. There are many previous studies regarding the regulation of microglial M1/M2 polarization. Interleukin-4 (IL-4) and interleukin-10 (IL-10) are well-known cytokines that induce M2 microglia, and tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) are representative inducers of M1 microglia [
4,
7,
8]. Substance P (SP) is a neurotransmitter composed of 11 amino acids regulating immune modulation [
9]. In our previous study, the injection of SP into a rat spinal cord injury model increased the number of M2 microglia/macrophages at the injury site [
10]. Moreover, bone marrow monocytes/macrophages can be activated and induced to proliferate by GM-CSF treatment, and induced to polarize into either of the M1 or M2 phenotypes by pro-inflammatory cytokines, such as TNF-α and IFN-γ or anti-inflammatory cytokines, such as IL-4/13 and IL-10, respectively, where SP, known as a neurotransmitter or neurohormone, has been identified as a novel anti-inflammatory cytokine [
11]. Microglia and macrophages play similar roles at the tissue level and share many markers. However, SP’s effect on microglia is unclear.
Astrocytes are the most abundant cells in the brain, and they maintain CNS homeostasis, provide structural support, and regulate ion, nutrient, and gas concentrations [
12,
13]. Astrocytes become reactive astrocytes after injury. Reactive astrocytes act as a double-edged sword because they play a dual role in CNS disease. Reactive astrocytes enhance damage by releasing pro-inflammatory cytokines, forming a glial scar, or alleviating the inflammation, and boosting tissue regeneration [
14,
15]. Just like microglia, recently, reactive astrocytes were also distinguished as two different types: A1 astrocytes play a harmful role, while A2 astrocytes are neuroprotective [
16,
17]. Although the roles of A1/A2 astrocytes are well known, more research on the inducers of A1/A2 astrocytes and the makers of both reactive astrocytes is needed. Furthermore, the crosstalk between astrocytes and neurons and microglia and neurons has been vigorously studied [
18,
19], whereas the crosstalk between astrocytes and microglia has been less studied. During CNS disease, astrocytes are activated later than microglia. Thus, studies of the crosstalk between microglia and astrocytes focused on astrocytes; studies on the crosstalk between these two cells with a focus on microglia and the correlation of both A1/A2 astrocytes and microglia are needed.
In this study, we aimed to elucidate whether astrocytes and microglia could crosstalk, especially focusing on the microglial phenotypic change and microglial polarization induced by SP. We found that microglia co-cultured with mixed glial cells for 14 days were polarized to the M2 phenotype and that microglial proliferation increased. Moreover, to further demonstrate the key cells that induced these microglial changes among the mixed glial cells, we co-cultured microglia and astrocytes. The microglia–astrocyte co-culture showed more proliferating cells, as confirmed by a BrdU-incorporation assay. In addition, to increase microglial proliferation, we treated the microglia with various cytokines and demonstrated that GM-CSF increased the proliferation of microglia and induced M1 polarization. This polarization was inhibited by sequential treatment with SP, IL-4, or IL-10 after GM-CSF treatment. Furthermore, we demonstrated that the reactive astrocytes of the co-culture were skewed to the A2 type by confirming A1/A2 specific marker expression. In conclusion, crosstalk between microglia and astrocytes increased microglial proliferation and M2 polarization, and SP was able to suppress the M1 polarization of microglia.
3. Discussion
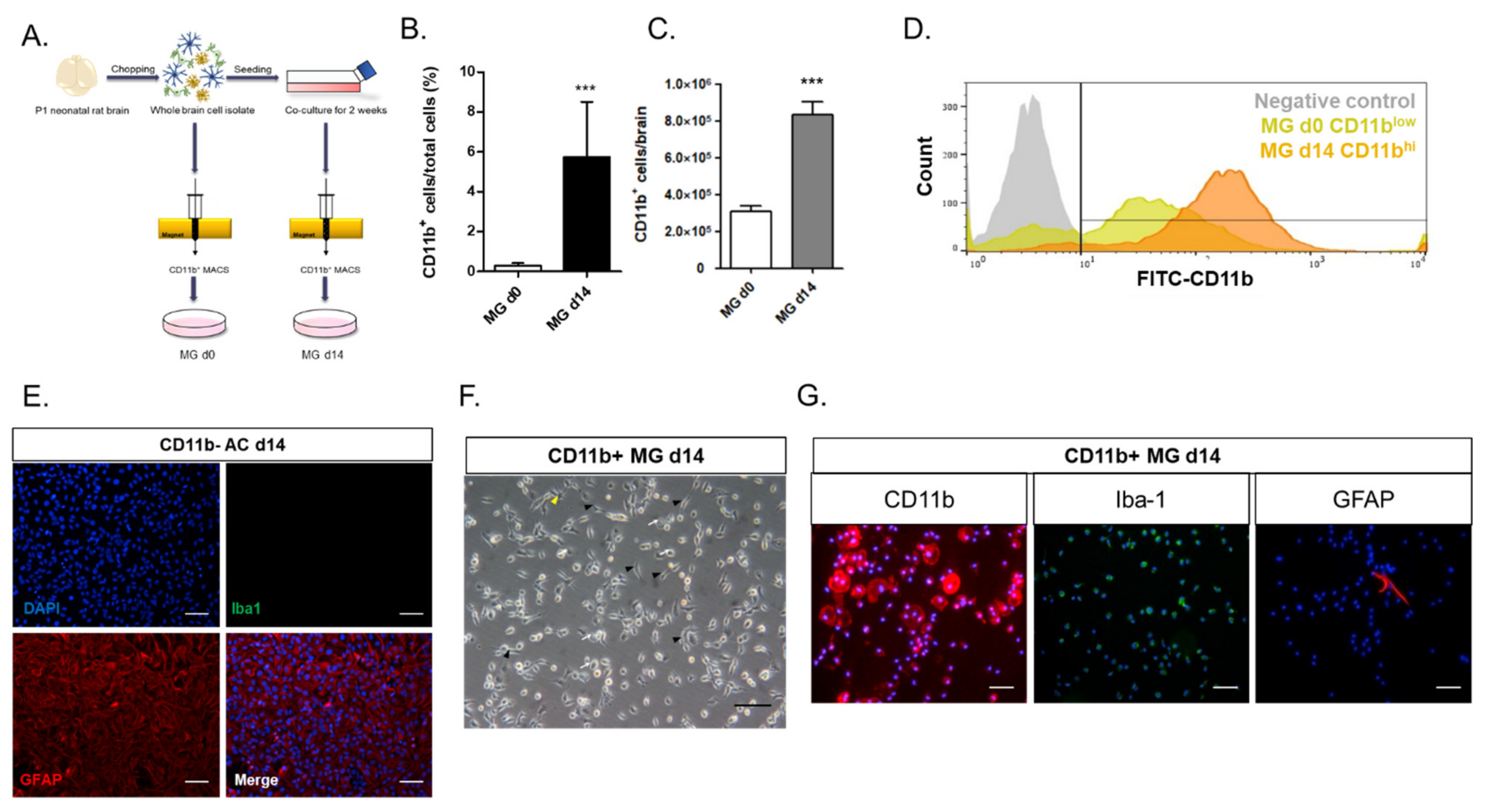
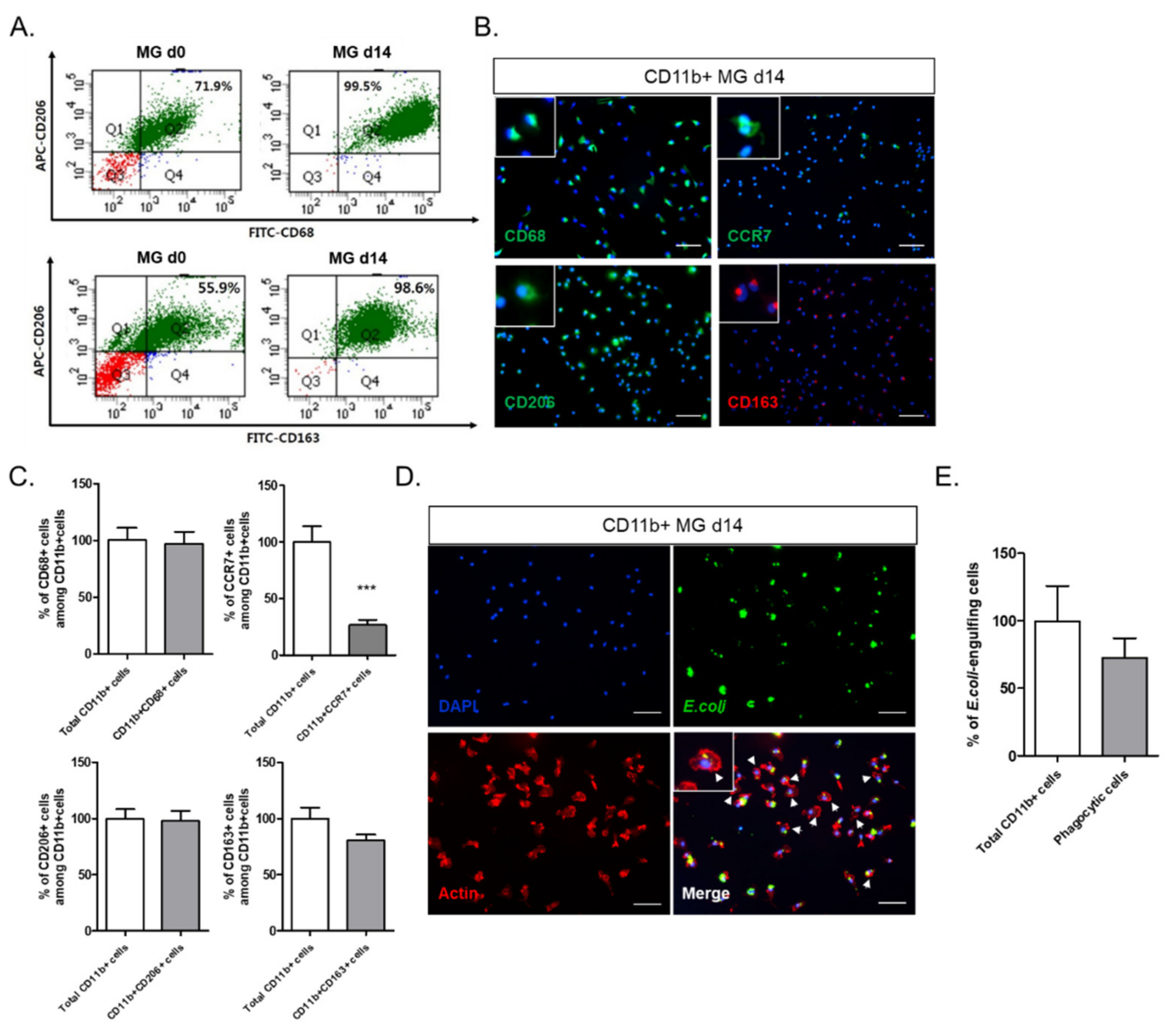
In this study, we investigated the crosstalk between microglia and astrocytes and their phenotypic conversion in vitro. Over two weeks of culture, microglia isolated from neonatal rat brains by CD11b
+ MACS isolation showed an increase in cell number (
Figure 1), cells positive for M2 microglial markers, and phagocytotic microglia (
Figure 2). This supports the concept that microglia derived from the neonatal rat brain can proliferate in a mixed culture of whole brain isolates and preferentially transform to M2-type microglia with phagocytic function. In addition, we characterized the non-microglial cell population in the mixed glial cell culture by staining with microglia- and astrocyte-specific markers. Almost 99% of the CD11b
− cells were found to be GFAP-positive astrocytes (
Figure 1E). Thus, astrocytes are main cellular components in mixed cultures of whole brain cell isolates, which may provide a favorable microenvironment for microglial survival and proliferation. Moreover, the stimulation of astrocytes was demonstrated with the reconstitution of astrocytes in the CD11b
+ MACS-sorted microglial culture and enhanced by astrocyte conditioned medium (
Figure 3B–D). However, microglial cell proliferation was significantly higher in direct cell–cell contact in the microglia–astrocyte co-culture than in the conditioned medium. Therefore, astrocytes clearly secrete some factors stimulating microglial proliferation, but direct cellular contact with astrocytes further stimulates microglial proliferation. Additionally, cytokine arrays for microglial culture, astrocyte culture, and microglial and astrocyte co-culture in different cell ratios showed that both cells secrete many cytokines involved in immune responses, neurotrophic responses, tissue repair, and tissue remodeling (
Figure 4), which may work in coordination in influencing microglial proliferation as well as M2 polarization. In particular, astrocytes in the mixed glial culture also seemed to acquire the A2 phenotype. Finally, it was shown that M2 microglia and A2 astrocytes could be further converted to M1 microglia and A1 astrocytes under an inflammatory microenvironment and stroke-mimicking environment, and TNF-α and IFN-γ increased Bcl-xL expression of microglia, suggesting self-defense mechanism from NO-induced apoptosis [
22] (
Figure 5,
Figure 6 and
Figure 7). In addition, astrocytes in microglia co-culture could be very susceptibly induced to A1 astrocyte phenotype by IFN-γ or TNF-α and CINC-1/2 expression could be applied as a whole marker for TNF-α -inducible A1 astrocyte. Thus, this study demonstrated crosstalk between microglia and astrocytes facilitating M2 microglia and A2 astrocytes, which can be further regulated under a variety of cytokine microenvironments, influencing their behavior in the in vivo environment after transplantation as adoptive cell therapy.
In addition, M2 microglia are not terminally differentiated but can proliferate and polarize to M1 microglia upon exposure to astrocyte-secreted cytokines, such as GM-CSF; M1 microglia have a phenotype that can also be further modulated by anti-inflammatory cytokines. Therefore, M1/M2 polarization may be specifically interpreted in the context of the cytokine milieu in the vicinity. In the brain microenvironment after ischemic injury, many inflammatory cytokines can activate M1 microglial polarization [
23,
24]. As shown by our data, M2 microglia can be polarized to M1 microglia upon exposure to inflammatory cytokines such as TNF-α and IFN-γ (
Figure 5). M2 microglia play an anti-inflammatory role in CNS injury by the secretion of many growth factors and cytokines that promote tissue repair. However, applying M2 microglia as a cell therapy is quite challenging because a microglial cell source, such as the brain, is difficult to isolate from humans, the number of microglia in the brain is too low, and the injury environment can polarize the injected M2 microglia to M1 microglia. Thus, for adoptive cell therapy with M2 microglia to treat a variety of neurodegenerative diseases that have chronic inflammatory backgrounds, it may be technically important to increase M2 microglial cell populations by in vitro astrocyte co-culture, GM-CSF priming, and the subsequent induction of M2 polarization with IL-4, IL-10, and SP. However, the therapeutic effect of injecting M2 microglia to the stroke model and the maintenance of their phenotypes must be further demonstrated. In addition, SP shows an immunosuppressive role in both microglia and astrocytes, but further research is still needed to determine whether SP can skew M1 microglia to M2 microglia in conjunction with different cytokines and if SP can be a novel inducer of A2 astrocytes.
In a previous study, the neuroprotective crosstalk between astrocytes and microglia modulated TGF-β and IL-10 under an LPS-induced injury environment [
25]. According to this study, the astrocytes in the microglial co-culture were most likely skewed to A2 astrocytes, and IL-10 secretion was increased in the co-culture condition, demonstrating the neuroprotective crosstalk between astrocytes and microglia under normal status. These cultured A2 astrocytes could be induced to the A1 phenotype by TNF-α and IFN-γ in the OGD condition; IFN-γ may be the most potent A1 inducer in the stroke environment (
Figure 7). Moreover, CINC-1 and CINC-2, which were shown to be major cytokines released from astrocytes based on a cytokine array (
Figure 4), may be considered to be novel markers of A1 astrocytes, since TNF-α, a known A1-inducing cytokine, strongly stimulated CINC-1/2 secretion and expression (
Figure 7B). Therefore, this microglia–astrocyte co-culture system can be further applied to elucidate the phenotypic conversion of the A1/A2 astrocytes and the bidirectional communication between microglia and A1 and A2 reactive astrocytes in a variety of neuropathology-mimicking microenvironments.
In many previous studies, the roles of the Th 1 and Th 2 cytokines were well investigated under many neurodegenerative and autoimmune diseases. Th 1 cytokines, such as TNF-α and IFN-γ, are known to lead the progression of autoimmune diseases such as type 1 diabetes and multiple sclerosis (MS) [
26,
27,
28], while IL-4, IL-10, and IL-13 refer to Th 2 cytokines and inhibit the pro-inflammatory response and reduce damage [
29,
30,
31]. Furthermore, IL-37 plays an anti-inflammatory role and suppresses the pathogenesis of MS [
32,
33]. Moreover, the macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine produced by epithelial cells, immune cells, astrocytes, and neurons. In neurodegenerative conditions, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS), MIF plays a dichotomic role. In PD and ALS, MIF inhibits the apoptosis of neuronal cells via suppressing inflammation by reducing the number of microglia/macrophages, which can result in neuroprotection [
34]. However, in the case of AD, MIF induces pathogenic progression [
35]. These cytokines may affect the microglial and astrocyte polarization under the injury environment, causing inflammation or suppression. The in vitro co-culture system in this study can be a good tool with which to develop expectations for how microglia and astrocytes may behave in the disease milieu after in vivo transplantation under brain injury and how these cells directly crosstalk and regulate the inflammatory responses to various cytokine stimuli.
4. Materials and Methods
4.1. Animals
All the animal experiments were approved by the Animal Studies Committee of Kyung Hee University (KHUASP #16-012 approved on 1 July 2016) in Yong In, Korea, and performed under the Institutional Animal Care and Use Committee (IACUC) guidelines.
4.2. Mixed Glial Cell Culture and Isolation of Microglia
The mixed glial cell culture was prepared from the brain of postnatal day (P) 1–3 inbred Lewis rats (Orientbio, Sungnam, Korea), modified as previously described [
36]. Briefly, the meninges and the blood vessels of the whole brain were removed under a dissecting microscope. Then, the brain was minced into small pieces in serum-free Dulbecco’s modified Eagle’s medium/nutrient mixture F12 mixture (1:1) (DMEM/F12, Gibco, Grand Island, NY, USA) and centrifuged. The pellet was re-suspended in DPBS (Welgene, Daegu, Korea). After washing, the cells were seeded in 75 cm
2 culture flasks in 15 mL of DMEM/F12 (1:1) containing 10% FBS (Gibco, Grand Island, NY, USA), 2 mM L-glutamine (Welgene, Daegu, Korea), 50 U/mL penicillin, and 50 mg/mL streptomycin (Welgene, Daegu, Korea). The cultures were maintained at 37 °C in a humidified atmosphere of 5% CO
2 and 95% air. The medium was changed every three days, and the culture was maintained for 14 days.
To harvest microglia, microglia were isolated from the mixed glial cell culture on Day 14 using magnetic-activated cell sorting (MACS). All the glial cells were detached using 0.25% trypsin–EDTA, which was then neutralized with complete culture medium. Then, the cells were washed with PBS and re-suspended to a cell density of 1 × 107 cells/mL. The cells were incubated with a CD11b antibody (1:100, BD Bioscience, San Jose, CA, USA) for 5 min at 4 °C and anti-mouse IgG microbeads (1:5, Miltenyi, Bergisch Gladbach, Germany) for 15 min at 4 °C. The cells were washed twice with PBS. During washing, the MACS column was equilibrated by applying 10 mL of autoMACS rinsing solution (Miltenyi, Bergisch Gladbach, Germany). The cell suspension was applied to the LS column (Miltenyi, Bergisch Gladbach, Germany) and allowed to pass through the resin by gravity flow. The column was washed twice with autoMACS rinsing solution. Then, the column was taken off from the magnet, and the CD11b+ microglia were eluted. The microglia were seeded into 6-well plates at a density of 1 × 105 cells/mL or seeded into 24-well plates at a density of 5 × 104 cells/mL and incubated with DMEM/F12 (1:1) supplemented with 10% FBS (Gibco, Grand Island, NY, USA), 2 mM L-glutamine (Welgene, Daegu, Korea), 50 U/mL penicillin, and 50 mg/mL streptomycin (Welgene, Daegu, Korea) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
4.3. Phenotype Conversion of M1 and M2 Microglia In Vitro
To investigate the phenotypical changes of microglia, MG d14 were pre-treated with 10 ng/mL GMCSF (R&D Systems, Minneapolis, MN, USA) for 5 days and then incubated with the following cytokines for 3 days: 10 ng/mL GMCSF (R&D systems, Minneapolis, MN, USA), 100 nM Substance P (SP, Sigma, St. Louis, MO, USA), 10 ng/mL rat IL-4 (R&D systems, Minneapolis, MN, USA), 10 ng/mL rat IL-10 (R&D systems, Minneapolis, MN, USA), 10 ng/mL TNF-α (Sigma, St. Louis, MO, USA), and 20 ng/mL IFN-γ (R&D systems, Minneapolis, MN, USA).
4.4. Astrocyte Culture and OGD In Vitro
CD11b− astrocytes were collected by CD11b MACS. The cells were incubated with DMEM/F12 (1:1) supplemented with 10% FBS (Gibco, Grand Island, NY, USA), 2 mM L-glutamine (Welgene, Daegu, Korea), 50 U/mL penicillin, and 50 mg/mL streptomycin (Welgene, Daegu, Korea) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The culture was maintained for 14 days.
To mimic the in vivo stroke environment, CD11b− astrocytes isolated by CD11b MACS were seeded into 6-well plates at a density of 1 × 106 cells/mL and incubated with no-glucose DMEM (Gibco, Grand Island, NY, USA) at 37 °C in a humidified atmosphere of 1% O2 and 5% CO2 air for 24 h using a Galaxy 48R incubator (Eppendorf, Hamburg, Germany). Then, the cells were treated with 10 ng/mL TNF-α (Sigma, St. Louis, MO, USA) and 10 ng/mL IFN-γ (R&D systems, Minneapolis, MN, USA) for 4 h at 37 °C in a humidified atmosphere of 5% CO2 and 95% air, mimicking the reperfusion following ischemic stroke.
4.5. Phagocytosis Assay for Microglia
On Day 1, the MG d14 were incubated with 25 μg/mL E. coli BioParticle (Thermo, Waltham, MA, USA) for 30 min and fixed with 3.7% formaldehyde. The cells were permeabilized with 0.2% Triton X-100 for 5 min and incubated with TRITC-conjugated phalloidin (Sigma, St. Louis, MO, USA, 1:1000) for 30 min at RT. Then, the cells were mounted with Vectashield mounting medium containing DAPI (Vector, Burlingame, CA, USA), and immunofluorescence images were obtained using the Leica DMI 4000 B fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
4.6. Reconstitution of Astrocyte Feeder on Microglial Culture
Astrocytes were cultured as described above. When the cells reached confluence, 10 μM Ara-C (Sigma, St. Louis, MO, USA) was added for 4 days, and the cells were washed with PBS. Then, the MG d14 isolated from the mixed glial cell culture was added on the top of the astrocyte feeder and incubated for 24 h in DMEM/F12 (1:1) supplemented with 10% FBS (Gibco, Grand Island, NY, USA), 2 mM L-glutamine (Welgene, Daegu, Korea), 50 U/mL penicillin, and 50 mg/mL streptomycin (Welgene, Daegu, Korea) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
4.7. Flow Cytometry
To harvest MG d0, the brains of P1–3 inbred Lewis rats (Orientbio, Sungnam, Korea) were minced and the CD11b+ cells isolated using MACS without a mixed glial cell culture. The MG d14 were isolated from the mixed glial cell culture and trypsinized. Both groups of cells were blocked with 1% bovine serum albumin (BSA, Sigma, St. Louis, MO, USA) and incubated with primary antibody for 30 min at 4 °C: CD11b (BD Bioscience, San Jose, CA, USA, 1:100), CD206 (Abcam, Cambridge, MA, USA, 1:200), CD163 (AbD Serotec, Oxford, UK, 1:100), and CD68 (Millipore, Burlington, MA, USA, 1:200). After washing the cells with 0.1% BSA solution, the cells were incubated with secondary antibody for 30 min at 4 °C: APC donkey anti-rabbit (Jackson ImmunoResearch, W Baltimore Pike, PA, USA, 1:500) and FITC goat anti-mouse (Abcam, Cambridge, MA, USA, 1:500). The data were acquired with the BD FACSDiva 7.0 software.
4.8. Immunofluorescence Staining
The microglia and astrocytes were fixed with 3.7% formaldehyde (Sigma, St. Louis, MO, USA) and permeabilized by treatment with 0.2% Triton X-100 in PBS for 5 min. Then, the samples were blocked using 20% normal goat serum (Vector, Burlingame, CA, USA) for 1 h at RT. For immunofluorescence staining, the samples were incubated with primary antibodies at 4 °C overnight: CD11b (BD Bioscience, San Jose, CA, USA, 1:100), ionized calcium-binding adapter molecule-1 (Iba-1, Sigma, St. Louis, MO, USA, 1:200), CD163 (AbD Serotec, Oxford, UK, 1:100), CD206 (Abcam, Cambridge, MA, USA, 1:200), CD68 (Millipore, Burlington, MA, USA, 1:500), CCR7 (Abcam, Cambridge, MA, USA, 1:500), and GFAP (Abcam, Cambridge, MA, USA, 1;500). The samples were then incubated with secondary antibodies for 1 h at RT: Alexa 488 goat anti-mouse (Invitrogen, Waltham, MA, USA, 1:1000), Alexa 488 goat anti-rabbit (Jackson ImmunoResearch, W Baltimore Pike, PA, USA, 1:1000), Cy3 goat anti-mouse (Jackson ImmunoResearch, W Baltimore Pike, PA, USA, 1:1000), and Cy3 goat anti-rabbit (Jackson ImmunoResearch, W Baltimore Pike, PA, USA, 1:1000). The cells were mounted with Vectashield mounting medium containing DAPI (Vector, Burlingame, CA, USA), and immunofluorescence images were obtained using a Leica DMI 4000 B fluorescence microscope (Leica Microsystems, Wetzlar, Germany). The cell counting was performed at 200x magnification using Adobe Photoshop CS6 software.
4.9. BrdU-Incorporation Assay
The ACM was collected from CD11b− astrocyte culture and mixed with fresh MCM at two different ratios (i.e., 1:1 and 1:5). The diluted conditioned medium was used to treat microglia, which were incubated for 24 h at 15 h after seeding; then, 20 μM BrdU (Sigma, St. Louis, MO, USA) was added to the culture medium, and the cells were incubated for 9 h. The microglia were fixed with ice-cold 100% absolute methanol (Merck, Darmstadt, Germany) for 5 min. Then, the microglia were incubated with anti-BrdU antibody (Roche, Basel, Schweiz, 1:25) overnight at 4 °C and with Alexa 488 goat anti-mouse antibody (Invitrogen, Waltham, MA, USA, 1:1000) for 1 h at RT. The nucleus was stained with propidium iodide (PI) (Sigma, St. Louis, MO, USA, 1:1000) for 30 min at RT. The sample was mounted with Vectashield mounting medium (Vector, Burlingame, CA, USA), and immunofluorescence images were obtained using the Leica DMI 4000 B fluorescence microscope (Leica Microsystems, Wetzlar, Germany). The BrdU-positive cells were counted at 100x magnification using Adobe Photoshop CS6 software.
The MG d14 were incubated with the following cytokines for 5 days: 10 ng/mL GMCSF (R&D systems, Minneapolis, MN, USA), 100 nM SP (Sigma, St. Louis, MO, USA), 10 ng/mL rat IL-4 (R&D systems, Minneapolis, MN, USA), 10 ng/mL rat IL-10 (R&D systems, Minneapolis, MN, USA), 10 ng/mL TNF-α (Sigma, St. Louis, MO, USA), and 20 ng/mL IFN-γ (R&D systems, Minneapolis, MN, USA). 20 μM of BrdU were added 24 h before cell fixation. The staining procedure was the same as described above.
4.10. Real-Time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s protocol. The template cDNAs were synthesized using a Superscript III RT-PCR kit (Invitrogen, Waltham, MA, USA). Then, the qPCR was performed by using TB Green®® Premix Ex Taq™ II (Takara Bio. Inc., Shiga, Japan) with 1 μg of total RNA and the following primer sequences for RT-PCR: rat TGF-β (Forward: CACTCCCGTGGCTTCTAGTG; Reverse: CTGGCGAGCCTTAGTTTGGA), rat IGF2 (Forward: TCTTCTACCTGGCACTCTGCT; Reverse: ACGAACTGAAGAGCGTCCAC), rat iNOS (Forward: AGCTTCTGCCTCAAGCCATT; Reverse: TTTGTTACGGCTTCCAGCCT), rat IL-1β (Forward: TGGCAACTGTCCCTGAACTC; Reverse: CCCAAGTCAAGGGCTTGGAA), rat CXCL10 (Forward: CCTGCAAGTCTATCCTGTCCG; Reverse: CCTTCTTTGGCTCACCGCTT), rat Amigo2 (Forward: TGCCTAGAGCTGTCAAACCG; Reverse: TGTCAGTGGCACAGATGCAA), rat Serping1 (Forward: TGCTGCTAGCTGGGGATAGA; Reverse: TCCTGTGTGCTCAATGGGAC), rat CD109 (Forward: TGACAACCCCACGAGAGAGA; Reverse: GGAGCTCACTGGAATCGGAC), rat Emp1 (Forward: TCTCCACCATTGCCAACGTC; Reverse: TAGCTCAGAGAGCCGTCACA), rat CINC-2 (Forward: ACCAGCCTTCAGGGACTGT; Reverse: GGCTATGACTTCTGTCTGGGT).
4.11. Cytokine Array
MG d14 were co-cultured with astrocyte feeder cells in various ratios (1:1, 1:3, and 1:10), and, as a control, an MG d14 single culture and astrocyte single culture were performed. Since the cytokine array recommended serum-free media or low-serum media, the medium for all the samples was changed to serum-free DMEM/F12, and the cells were incubated overnight. Then, the medium was collected and centrifuged at 10,000 rpm for 5 min. The cytokine array (RayBiotech, Inc., Norcross, GA, USA) was performed according to the manufacturer’s protocol. Briefly, NC membranes were blocked with blocking buffer for 30 min and incubated with the samples overnight at 4 °C. The detection antibodies were added and incubated with the samples overnight at 4 °C. The membranes were incubated with 1x HRP–Streptavidin for 2 h at RT, and the signals were visualized by the exposure of an X-ray film (AGFA, Mortsel, Belgium).
4.12. NO Assay
The production of NO was measured by using the Griess reagent system (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Microglia-conditioned media were collected at 3, 24, and 36 h after SP or cytokine treatment. Fifty microliters of the sulfanilamide solution were added to 50 μL of the NO2− standard and 50 μL of conditioned medium; the mixture was incubated for 10 min at RT. Then, 50 μL of the NED was added, and the mixture was incubated for 10 min at RT. The OD at a wavelength of 550 nm was measured.
4.13. Western Blot
In order to examine the levels of apoptotic proteins of the microglia, microglia were treated with four conditions: control, 100 nM SP, 10 ng/mL TNF-α + 10 ng/mL IFN-γ, 10 ng/mL TNF-α + 10ng/mL IFN-γ + 100nM SP, and 10 ng/mL TNF-α + 10 ng/mL IFN-γ + 100 nM SP + 1 μM RP67580, for 24 h. The cells were lysed for Western blot analysis. The cell lysates were prepared using lysis buffer (Cell Signaling, Danvers, MA, USA) containing 1% SDS (Sigma, St. Louis, MO, USA) and 2 mM PMSF (Sigma, St. Louis, MO, USA). The proteins in the lysates and conditioned medium were separated by 6–10% SDS–PAGE and transferred to nitrocellulose membranes. The blots were blocked with 5% skim milk in TBS-T and then incubated with primary antibodies at 4 °C overnight: α-Tubulin (Sigma, St. Louis, MO, USA, 1:4000), Bcl-xL (Cell Signaling, Danvers, MA, USA, 1:2000), IκB (Cell Signaling, Danvers, MA, USA, 1:2000), and BAD (Cell Signaling, Danvers, MA, USA, 1:2000). The blots were then incubated with the secondary antibodies goat anti-rabbit HRP (Bio-Rad, Hercules, CA, USA, 1:5000) and goat anti-mouse HRP (Bio-Rad, Hercules, CA, USA, 1:3000) for 1 h. The ECL signal was visualized by exposing an X-ray film to the membrane (AGFA, Mortsel, Belgium).
4.14. ELISA
The astrocytes were incubated with the following cytokines: 100 nM SP, 10 ng/mL IL-4, 10 ng/mL IL-10, 10 ng/mL TNF-α, or 20 ng/mL IFN-γ. The conditioned medium was collected 24 h after the cytokine treatment. The CINC-1, CINC-2, and VEGF levels were measured using rat CINC-1, CINC-2, and VEGF ELISA kits (R&D Systems, Minneapolis, MN, USA). Data from three independent experiments were statistically analyzed.
4.15. Statistics
All the data are presented as the mean ± SEM values. Statistical analysis of all the data was carried out using the GraphPad Prism software. The unpaired, two-tailed Student’s t-test was used to determine statistically significant differences (* p-value < 0.05).