Abstract
From all types of cancer, cervical cancer manages to be in top four most frequent types, with a 6.5% rate of occurrence. The infectious vector that induces the disease, the high-risk Human papillomavirus (HPV), which is a sexually transmitted virus, is capable of transforming the host cell by modulating some of the principal signaling pathways responsible for cell cycle arrest, proliferation, and survival. Fortunately, like other cancer types, cervical cancer can be treated by chirurgical interventions or chemoradiotherapy, but these methods are not exactly the lucky clover of modern medicine because of the adverse effects they have. That is the reason why in the last years the emphasis has been on alternative medicine, more specifically on phytochemicals, as a substantial number of studies showed that diet contributes to cancer prevention and treatment. All these studies are trying to find new chemopreventive agents with less toxicity but high effectiveness both in vitro and in vivo. The aim of this review is to evaluate the literature in order to underline the advantages and disadvantages of polyphenols, a class of dietary compounds, as chemopreventive and chemotherapeutic agents. This review also aims to present polyphenols from different perspectives, starting with mechanisms of action and ending with their toxicity. The bigger picture illustrates that polyphenols have great potential in cervical cancer prevention, with strong effects on gene modulation.
1. Introduction
Cancer is one of the leading causes of death, with cervical cancer being the fourth most common cancer type among women worldwide [1]. The incidence and mortality rate varies with geographical location (Figure 1) [2]. Numerous studies have shown that cervical cancer cannot be triggered only by one factor [3]. Besides HPV, which represents the primary risk factor for the development of cervical cancer, socioeconomic status, venereal diseases, reproductive factors, long-term oral contraceptives, smoking, and obesity have also been highlighted as risk factors for this type of cancer [3,4]. In addition, genetic changes and epigenetic aberrations play an important role in the progression of cervical cancer [4]. The most commonly used therapy for cervical cancer is surgery, more specifically pelvic lymphadenectomy and radical hysterectomy. Of course, radiotherapy and chemotherapy are also used to treat this type of cancer. However, all these therapies have shown signs of major side effects such as bleeding, damage to the organs around the surgery, and the risk of clots. Radiotherapy could yield menopause, discomfort, pain with intercourse, or maybe infertility, while chemotherapy may induce cytotoxicity in the whole body, not only in tumoral cells. Furthermore, cisplatin or other drugs that are usually prescribed for cervical cancer can also lead to major side effects or even drug resistance [5].
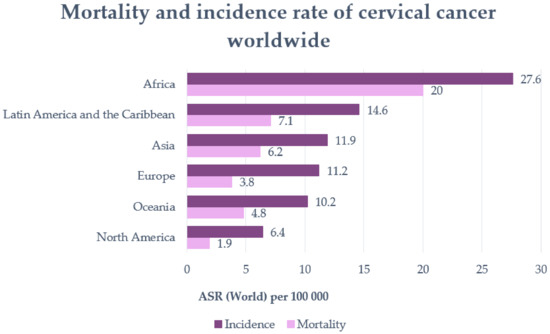
Figure 1.
Mortality and incidence rate of cervical cancer worldwide (per 100,000 individuals) (adapted after Khazaei et al., 2019 [2]).
In the last decade, studies have shown strong evidence that natural compounds such as polyphenols or other phytochemicals can potentially regulate gene expression by targeting different components of the genetic and epigenetic machinery [4]. Although polyphenols are promising anti-cervical cancer agents, their poor solubility and low oral bioavailability obstruct their potential clinical application [3].
2. Cervical Cancer
Cervical cancer is a sexually transmitted infection that is caused by high-risk Human papillomavirus (HPV) and according to current data, it is ranked fourth among all cancer types in women worldwide (Figure 2) [6,7]. In the past 30 years, the increasing percentage of young women diagnosed with cervical cancer has ranged from 10% to 40%. Of all the women diagnosed, the age range at which the incidence is the highest is 20–50 years [8].
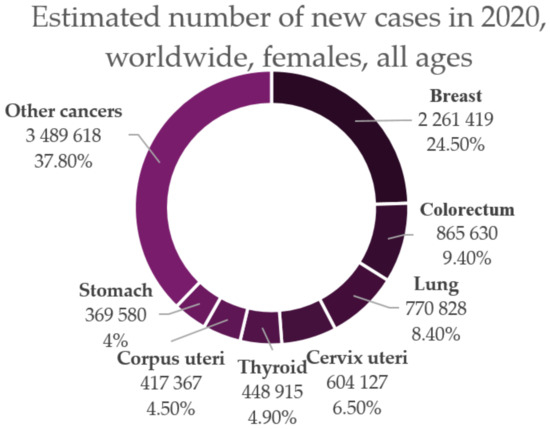
Figure 2.
Estimated number of new cases of cancer in 2020 among women of all ages worldwide (adapted after “GLOBOCAN 2020: New Global Cancer Data | UICC.” [1]).
Regarding the methods of preventing the occurrence of cervical cancer, there are two major methods currently used: anti-HPV vaccination and cervical cancer screening. HPV vaccination prevents over 95% of HPV infections with HPV16 and HPV18 types, while screening detects the early curable phase of cancer, decreasing the mortality associated with cervical cancer. That is the reason why in less developed countries, in which screening and vaccination is not that accessible, about nine out of ten women (89%) die from cervical cancer [8].
In addition to cisgender women, who are the main subjects of cervical cancer studies, it is important to spread awareness about transgender people who may also be victims of cervical cancer because cancer does not discriminate between cis and trans-individuals [9]. In this context, transgender is an umbrella term that describes a diverse group of individuals whose gender identity differs from their sexual identity. Someone who is born a woman but identifies as a man is called a female-to-male, transmasculine, or transgender man. Someone who is born male but identifies as a woman is called a male-to-female, transfeminine, or transgender woman [10]. Both these parts of the LGBTQ+ spectrum can be diagnosed with cervical cancer because not all trans people want to undergo gender-affirming treatment or have the resources to do so. According to many reports, transmen who retain their female genitalia are more likely to miss their screening or other health services (9.2% fewer transmen patients were up to date on their cervical cancer screening than ciswomen patients) because they may not seek out or be included in the target list for screening [11]. This is one of the most important reasons why transmen can be at a higher risk of gynecological cancers including cervical cancer [12]. In contrast, transwomen have a considerable lower risk of cervical cancer than ciswomen because they do not have a proper cervix, as a “neo-cervix” is made of a different type of cells compared to the cervix of ciswomen [8].
3. HPV: Structure, Pathogenicity and Transformation Activity
HPV is a member of the Papillomaviridae family and appears to be one of the most common viral pathogens that can lead to sexually transmitted infections worldwide [13,14].
The HPV genome is represented by a small double-stranded and highly conserved DNA with a molecular weight of 5 × 106 Daltons and contains approximately 7906 base pairs, including two coding regions (E and L) and one non-coding region called the long control region or upstream regulatory region (URR) (Figure 3) [15,16]. The E region encodes six early proteins (E1, E2, E4, E5, E6, and E7), three of them being regulatory proteins (E1, E2, and E4) and three of them being oncoproteins (E5, E6, and E7) that participate in the processes of replication and transformation of the host cells [17]. E1 and E2 are specifically involved in transcription and replication, E4 is involved in the process of virion release, E5 modulates cell proliferation, and E6 and E7 control the principal signaling pathways in the host cell [18]. The L region encodes two late proteins (L1 and L2), which are the structural proteins that form the viral capsid; L1 is responsible for the major viral capsid and L2 is responsible for the minor viral capsid [15,17,18]. The long control region contains the viral open reading frame (ORF) and the promoter and enhancer elements that modulate the viral DNA replication and transcription [17,18].
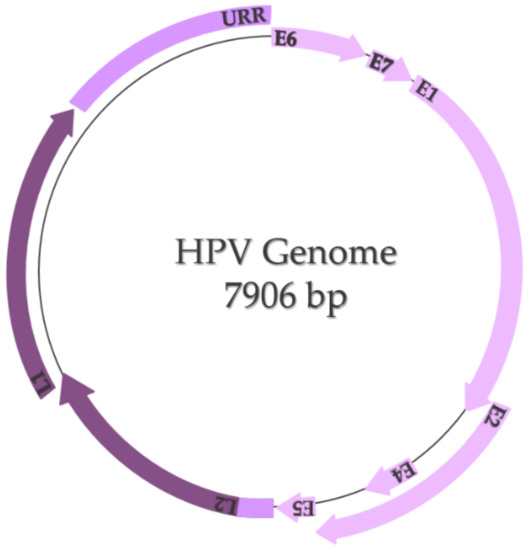
Figure 3.
HPV genome (adapted after Bowden and Kyrgiou, 2020 [18]).
In nature, HPVs represent only five out of all 39 genera of the Papillomaviridae family: alpha, beta, gamma, mu, and nu papillomaviruses, the alpha-papillomavirus being the one that causes genital warts [14]. In terms of viruses’ serotype, every HPV is genetically different based on the nucleotide sequence of the gene that encodes the L1 protein; thus, the classification is based on the chronological order of the dates on which they were found. Another form of classification is related to the carcinogenic potential of the HPVs-group 1 carcinogens (carcinogenic for humans), group 2A carcinogens (probably carcinogenic for humans), and group 2B carcinogens (potentially carcinogenic for humans) [16]. Of all high-risk HPVs, the most carcinogenic are HPV16 (approximately 50% of all cervical cancers are associated with this strain) and HPV18 because they are primarily involved in squamous epithelial lesions [19].
Infection with HPV is highly associated with sexual activity, non-sexual transmission, and transmission via fomites. Once the virus enter the body, it manages to interact with squamous epithelial cells via surface receptors such as α-6 integrines or heparin sulfate proteoglycans, infect them, and get access to basal cells during any form of abrasion. Here, in the basal cell, the expression of the E1 and E2 genes is induced, which means that the rolling circle replication begins [20]. The viral genome is integrated in the host genome, leading to the loss of E2’s ORF. This aspect is very important because E2 is the transcriptional repressor of E6 and E7 oncogenes. With that being said, when the replication is over, the E2’s ORF is missing and thus the E6 and E7 genes are overexpressed, which leads to cell transformation [21]. Another possible way to prevent the E2-mediated repression is by methylation of E2 binding sites within the URR [22]. After the transformation, L1 and L2 proteins will make the capsid and the mature virus ready to be released by the E4 protein to other cells [20].
From a molecular perspective, in order for HPV to transform the host cell, it must initiate a series of genetic changes. Thus, in order to prevent or even treat cervical cancer, it is necessary to understand not only the virus’ mechanism of invasion but also the mechanism by which the virus transforms the host cell into a cancer cell. To achieve this goal, it is necessary to visualize the overall image of the cell, focusing on the cellular signaling pathways responsible for the cell cycle, cell growth, and proliferation and induction of apoptosis. More specifically, it is necessary to analyze the possible mutations in the main proto-oncogenes and tumor suppressor genes (TSG), or the possible complexes that may occur due to the presence of HPV in the host cell (Figure 4):
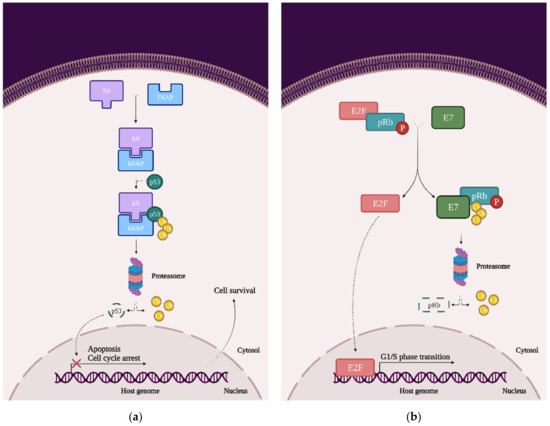
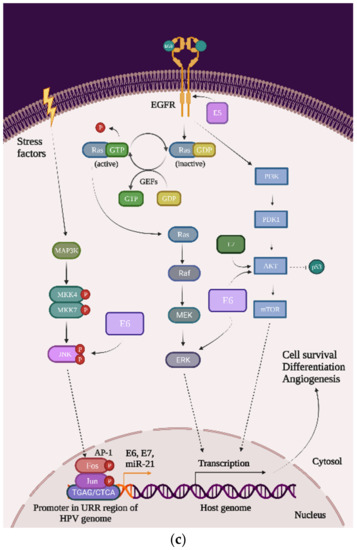
Figure 4.
The impact of HPV viral proteins on the main signaling pathways responsible for cell survival, proliferation, and differentiation: (a) the effect of the E6 oncoprotein on the p53 tran-scription factor; (b) the effect of the E7 oncoprotein on the pRb tumor-suppression protein; and (c) the effects of the E5, E6, and E7 oncoproteins on EGFR phosphorylation, the PI3K/Akt/mTOR pathway, JNK, ERK, and the AP-1 complex.
- p53: This transcription factor is involved in processes such as the cell cycle arrest, apoptosis, or induction of DNA damage response. In cervical cancer cells, HPVs are capable of inducing p53 ubiquitination via forming a complex between p53, the E6 oncoprotein, and the ubiquitin E3 ligase E6-associated protein (E6AP). This process will lead to p53 degradation by the proteasome and inevitably to chromosomal instability and avoidance of apoptosis and cell cycle arrest (Figure 4a) [23];
- pRb and pocket proteins: The retinoblastoma protein (pRb) is a tumor-suppressor protein and, together with p107 and p130, they form “the pocket proteins” that control the cell cycle. pRb needs to bind to the E2F transcription factor in order to reduce its expression and keep the cell in a G1/S phase. In cervical cancer cells, HPV’s E7 protein binds to the pRb-E2F complex and releases the E2F. E2F will be now expressed, which means that the cell will pass the G1/S phase and the pRb will be eventually degraded by the proteasome (this mechanism of degradation requires the binding to the cullin-2 ubiquitin ligase complex) (Figure 4b) [22];
- EGFR: The epidermal growth factor receptor (EGFR) is a transmembrane protein that contains an extracellular region that binds the ligands (such as the epidermal growth factor (EGF)), a transmembrane region, and an intracellular region, namely homodimers that have the catalytic site. Once the ligand is bound to the receptor, the EGFR homodimers autophosphorylate and activate some cellular pathways such as the mitogen-activated protein kinase (MAPK), phosphoinositide-3-kinase (PI3K), and protein kinase B (AKT). Primarily, EGFRs are involved in the signaling pathway that controls cell proliferation, differentiation, angiogenesis, and migration and survival, and the high expression of EGFR’s genes is associated with a poor prognosis in many cancer types. In cervical cancer, the HPV oncoprotein E5 increases the phosphorylation level of EGFRs, which lead to hyperproliferation (Figure 4c) [23];
- PI3K/Akt/mTOR: This signaling cascade targets some of the most important and complex intracellular processes, which are triggered by a series of internal and external stimuli such as cell proliferation, apoptosis, energy metabolism, growth, and migration. In cervical cancer cells, both E6 and E7 oncoproteins have the ability to upregulate the expression of PI3K and Akt, which will upregulate the expression of mTOR. Once mTOR is overexpressed, it will enhance cell proliferation, which will lead to carcinogenesis (Figure 4c) [24,25];
- MAPK/JNK: c-Jun N-terminal kinase (JNK) is a member of the subfamily Ser/Thr kinases (and is one of the three main classes of MAPK) and consists of ten isoforms encoded by three different genes, namely JNK1 (four isoforms ubiquitously expressed), JNK2 (four isoforms ubiquitously expressed), and JNK3 (two isoforms). The JNK signaling pathway can modulate oncogenic and tumor suppressive functions but it depends on the tissue in which it exercises its function. In cervical cancer cells, the E6 oncoprotein manages to increase JNK1/2 phosphorylation via the PDZ-binding motif. With that being said, when JNK1/2 is phosphorylated, c-Jun expression is activated, which induces the proliferation and expression of viral oncoproteins (Figure 4c) [23,26];
- MAPK/ERK: The extracellular signal-regulated kinase (ERK) represents another one of the three major classes of MAPK. The ERK pathway is associated with a large variety of processes such as proliferation, senescence, angiogenesis, survival, apoptosis, and differentiation. In cervical cancer cells, the E6 oncoprotein can upregulate the expression of ERK and both the E6 and E7 oncoproteins can regulate hypoxia-inducible factor 1α (HIF-1α), interleukine-8 (IL-8), and the vascular endothelial growth factor (VEGF), which can lead to high rates of proliferation, differentiation, and angiogenesis (Figure 4c) [23,27,28,29];
- AP-1: The activating protein-1 (AP-1) is an early transcription factor that plays an essential role in the transcription regulation of the HPV genome. Unlike normal cells, cervical cancer cells have high levels of AP-1 binding activity. AP-1 also represents a transcription factor family, with c-Fos and c-Jun as one of the crucial members. They bind to many consensus DNA-binding sequences (TGAG/CTCA) that are located in the promotor region of the genes and organize a series of gene expression processes of transformation, invasion, and metastasis. Furthermore, in cervical cancer cells, AP-1 upregulated microRNA miR-21 expression, which can contribute to an oncogenic potential. In cervical cancer cells, AP-1 binds to the HPV promoter located in the URR and thereby increases the expression of E6 and E7 oncoproteins, leading to carcinogenesis (Figure 4c) [23,30,31,32].
4. Cervical Cancer Treatments
Once the diagnosis is made, it is important to determine the patient’s clinical stage so that the treatment can be chosen appropriately [33]. By cancer staging, the degree of disease progression is determined, this being measured from 0 to IV, wherein 0 is the pre-cancerous/non-invasive stage and IV is the stage in which tumor cells can be found in certain areas of the body (the tumor is metastasized) [34].
For patients in the early stage (clinical stage IA), the generally accepted treatment is surgical. If patients show signs of relapse, it is helpful to receive chemotherapy at the same time. For patients in more advanced stages of the disease (clinical stages from IB2 to IVA), it is recommended to receive concomitant chemotherapy and radiotherapy [33]. For the chemotherapy, the most used chemical antitumor agent is cisplatin, which can be used as a unit agent or can be administered in combination with other agents including ifosfamide, paclitaxel, gemcitabine, topotecan, or vinorelbine [35].
Besides that, there are other therapies administered on a large scale such as monoclonal antibodies. A good example is Nivolumab, an anti-PD-1 monoclonal antibody which is capable of targeting the programmed death ligand 1 (PD-L1). In this context, in order for HPV to be maintained in the host cell and potentially develop a tumor, it needs to overexpress PD-L1. Therefore, once the immunotherapy starts, PD-L1 will be blocked by Nivolumab and the antitumor activity in cervical cells will be exerted [33].
Although classic treatments offer patients an extension of life without tumor progression, these invasive and non-invasive procedures also have their own disadvantages, namely the side effects. Many existing studies managed to present the dark side of concomitant chemoradiotherapy, highlighting the toxicity of the cervical cancer therapy on a large number of organs. Fundamentally, the main idea of the studies was that chemoradiotherapy can disrupt the long-term quality of life [36]. The following main side effects have been reported:
- urologic complications: bladder compliance, incontinence of urine, dysuria, hematuria, hemorrhagic cystitis, ureteral stricture, bowel obstruction, ureteric fibrosis, and vesicovaginal and ureterovaginal fistula [36,37,38];
- gastrointestinal symptoms: diarrhea, malaise, ulceration, fecal urgency, tenesmus, fecal incontinence, and rectal bleeding [36,37,39];
- cardiovascular symptoms: pulmonary embolus [38];
- hematological toxicity: anemia, neutropenia, and thrombocytopenia [38,39];
- sexual dysfunctions: sexual discomfort, pain with penetration, hot flashes, vaginal dryness and bleeding, and reproductive concerns [36];
- lymphedema: especially lower-extremity lymphedema [36];
- psychosocial problems: mood and stress disorders, reduced daily activities and decreased performance of social activity, depression and anxiety, body image concerns, and fear of recurrence [36].
Therefore, due to these side effects caused by the treatment of cervical cancer, the paradigm has changed over time and the emphasis has begun to be placed not on treatment but on prevention. Although this disease is largely preventable, in low-income or middle-income countries, this type of cancer occurs because of a lack of screening and HPV vaccination programs [40].
As far as is known, diet has a major impact on specific neoplasia and the most important diet is based on phytochemicals. Polyphenols, for example, can be used in the prevention and treatment of cervical cancer because of their properties, including the induction of apoptosis in HPV cells, inhibition of DNA synthesis, growth arrest, and modulation of signal transduction pathways [41].
In addition to its chemopreventive properties, polyphenols also play the role of sensitizers of cancer cells. Considering the toxic side effects of current therapy, achieving radio and chemo-sensitization of cancer cells, along with minimal toxicity overall, represents a goal in the oncological field. Furthermore, our work will present more information regarding polyphenols and both their chemopreventive and chemotherapeutic properties, studied on cervical cancer cell lines.
5. Polyphenols
Polyphenols compose one of the most diverse groups of plant metabolites [42] and, along with vitamins and enzymes, they represent a defense mechanism against oxidative stress caused by excess reactive oxygen species (ROS) [43,44,45]. These compounds are also the subject of many studies that focus on oxidative stress and its associated diseases such as cancer, diabetes, asthma, cardiovascular diseases, or even aging. All these studies aimed to find new chemopreventive agents that are less toxic than classical therapies but still effective [43,46].
In order to reveal their characteristics, most specifically their anticarcinogenic properties, scientists tested the polyphenols on multiple cell lines. The results emphasis that the phenolic compounds have a lot of health-promoting properties including antiproliferative, antineoplastic, proapoptotic, and anti-inflammatory activities [43,46,47]. These are the reasons why natural compounds have gained more attention over the last years, especially in the field of cancer. Polyphenols have great potential to act as anticancer drugs not only because of their properties but also because of their availability and toxicity statuses [48].
5.1. Polyphenols’ Classification
Although they are characterized as compounds with phenolic structural features, this group of dietary phenolics is diverse and contains sub-groups of phenolic compounds. Therefore, polyphenols are classified by their chemical structure (the number of phenol rings or the structural elements that bind these rings to one another) in four major classes: flavonoids, phenolic acids, lignans, and stilbenes [43,44,49]. Each class sums up a series of subclasses, all mentioned in Figure 5. In addition to these four classes, there are many more compounds that cannot be categorized into a specific class [4,44,49].
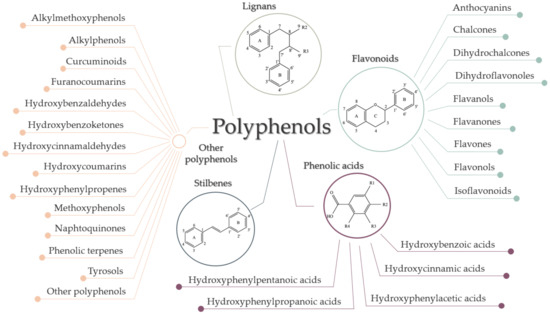
Figure 5.
Classification of polyphenols.
5.2. Polyphenols’ Mechanisms of Action
Fundamentally, the primary role of polyphenols is to protect plants from photosynthetic stress, ROS, and consumption by herbivores. In addition, polyphenols represent a significant part of the human diet, namely flavonoids, and phenolic acids are the most common in our food. Numerous studies have been performed to understand the molecular mechanisms underlying their chemotherapeutic and chemopreventive properties on cervical cancer lines [50]. Following these studies, three major mechanisms of action were determined: modulation of gene expression by involving epigenetic pathways, suppression of cancer stem cells (CSCs), and modulation of the cellular redox status [51,52].
5.2.1. Modulation of Gene Expression by Involving Epigenetic Pathways
Epigenetic refers to a series of reversible heritable changes that are not encoded in the DNA but have an important role when it comes to modulating the gene expression [50]. The three main epigenetic mechanisms studied in mammalian cells are DNA methylation, post-transcriptional gene regulation by non-coding RNA (microRNAs/miRNAs), and histone modification [53].
In the normal cells, all induce chromatin remodeling, which leads to variations of cell phenotypes, but, when these mechanisms get to be aberrant, they can induce alterations in the expression of oncogenes and tumor suppressor genes. These alterations can accumulate throughout life and eventually affect the transcript stability, the complete nuclear organization of the genetic material, and lastly can initiate tumorigenesis [50,53].
Studies have shown that polyphenols are involved in epigenetic processes that influence the behavior of tumor cells and, not only that, they also are involved in the protection of normal cells by enhancing the cytotoxicity of other therapies in tumor cells [4,54].
DNA Methylation
DNA methylation is believed to be the most studied epigenetic modification in mammalian cells [55]. It occurs more specifically to regulate tumor growth and the development of carcinogenesis by activation of oncogenes, in addition to silencing TSGs [4]. Consequently, this mechanism of epigenetic machinery is responsible for X-chromosome inactivation and genomic imprinting of even the repression of repeated elements [53]. DNA methylation appears in CpG islands, which are areas of DNA in which a cytosine nucleotide is followed by a guanine nucleotide in 5′ → 3′ direction. These CpG islands are located mostly in promoter regions of the genes as well as in intergenic regions or in regions of large repetitive sequences [4,50]. The key enzymes that modulate this process are DNA methyltransferase enzymes (DNMT). They transfer a methyl group to the 5′ carbon position of cytosine to form 5-methylcytosine [56].
Natural polyphenols, such as resveratrol, genistein, quercetin, or epigallocatechin-3-gallate (EGCG), induce changes in the levels of DNMTs by the direct or indirect effect on DNMT activity (Figure 6) [4,57]. EGCG, for example, is well-known for its capacity to bind directly to the DNMTs, inactivating the enzymes [57]. Conversely, quercetin not only acts as a competitive inhibitor for various members of DNMT families and downregulates their gene expression, but it can restore the expression of TSGs by reducing the methylation of their promoters [57].
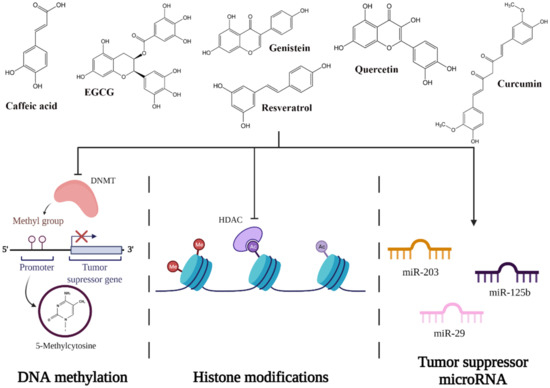
Figure 6.
Potential mechanisms of action of polyphenols on epigenetic pathways.
Histone Modifications
The process of histone modification occurs because of the translational and post-translational modifications (PTMs) [56]. These PTMs occur mostly within the histones’ N-terminal tail or within their globular domain and include a variety of processes such as acetylation, biotinylation, phosphorylation, ubiquitination, SUMOylation, ADP ribosylation, proline isomerization, citrullination, butyrylation, propionylation, and glycosylation [57]. Thus, these mechanisms interrupt the chromatin organization and add new binding sites in a specific region of chromatin. The key enzymes that modulate these processes are histone acetyltransferases (HATs), histone deacetylases (HDACs), and histone methyltransferases (HMTs) [56].
Dietary polyphenols can modulate histone modification to prevent cancer by inhibiting HDAC [4,51]. Quercetin (inhibits HDAC2, HDAC4, HDAC7, and HDAC8), genistein (inhibits HDAC6 and tyrosine kinases), caffeic acid, and curcumin are the most well known for their capacity of inhibiting HDACs (Figure 6) [58].
Non-Coding RNA: MicroRNA
MicroRNAs (miRNAs) are short, non-coding single-stranded RNA fragments that regulate cellular processes through transcriptional repression and degradation of messenger RNA (mRNA) [59]. miRNA binds to the mRNA by sequence-specific base pairing with both 3’-untranslated regions of the target fragment and regions that can be realized by complete or partial complementary [5,57,59]. When miRNA binds to mRNA via imperfect complementary, translational repression will occur not only in a single fragment of mRNA, but also in tens to hundreds of different mRNAs. When miRNA binds to mRNA via perfect complementary, degradation of mRNA will occur [59]. In order to achieve this, miRNA needs a protein complex called the RNA-induced silencing complex (RISC), which is a complex that degrades the mRNA after the miRNA is fixed [57]. In vitro and in vivo studies emphasized that miRNAs are classified both as tumor suppressors and oncogenes, and their expression is downregulated or upregulated based on the tumor needs [60].
Various studies have shown that polyphenols also have a significant role in modulating mRNA function. One of the promising polyphenols in cervical cancer therapy is EGCG, a phytochemical found in green tea that has the potential to induce apoptosis in cervical cell lines. Zhu et al. tested the effect of EGCG on multiple cervical cell lines and noticed that in the CaSki cell line, EGCG upregulates miR-203, miR-125b, and mir-29, which are tumor suppressors in cervical cancer cells (Figure 6) [61,62,63,64,65].
5.2.2. Modulation of the Cellular Redox Status
Oxidative stress represents an imbalance between two processes, namely the formation and elimination of oxidative species such as superoxide anion, hydroxyl radical, and hydrogen peroxide [66]. These compounds are primarily the result of cytochrome P450 and peroxisome actions, and when they accumulate, they lead to a series of dysfunctions in the cell [67]. In normal cells, antioxidant compounds come in handy because they are capable of restoring the redox homeostasis by modulating the formation and degradation of ROS [68]. In contrast, in cancer cells, oxidative stress plays an important role in the epigenetic reprogramming of the expression of oncogenes and TSGs [51]. It is well known that cancer cells are usually under greater oxidative stress than normal cells but this property may be an advantage for the discovery of pro-oxidants that induce selective cytotoxicity in tumor cells [68].
What is fascinating about polyphenols is the fact that they can manifest their pro-oxidant properties only in tumor cells, not in normal ones, by decreasing cell viability precisely through the promotion of ROS [68,69]. A good example is curcumin; it has the potential to increase ROS levels in cervical cancer cells, which triggers endoplasmic reticulum stress (ER stress). Once ER stress is initiated, it induces ER stress-mediated apoptosis through activation of the C/EBP Homologous Protein CHOP (transcription factor involved in apoptosis) [70].
5.2.3. Suppression of Cancer Stem Cells
Cancer stem cells (CSCs) are a small subpopulation of tumor cells that play an important role in many processes such as tumorigenicity, tumorigenesis, defining tumor size, the speed of development, trans-differentiating into vascular endothelial cells or other stromal cells associated with the tumor, self-renewal, slow-cycling capacity, metastasis, and the level of regression following treatment [71,72,73]. Furthermore, in contrast with the differentiated cancer cells, CSCs are known for their low ROS levels, more efficient DNA repair responses, and promotion of glycolysis and autophagy [51]. Many studies state that the elimination of CSCs can represent a permanent cure for cancer [73]. The problem is that in cervical cancer, CSCs are associated with chemoradio-resisting properties [52,72].
Shin et al. [52] managed to prove that polyphenols might be a natural alternative to chemoradiotherapy, contributing to tumor cell destruction. More specifically, they showed that pterostilbenes are not only a promising therapy for cervical CSCs, but they also are greater inhibitors than other polyphenols such as resveratrol [52]. Their study demonstrated that pterostilbenes can:
- induce cycle cell arrest at the S/G1 phase via the induction of p53 and p21 (both TSGs), and the reduction of cyclin E1 and cyclin B1;
- induce apoptosis via the downregulation of Bcl-2 and Bcl-XL (antiapoptotic proteins), and ROS-mediated activation of caspase-3 and caspase-9;
- inhibit MMP-2 and MM-9 expression (matrix metalloproteinases) [52].
Despite all this information, polyphenols are involved in many signaling pathways, which will not be detailed in this review. However, in Table 1, the most used polyphenols in cervical cancer research and their main effects observed in both in vitro and in vivo studies are summarized.

Table 1.
Summary of the most used polyphenols in cervical cancer therapy. ↑ indicates upregulation/induction/increasing, while ↓ indicates downregulation/reduction/decreasing.
5.3. A Perspective on Polyphenols’ Toxicity
So far, we have deduced that polyphenols are indeed promising agents against cervical cancer but their accelerated metabolism and reduced bioavailability are obstacles in accomplishing their activity. That is the reason why, in order to achieve the desired results, a high dose of polyphenols is needed [157]. Nevertheless, there is still a question that should be addressed: is a high dose of polyphenols equal to a high rate of absorption or a high efficiency?
It is well known that in any drug development process, a crucial part is represented by the toxicological study [158]. As for polyphenols, it seems that dose, which is linked to bioavailability in most of the cases, can be just as beneficial as it can be harmful for the body [159]. Most of the studies confirm that a higher dose of polyphenols is usually linked with toxicity, but why is this association widely recognized? The shortest and simplest answer is the notion of hormesis [160]. From a biological perspective, hormesis is an adaptive response to stress. When the cell is exposed to a lower concentration of stress-inducing agents, some signaling pathways will be activated in order to confer resistance to higher concentrations of the same agents or for other ones. From a chemical perspective, hormesis is a phenomenon that is characterized by a biphasic dose-response curve. Essentially, a chemical (in our case, a polyphenol) can act as a stimulant when given in small doses and as a toxic agent when given in high doses [161].
In this context, some of the risky doses of polyphenols that have been used in cervical cancer research, together with their harmful effects, are summarized in Table 2.

Table 2.
Polyphenols’ main toxic effects based on their high administration doses.
6. Conclusions and Perspectives
To sum up everything that has been stated so far, it is safe to say that polyphenols can be cataloged as a double-edged therapeutic agent with both advantages and disadvantages.
The main advantage of polyphenols is their ability to fight with cancer cells in many ways. Polyphenols have the capacity to modulate the expression of many oncogenes/TSGs and therefore to change the cell dynamics in order to finally maintain the integrity of the host cell intact. Another advantage is that polyphenols can be found in a large variety of plants in both higher or lower concentrations, which gives them their quality of dietary compounds used in cancer prevention.
The main disadvantage is their low bioavailability, which makes them hard to work with because they need high concentrations in order to do their job. This leads us to the second disadvantage, which concerns the polyphenols’ toxicity: in high concentrations, polyphenols can induce toxicity in the organism. With a moderate plant-based diet, though, this disadvantage can be avoided. These disadvantages limit and/or compromise the effectiveness of the compound. Recent studies emphasized that approximately 50–60% of cancer patients from the US choose to use plant-based or alternative medicine rather than chemoradiotherapy; thus, there is a pressing need to find solutions in order to enhance the efficacy of treatment and reduce the side effects [74].
A considerable solution is to encapsulate the polyphenols in various systems such as nanoparticles. Accordingly, the polyphenols can be protected from the destructive action of external media and be carried in an improved delivery system that can optimize and maximize their performance by modifying their composition, morphology, and size by reducing the side effects and overcoming drug resistance [171]. Therefore, the use of nano-sized phytochemicals is desired because they have high biocompatibility, biodegradability, and stability in the biological environment, and also enhance drug specificity, improve absorption rates, and reduce drug degradation and systemic toxicity [74].
Although this might be a promising approach, manufacturing this kind of nanotechnology remains an issue for clinical success. In the future, the emphasis will be on the safety of nanocarriers, on achieving effectiveness by improving the pharmaceutical properties of therapeutic molecules, and on the determination of optimal physicochemical parameters [171].
Funding
This research was funded by the Romanian National Authority for Scientific Research (UEFISCDI), grant numbers PN-III-P1-1.1-TE-2019-0960 a178TE/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| HPV | Human papillomavirus |
| URR | upstream regulatory region |
| Rb | retinoblastoma gene |
| ORF | open reading frame |
| E6AP | E6-associated protein |
| TSG | tumor suppressor genes |
| pRb | retinoblastoma protein |
| EGFR | epidermal growth factor receptor |
| EGF | epidermal growth factor |
| ROS | reactive oxygen species |
| MAPK | mitogen-activated protein kinase |
| PI3K | phosphoinositide-3-kinase |
| AKT | protein kinase B |
| JNK | c-Jun N-terminal kinase |
| ERK | extracellular signal-regulated kinase |
| PD-L1 | programmed death ligand 1 |
| CHOP | C/EBP Homologous Protein |
| CSCs | cancer stem cells |
| DNMT | DNA methyltransferase |
| EGCG | epigallocatechin-3-gallate |
| HAT | histone acetyltransferase |
| HDAC | histone deacetylase |
| HMT | histone methyltransferase |
| PTMs | post-translational modifications |
| miRNA | microRNA |
| mRNA | messenger RNA |
| RISC | RNA-induced silencing complex |
| ER stress | endoplasmic reticulum stress |
| CSCs | cancer stem cells |
References
- UICC. GLOBOCAN 2020: New Global Cancer Data. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 25 April 2021).
- Goodarzi, E.; Khazaei, Z.; Sohrabivafa, M.; Mansori, K.; Naemi, H. Incidence and mortality of cervix cancer and their relationship with the human development index in 185 countries in the world: An ecology study in 2018. Adv. Hum. Biol. 2019, 9, 222. [Google Scholar] [CrossRef]
- Venkatas, J.; Singh, M. Cervical cancer: A meta-analysis, therapy and future of nanomedicine. Ecancermedicalscience 2020, 14, 1111. [Google Scholar] [CrossRef] [PubMed]
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 4567. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kim, M.; Lee, S.; Jung, W.; Kim, B. Therapeutic Potential of Natural Products in Treatment of Cervical Cancer: A Review. Nutrients 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Garey, S.L. Cancer, Cervical. In Encyclopedia of Behavioral Medicine; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 350–351. [Google Scholar]
- Koshiyama, M. The Effects of the Dietary and Nutrient Intake on Gynecologic Cancers. Healthcare 2019, 7, 88. [Google Scholar] [CrossRef]
- Ngoma, M.; Autier, P. Cancer prevention: Cervical cancer. Ecancermedicalscience 2019, 13, 952. [Google Scholar] [CrossRef]
- Dhillon, N.; Oliffe, J.L.; Kelly, M.T.; Krist, J. Bridging Barriers to Cervical Cancer Screening in Transgender Men: A Scoping Review. Am. J. Men’s Health 2020, 14. [Google Scholar] [CrossRef]
- Braun, H.; Nash, R.; Tangpricha, V.; Brockman, J.; Ward, K.; Goodman, M. Cancer in Transgender People: Evidence and Methodological Considerations. Epidemiol. Rev. 2017, 39, 93–107. [Google Scholar] [CrossRef]
- Sterling, J.; Garcia, M.M. Cancer screening in the transgender population: A review of current guidelines, best practices, and a proposed care model. Transl. Androl. Urol. 2020, 9, 2771–2785. [Google Scholar] [CrossRef]
- Weyers, S.; Garland, S.; Cruickshank, M.; Kyrgiou, M.; Arbyn, M. Cervical cancer prevention in transgender men: A review. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 822–826. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef]
- Calaf, G.M.; Urzúa, U.; Termini, L.; Aguayo, F. Oxidative stress in female cancers. Oncotarget 2018, 9, 23824–23842. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Hu, R.; Du, Y.; Tan, L.; Li, L.; Du, J.; Bai, L.; Ma, Y.; Cui, H. Immunodiagnosis and Immunotherapeutics Based on Human Papillomavirus for HPV-Induced Cancers. Front. Immunol. 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, X.; Zhang, Y. Involvement of Human Papillomaviruses in Cervical Cancer. Front. Microbiol. 2018, 9, 2896. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination—Review of Current Perspectives. J. Oncol. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Bowden, S.J.; Kyrgiou, M. Human papillomavirus. Obstet. Gynaecol. Reprod. Med. 2020, 30, 109–118. [Google Scholar] [CrossRef]
- Sammarco, M.L.; Tamburro, M.; Pulliero, A.; Izzotti, A.; Ripabelli, G. Human Papillomavirus Infections, Cervical Cancer and MicroRNAs: An Overview and Implications for Public Health. MicroRNA 2020, 9, 174–186. [Google Scholar] [CrossRef]
- Rai, B.; Bansal, A.; Singh, M.P. Human papillomavirus-associated cancers: A growing global problem. Int. J. Appl. Basic Med. Res. 2016, 6, 84–89. [Google Scholar] [CrossRef]
- Bechtold, V.; Beard, P.; Raj, K. Human Papillomavirus Type 16 E2 Protein Has No Effect on Transcription from Episomal Viral DNA. J. Virol. 2003, 77, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Scarth, J.A.; Patterson, M.R.; Morgan, E.L.; Macdonald, A. The human papillomavirus oncoproteins: A review of the host pathways targeted on the road to transformation. J. Gen. Virol. 2021, 102, 001540. [Google Scholar] [CrossRef]
- Medda, A.; Duca, D.; Chiocca, S. Human Papillomavirus and Cellular Pathways: Hits and Targets. Pathogens 2021, 10, 262. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Ling, M.T.; Zhao, L.; Zhao, K.-N. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer 2015, 14, 1–13. [Google Scholar] [CrossRef]
- Bossler, F.; Hoppe-Seyler, K.; Hoppe-Seyler, F. PI3K/AKT/mTOR Signaling Regulates the Virus/Host Cell Crosstalk in HPV-Positive Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 2188. [Google Scholar] [CrossRef]
- Morgan, E.L.; Scarth, J.A.; Patterson, M.R.; Wasson, C.W.; Hemingway, G.C.; Barba-Moreno, D.; Macdonald, A. E6-mediated activation of JNK drives EGFR signalling to promote proliferation and viral oncoprotein expression in cervical cancer. Cell Death Differ. 2021, 28, 1669–1687. [Google Scholar] [CrossRef]
- Bonab, F.R.; Baghbanzadeh, A.; Ghaseminia, M.; Bolandi, N.; Mokhtarzadeh, A.; Amini, M.; Dadashzadeh, K.; Hajiasgharzadeh, K.; Baradaran, B.; Baghi, H.B. Molecular pathways in the development of HPV-induced cervical cancer. EXCLI J. 2021, 20, 320–337. [Google Scholar]
- Liu, F.; Lin, B.; Liu, X.; Zhang, W.; Zhang, E.; Hu, L.; Ma, Y.; Li, X.; Tang, X. ERK Signaling Pathway Is Involved in HPV-16 E6 but not E7 Oncoprotein-Induced HIF-1α Protein Accumulation in NSCLC Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2016, 23, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Anerillas, C.; Abdelmohsen, K.; Gorospe, M. Regulation of senescence traits by MAPKs. GeroScience 2020, 42, 397–408. [Google Scholar] [CrossRef]
- Mirzaei, H.; Khodadad, N.; Karami, C.; Pirmoradi, R.; Khanizadeh, S. The AP-1 pathway; A key regulator of cellular transformation modulated by oncogenic viruses. Rev. Med. Virol. 2019, 30, e2088. [Google Scholar] [CrossRef] [PubMed]
- Prusty, B.K.; Das, B.C. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer 2005, 113, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Díaz-González, S.D.M.; Rodríguez-Aguilar, E.D.; Meneses-Acosta, A.; Valadez-Graham, V.; Deas, J.; Gómez-Cerón, C.; Tavira-Montalván, C.A.; Arizmendi-Heras, A.; Ramírez-Bello, J.; Zurita-Ortega, M.E.; et al. Transregulation of microRNA miR-21 promoter by AP-1 transcription factor in cervical cancer cells. Cancer Cell Int. 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Porras, G.O.R.; Nogueda, J.C.; Chacón, A.P. Chemotherapy and molecular therapy in cervical cancer. Rep. Pract. Oncol. Radiother. 2018, 23, 533–539. [Google Scholar] [CrossRef]
- Šarenac, T.; Mikov, M. Cervical Cancer, Different Treatments and Importance of Bile Acids as Therapeutic Agents in This Disease. Front. Pharmacol. 2019, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- P.A.T.E. Board. Cervical Cancer Treatment (PDQ®). PDQ Cancer Inf. Summ. May 2020. pp. 1–22. Available online: https://www.ncbi.nlm.nih.gov/books/NBK65985/ (accessed on 12 July 2021).
- Pfaendler, K.S.; Wenzel, L.; Mechanic, M.B.; Penner, K.R. Cervical Cancer Survivorship: Long-term Quality of Life and Social Support. Clin. Ther. 2015, 37, 39–48. [Google Scholar] [CrossRef]
- Jones, B. Toxicity after Cervical Cancer Treatment using Radiotherapy and Chemotherapy. Clin. Oncol. 2009, 21, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.M.; Symonds, P.; Green, J.A.; Tierney, J.; Collingwood, M.; Williams, C.J. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother. Oncol. 2003, 68, 217–226. [Google Scholar] [CrossRef]
- Tan, L.; Russell, S.; Burgess, L. Acute Toxicity of Chemo-radiotherapy for Cervical Cancer: The Addenbrooke’s Experience. Clin. Oncol. 2004, 16, 255–260. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Mironescu, A.; Dracea, L.; Ples, L. The Role of Natural Polyphenols in the Prevention and Treatment of Cervical Cancer—An Overview. Molecules 2016, 21, 1055. [Google Scholar] [CrossRef]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Ranga, F.; Rugina, D.; Leopold, L.; Pop, O.; Vodnar, D.; Cuibus, L.; Socaciu, C. Phenolic Content and Their Antioxidant Activity in Various Berries Cultivated in Romania. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2015, 72, 99–103. [Google Scholar] [CrossRef]
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Diaconeasa, Z.; et al. Phytochemical Characterization of Five Edible Purple-Reddish Vegetables: Anthocyanins, Flavonoids, and Phenolic Acid Derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Rampogu, S.; Ravinder, D.; Pawar, S.C.; Lee, K.W. Natural Compound Modulates the Cervical Cancer Microenvironment—A Pharmacophore Guided Molecular Modelling Approaches. J. Clin. Med. 2018, 7, 551. [Google Scholar] [CrossRef]
- Rothwell, J.; Pérez-Jiménez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell. Longev. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene Suppresses both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228. [Google Scholar] [CrossRef]
- Carlos-Reyes, Á.; Lopez-Gonzalez, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.; La Vega, H.A.-D.; De La Cruz, O.N.H.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef]
- Sayed, M.; ElHamid Mahmou, A.A. Cancer Chemoprevention by Dietary Polyphenols. In Carcinogenesis; InTech: London, UK, 2013. [Google Scholar]
- Park, L.K.; Friso, S.; Choi, S.-W. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2011, 71, 75–83. [Google Scholar] [CrossRef]
- Arora, I.; Sharma, M.; Sun, L.Y.; Tollefsbol, T.O. The Epigenetic Link between Polyphenols, Aging and Age-Related Diseases. Genes 2020, 11, 1094. [Google Scholar] [CrossRef]
- Russo, G.L.; Vastolo, V.; Ciccarelli, M.; Albano, L.; Macchia, P.E.; Ungaro, P. Dietary polyphenols and chromatin remodeling. Crit. Rev. Food Sci. Nutr. 2017, 57, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, N.L.; Deberaldini, M.G.; Gomes, D.; Pavan, A.R.; Sousa, Â.; Dos Santos, J.L.; Soares, C.P. Histone Deacetylase Inhibitors as Therapeutic Interventions on Cervical Cancer Induced by Human Papillomavirus. Front. Cell Dev. Biol. 2021, 8, 592868. [Google Scholar] [CrossRef]
- Pedroza-Torres, A.; López-Urrutia, E.; Garcia, V.; Jacobo-Herrera, N.; Herrera, L.A.; Peralta-Zaragoza, O.; López-Camarillo, C.; De Leon, D.C.; Fernández-Retana, J.; Cerna-Cortés, J.F.; et al. MicroRNAs in Cervical Cancer: Evidences for a miRNA Profile Deregulated by HPV and Its Impact on Radio-Resistance. Molecules 2014, 19, 6263–6281. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Arora, S.; Averett, C.; Singh, S.; Singh, A.P. Modulation of MicroRNAs by Phytochemicals in Cancer: Underlying Mechanisms and Translational Significance. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, Y.; Liu, M.; Yan, Q.; Zhao, W.; Yang, P.; Gao, Q.; Wei, J.; Zhao, W.; Ma, L. Epigallocatechin gallate inhibits cell growth and regulates miRNA expression in cervical carcinoma cell lines infected with different high-risk human papillomavirus subtypes. Exp. Ther. Med. 2018, 17, 1742–1748. [Google Scholar] [CrossRef]
- Cui, F.; Li, X.; Zhu, X.; Huang, L.; Huang, Y.; Mao, C.; Yan, Q.; Zhu, J.; Zhao, W.; Shi, H. MiR-125b Inhibits Tumor Growth and Promotes Apoptosis of Cervical Cancer Cells by Targeting Phosphoinositide 3-Kinase Catalytic Subunit Delta. Cell. Physiol. Biochem. 2012, 30, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, A.J.; Liu, Y.; Fang, Y.; Ding, X.; Liang, M. The miR-29 family: Genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol. Genom. 2012, 44, 237–244. [Google Scholar] [CrossRef]
- Chan, Y.C.; Banerjee, J.; Choi, S.Y.; Sen, C.K. miR-210: The Master Hypoxamir. Microcirculation 2011, 19, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Er, K.; Mao, C.; Yan, Q.; Xu, H.; Zhang, Y.; Zhu, J.; Cui, F.; Zhao, W.; Shi, H. miR-203 Suppresses Tumor Growth and Angiogenesis by Targeting VEGFA in Cervical Cancer. Cell. Physiol. Biochem. 2013, 32, 64–73. [Google Scholar] [CrossRef]
- Rugina, D.O.; Diaconeasa, Z.; Coman, C.; Bunea, A.; Socaciu, C.; Pintea, A. Chokeberry Anthocyanin Extract as Pancreaticβ-Cell Protectors in Two Models of Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Silva, G.Á.F.; Nunes, R.A.L.; Morale, M.G.; Boccardo, E.; Aguayo, F.; Termini, L. Oxidative stress: Therapeutic approaches for cervical cancer treatment. Clinies 2018, 73, e548s. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, H.S.; Jung, E.-J.; Lee, J.Y.; Tsang, B.K.; Lim, J.M.; Song, Y.S. Curcumin induces ER stress-mediated apoptosis through selective generation of reactive oxygen species in cervical cancer cells. Mol. Carcinog. 2016, 55, 918–928. [Google Scholar] [CrossRef]
- Huang, R.; Rofstad, E.K. Cancer stem cells (CSCs), cervical CSCs and targeted therapies. Oncotarget 2017, 8, 35351–35367. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, G.; Rocha-Zavaleta, L.; Esparza-Ibarra, E.; Olmos, J. Cervical cancer stem cells and other leading factors associated with cervical cancer development (Review). Oncol. Lett. 2019, 18, 3423–3432. [Google Scholar] [CrossRef]
- Chhabra, R. Cervical cancer stem cells: Opportunities and challenges. J. Cancer Res. Clin. Oncol. 2015, 141, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.-H.; Yang, S.-F.; Tsai, S.-J.; Hsieh, S.-C.; Huang, Y.-C.; Bau, D.-T.; Hsieh, Y.-H. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch. Toxicol. 2012, 86, 263–273. [Google Scholar] [CrossRef]
- Chien, S.-T.; Shi, M.-D.; Lee, Y.-C.; Te, C.-C.; Shih, Y.-W. Galangin, a novel dietary flavonoid, attenuates metastatic feature via PKC/ERK signaling pathway in TPA-treated liver cancer HepG2 cells. Cancer Cell Int. 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Kumar, R.; Tiku, A. Galangin induces cell death by modulating the expression of glyoxalase-1 and Nrf-2 in HeLa cells. Chem. Interact. 2018, 279, 1–9. [Google Scholar] [CrossRef]
- Wei, J.; Su, H.; Bi, Y.; Li, J.; Feng, L.; Sheng, W. Anti-proliferative effect of isorhamnetin on HeLa cells through inducing G2/M cell cycle arrest. Exp. Ther. Med. 2018, 15, 3917–3923. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.-J.; Choi, E.O.; Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Hong, S.H.; Cheong, J.; Yun, S.J.; et al. Isorhamnetin Induces Cell Cycle Arrest and Apoptosis Via Reactive Oxygen Species-Mediated AMP-Activated Protein Kinase Signaling Pathway Activation in Human Bladder Cancer Cells. Cancers 2019, 11, 1494. [Google Scholar] [CrossRef]
- Cai, F.; Zhang, Y.; Li, J.; Huang, S.; Gao, R. Isorhamnetin inhibited the proliferation and metastasis of androgen-independent prostate cancer cells by targeting the mitochondrion-dependent intrinsic apoptotic and PI3K/Akt/mTOR pathway. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.P.; Bonfim-Mendonça, P.D.S.; Gimenes, F.; Ratti, B.A.; Kaplum, V.; Bruschi, M.L.; Nakamura, C.V.; Silva, S.O.; Maria-Engler, S.; Consolaro, M.E.L. Oxidative Stress Triggered by Apigenin Induces Apoptosis in a Comprehensive Panel of Human Cervical Cancer-Derived Cell Lines. Oxid. Med. Cell. Longev. 2017, 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Krajewski, R.; Makuch, S.; Agrawal, S. Phytochemicals in Gynecological Cancer Prevention. Int. J. Mol. Sci. 2021, 22, 1219. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Thakur, K.; Wang, J.; Wang, H.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. Molecular mechanism of anti-cancerous potential of Morin extracted from mulberry in Hela cells. Food Chem. Toxicol. 2018, 112, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Solairaja, S.; Andrabi, M.Q.; Dunna, N.R.; Venkatabalasubramanian, S. Overview of Morin and Its Complementary Role as an Adjuvant for Anticancer Agents. Nutr. Cancer 2021, 73, 927–942. [Google Scholar] [CrossRef]
- Mbaveng, A.; Zhao, Q.; Kuete, V. Harmful and Protective Effects of Phenolic Compounds from African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 577–609. [Google Scholar]
- Yi, J.-L.; Shi, S.; Shen, Y.-L.; Wang, L.; Chen, H.-Y.; Zhu, J.; Ding, Y. Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against HeLa cervical cancer cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 1116–1127. [Google Scholar] [PubMed]
- Jiang, M.; Zhu, M.; Wang, L.; Yu, S. Anti-tumor effects and associated molecular mechanisms of myricetin. Biomed. Pharmacother. 2019, 120, 109506. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Rajavel, T.; Habtemariam, S.; Nabavi, S.F. Molecular mechanisms underlying anticancer effects of myricetin. Life Sci. 2015, 142, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Moutinho, M.S.; Aragão, S.; Carmo, D.; Casaca, F.; Silva, S.; Ribeiro, J.; Sousa, H.; Pires, I.; Queiroga, F.; Colaço, B.; et al. Curcumin and Rutin Down-regulate Cyclooxygenase-2 and Reduce Tumor-associated Inflammation in HPV16-Transgenic Mice. Anticancer Res. 2018, 38, 1461–1466. [Google Scholar] [CrossRef]
- Deepika, M.S.; Thangam, R.; Sheena, T.S.; Sasirekha, R.; Sivasubramanian, S.; Babu, M.D.; Jeganathan, K.; Thirumurugan, R. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed. Pharmacother. 2019, 109, 1181–1195. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Hussain, A.; Haque, S.; Raina, R.; Afroze, N. Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell. Biochem. 2019, 120, 18357–18369. [Google Scholar] [CrossRef]
- Zhang, E.; Zhang, Y.; Fan, Z.; Cheng, L.; Han, S.; Che, H. Apigenin Inhibits Histamine-Induced Cervical Cancer Tumor Growth by Regulating Estrogen Receptor Expression. Molecules 2020, 25, 1960. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-N.; Shi, J.; Fu, Y.; Zhao, X.-H. The Stability and Activity Changes of Apigenin and Luteolin in Human Cervical Cancer Hela Cells in Response to Heat Treatment and Fe2+/Cu2+ Addition. Foods 2019, 8, 346. [Google Scholar] [CrossRef]
- Yu, X.; Yang, Y.; Li, Y.; Cao, Y.; Tang, L.; Chen, F.; Xia, J. Baicalein inhibits cervical cancer progression via downregulating long noncoding RNA BDLNR and its downstream PI3 K/Akt pathway. Int. J. Biochem. Cell Biol. 2018, 94, 107–118. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Wang, Y.; Mao, X.; Zhang, Y.; Xia, J. Baicalein induces cervical cancer apoptosis through the NF-κB signaling pathway. Mol. Med. Rep. 2018, 17, 5088–5094. [Google Scholar] [CrossRef]
- Xiaolan, Y.; Lian, H.; Hui, Y.; Xiaoping, T.; Wei, T.; Jiyi, X. Baicalein suppresses the proliferation of human cervical cancer cells via Notch 1/Hes signaling pathway. J. Cancer Res. Ther. 2019, 15, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, R.; Liu, Y.; Zhou, J.; Shen, K.; Dai, Y. Baicalein Represses Cervical Cancer Cell Growth, Cell Cycle Progression and Promotes Apoptosis via Blocking AKT/mTOR Pathway by the Regulation of circHIAT1/miR-19a-3p Axis. OncoTargets Ther. 2021, 14, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Chen, L.; Hu, Y.; Yuan, Z.; Luo, Y.; Xiong, Y. Antitumor Effects of Baicalein and Its Mechanism via TGFβ Pathway in Cervical Cancer HeLa Cells. Evid. Based Complement. Altern. Med. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The Fascinating Effects of Baicalein on Cancer: A Review. Int. J. Mol. Sci. 2016, 17, 1681. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Q.; Li, K.; Zhao, H.; Han, Z.; Li, F.; Sun, M.; Zhang, Y. Antitumor activity of baicalein on the mice bearing U14 cervical cancer. Afr. J. Biotechnol. 2011, 10, 14169–14176. [Google Scholar] [CrossRef]
- Yang, H.; Xia, J.; Li, Y.; Cao, Y.; Tang, L.; Yu, X. Baicalein inhibits the invasion of human cervical cancer cells by inhibiting the hedgehog/Gli signaling pathway. Trop. J. Pharm. Res. 2020, 19, 115–120. [Google Scholar] [CrossRef]
- Khoo, B.Y.; Chua, S.L.; Balaram, P. Apoptotic Effects of Chrysin in Human Cancer Cell Lines. Int. J. Mol. Sci. 2010, 11, 2188–2199. [Google Scholar] [CrossRef]
- Laishram, S.; Moirangthem, D.S.; Borah, J.C.; Pal, B.C.; Suman, P.; Gupta, S.; Kalita, M.C.; Talukdar, N.C. Chrysin rich Scutellaria discolor Colebr. induces cervical cancer cell death via the induction of cell cycle arrest and caspase-dependent apoptosis. Life Sci. 2015, 143, 105–113. [Google Scholar] [CrossRef]
- Jung, J. Emerging Utilization of Chrysin Using Nanoscale Modification. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Lee, K.; Lee, D.H.; Jung, Y.J.; Shin, S.Y.; Lee, Y.H. The natural flavone eupatorin induces cell cycle arrest at the G2/M phase and apoptosis in HeLa cells. Appl. Biol. Chem. 2016, 59, 193–199. [Google Scholar] [CrossRef]
- Razak, N.A.; Yeap, S.K.; Alitheen, N.B.; Ho, W.Y.; Yong, C.Y.; Tan, S.W.; Tan, W.S.; Long, K. Eupatorin Suppressed Tumor Progression and Enhanced Immunity in a 4T1 Murine Breast Cancer Model. Integr. Cancer Ther. 2020, 19. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Líšková, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zou, B.; Zhang, X.; Peng, X. Eupatilin regulates proliferation and cell cycle of cervical cancer by regulating hedgehog signalling pathway. Cell Biochem. Funct. 2020, 38, 428–435. [Google Scholar] [CrossRef]
- Tousi, M.S.; Sepehri, H.; Khoee, S.; Farimani, M.M.; Delphi, L.; Mansourizadeh, F. Evaluation of apoptotic effects of mPEG-b-PLGA coated iron oxide nanoparticles as a eupatorin carrier on DU-145 and LNCaP human prostate cancer cell lines. J. Pharm. Anal. 2021, 11, 108–121. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, G.; Peng, L.; Tian, J.; Zhang, H. Calycosin inhibits viability, induces apoptosis, and suppresses invasion of cervical cancer cells by upregulating tumor suppressor miR-375. Arch. Biochem. Biophys. 2020, 691, 108478. [Google Scholar] [CrossRef]
- Guo, J.M.; Kang, G.Z.; Xiao, B.X.; Liu, D.H.; Zhang, S. Effect of daidzein on cell growth, cell cycle, and telomerase activity of human cervical cancer in vitro. Int. J. Gynecol. Cancer 2004, 14, 882–888. [Google Scholar] [CrossRef]
- Ju, Y.H.; Fultz, J.; Allred, K.F.; Doerge, D.R.; Helferich, W.G. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis 2006, 27, 856–863. [Google Scholar] [CrossRef]
- Liu, H.; Lee, G.; Lee, J.I.; Ahn, T.-G.; Kim, S.A. Effects of genistein on anti-tumor activity of cisplatin in human cervical cancer cell lines. Obstet. Gynecol. Sci. 2019, 62, 322–328. [Google Scholar] [CrossRef]
- Wang, K.-L.; Yu, Y.-C.; Hsia, S.-M. Perspectives on the Role of Isoliquiritigenin in Cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Hirchaud, F.; Hermetet, F.; Ablise, M.; Fauconnet, S.; Vuitton, D.A.; Prétet, J.-L.; Mougin, C. Isoliquiritigenin Induces Caspase-Dependent Apoptosis via Downregulation of HPV16 E6 Expression in Cervical Cancer Ca Ski Cells. Planta Med. 2013, 79, 1628–1635. [Google Scholar] [CrossRef]
- Jia, L.; Hu, Y.; Yang, G.; Li, P. Puerarin suppresses cell growth and migration in HPV-positive cervical cancer cells by inhibiting the PI3K/mTOR signaling pathway. Exp. Ther. Med. 2019, 18, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Ramesh, E.; Periasamy, V.; Subash-Babu, P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam. Clin. Pharmacol. 2012, 27, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Zhang, J.; Gao, J.; Ge, X.; Lou, G. Hesperidin inhibits HeLa cell proliferation through apoptosis mediated by endoplasmic reticulum stress pathways and cell cycle arrest. BMC Cancer 2015, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhang, G.; Li, C.; Li, G.; Cao, Y.; Sun, F. Hesperidin Induces Mitochondria Mediated Intrinsic Apoptosis in HPV-Positive Cervical Cancer Cells via Regulation of E6/p53 Expression; Research Square: Durham, NC, USA, 2020. [Google Scholar] [CrossRef]
- Zeng, L.; Zhen, Y.; Chen, Y.; Zou, L.; Zhang, Y.; Hu, F.; Feng, J.; Shen, J.; Wei, B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NF-κB/COX-2-caspase-1 pathway in HeLa cervical cancer cells. Int. J. Oncol. 2014, 45, 1929–1936. [Google Scholar] [CrossRef]
- Ramesh, E.; Alshatwi, A. Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem. Toxicol. 2013, 51, 97–105. [Google Scholar] [CrossRef]
- Giuliani, A.; Cerretani, L.; Cichelli, A. Colors: Properties and Determination of Natural Pigments. In Encyclopedia of Food and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 273–283. [Google Scholar]
- Pratiwi, R.; Tunjung, W.A.S.; Rumiyati, R.; Amalia, A.R. Black Rice Bran Extracts and Fractions Containing Cyanidin 3-glucoside and Peonidin 3-glucoside Induce Apoptosis in Human Cervical Cancer Cells. Indones. J. Biotechnol. 2016, 20, 69–76. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Ayvaz, H.; Ruginǎ, D.; Leopold, L.F.; Stǎnilǎ, A.; Socaciu, C.; Tăbăran, F.; Luput, L.; Mada, D.C.; Pintea, A.; et al. Melanoma Inhibition by Anthocyanins Is Associated with the Reduction of Oxidative Stress Biomarkers and Changes in Mitochondrial Membrane Potential. Plant. Foods Hum. Nutr. 2017, 72, 404–410. [Google Scholar] [CrossRef]
- Bai, X.; Ma, Y.; Zhang, G. Butein suppresses cervical cancer growth through the PI3K/AKT/mTOR pathway. Oncol. Rep. 2015, 33, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.-Q.; Lv, W.; Luo, W.-Q.; Lu, W.-X.; Li, N. Treatment of human cervical cancer cells with butein leads to apoptosis and DNA damage. Int. J. Clin. Exp. Med. 2016, 9, 11084–11089. [Google Scholar]
- Yang, P.; Hu, D.; Kao, Y.; Lin, I.; Liu, F. Butein induces apoptotic cell death of human cervical cancer cells. Oncol. Lett. 2018, 16, 6615–6623. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.K.; Malek, S.N.A. Xanthohumol Induces Growth Inhibition and Apoptosis in Ca Ski Human Cervical Cancer Cells. Evid. Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid from Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Kłósek, M.; Kuropatnicki, A.K.; Szliszka, E.; Korzonek-Szlacheta, I.; Król, W. Chalcones Target the Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand (TRAIL) Signaling Pathway for Cancer Chemoprevention. In Nutrition and Functional Foods for Healthy Aging; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 233–244. [Google Scholar]
- Hsiao, Y.-H.; Hsieh, M.-J.; Yang, S.-F.; Chen, S.-P.; Tsai, W.-C.; Chen, P.-N. Phloretin suppresses metastasis by targeting protease and inhibits cancer stemness and angiogenesis in human cervical cancer cells. Phytomedicine 2019, 62, 152964. [Google Scholar] [CrossRef]
- De Oliveira, M.R. Phloretin-induced cytoprotective effects on mammalian cells: A mechanistic view and future directions. BioFactors 2016, 42, 13–40. [Google Scholar] [CrossRef]
- Wang, H.R.; Sui, H.C.; Zhu, B.T. Ellagic acid, a plant phenolic compound, activates cyclooxygenase-mediated prostaglandin production. Exp. Ther. Med. 2019, 18, 987–996. [Google Scholar] [CrossRef]
- Xia, J.; Xue, C.; Yu, J. Ellagic acid inhibited cervical cancer growth via blocking the AKT/mTOR/STAT3 pathway. Arch. Med. Sci. 2020, 16. [Google Scholar] [CrossRef]
- Li, L.; Na, C.; Tian, S.; Chen, J.; Ma, R.; Gao, Y.; Lou, G. Ellagic acid induces HeLa cell apoptosis via regulating signal transducer and activator of transcription 3 signaling. Exp. Ther. Med. 2018, 16, 29–36. [Google Scholar] [CrossRef]
- Chen, W.; Shen, X.; Ma, L.; Chen, R.; Yuan, Q.; Zheng, Y.; Li, C.; Peng, G. Phenolic Compounds from Polygonum chinense Induce Growth Inhibition and Apoptosis of Cervical Cancer SiHa Cells. BioMed Res. Int. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Kumar, D.; Basu, S.; Parija, L.; Rout, D.; Manna, S.; Dandapat, J.; Debata, P.R. Curcumin and Ellagic acid synergistically induce ROS generation, DNA damage, p53 accumulation and apoptosis in HeLa cervical carcinoma cells. Biomed. Pharmacother. 2016, 81, 31–37. [Google Scholar] [CrossRef]
- Tang, J.; Li, B.; Hong, S.; Liu, C.; Min, J.; Hu, M.; Li, Y.; Liu, Y.; Hong, L. Punicalagin suppresses the proliferation and invasion of cervical cancer cells through inhibition of the β-catenin pathway. Mol. Med. Rep. 2017, 16, 1439–1444. [Google Scholar] [CrossRef]
- Zhang, L.; Chinnathambi, A.; Alharbi, S.A.; Veeraraghavan, V.P.; Mohan, S.K.; Zhang, G. Punicalagin promotes the apoptosis in human cervical cancer (ME-180) cells through mitochondrial pathway and by inhibiting the NF-kB signaling pathway. Saudi J. Biol. Sci. 2020, 27, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, G.; Prasad, N.R. Anticancer Effect of Caffeic Acid on Human Cervical Cancer Cells. In Coffee in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 655–661. [Google Scholar]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Mukherjee, S.; Vanmanen, J.; Banerjee, P.; Fata, J.E. Dietary Polyphenols, Resveratrol and Pterostilbene Exhibit Antitumor Activity on an HPV E6-Positive Cervical Cancer Model: An in vitro and in vivo Analysis. Front. Oncol. 2019, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, Q.; Zeng, L.; Xie, L.; Zhao, Q.; Xu, H.; Wang, X.; Jiang, N.; Fu, P.; Sang, M. Resveratrol suppresses the growth and metastatic potential of cervical cancer by inhibiting STAT3 Tyr705 phosphorylation. Cancer Med. 2020, 9, 8685–8700. [Google Scholar] [CrossRef]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondria in Neuroprotection by Phytochemicals: Bioactive Polyphenols Modulate Mitochondrial Apoptosis System, Function and Structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef]
- Zaman, M.S.; Chauhan, N.; Yallapu, M.M.; Gara, R.K.; Maher, D.M.; Kumari, S.; Sikander, M.; Khan, S.; Zafar, N.; Jaggi, M.; et al. Curcumin Nanoformulation for Cervical Cancer Treatment. Sci. Rep. 2016, 6, 20051. [Google Scholar] [CrossRef]
- Sheng, S.-Q.; Yu, L.-Y.; Zhou, X.-W.; Pan, H.-Y.; Hu, F.-Y.; Liu, J.-L. Paeonol prevents migration and invasion, and promotes apoptosis of cervical cancer cells by inhibiting 5-lipoxygenase. Mol. Med. Rep. 2021, 23, 1–8. [Google Scholar] [CrossRef]
- Du, J.; Song, D.; Li, J.; Li, Y.; Li, B.; Li, L. Paeonol triggers apoptosis in HeLa cervical cancer cells: The role of mitochondria-related caspase pathway. Psychopharmacology 2021, 1–10. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, L.; Sun, X.; Zeng, F.; Pan, Q.; Xue, M. Scopoletin exerts anticancer effects on human cervical cancer cell lines by triggering apoptosis, cell cycle arrest, inhibition of cell invasion and PI3K/AKT signalling pathway. JBUON 2019, 24, 997–1002. [Google Scholar] [PubMed]
- Zhao, X.; Zhu, W.; Zhang, R.; Zhang, M.; Zhao, J.; Hou, J.; Zhang, W.; Zhua, W. Targeted juglone blocks the invasion and metastasis of HPV-positive cervical cancer cells. J. Pharmacol. Sci. 2019, 140, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, H.; Zheng, X.-M.; Chen, M.-L. Experimental study on the apoptosis of cervical cancer Hela cells induced by juglone through c-Jun N-terminal kinase/c-Jun pathway. Asian Pac. J. Trop. Med. 2017, 10, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, X.; Zhao, J.; Zhu, W.; Hou, J.; Wang, Y.; Zhang, W. Juglone Inhibits Proliferation of HPV-Positive Cervical Cancer Cells Specifically. Biol. Pharm. Bull. 2019, 42, 475–480. [Google Scholar] [CrossRef]
- Su, K.; Wang, C.-F.; Zhang, Y.; Cai, Y.-J.; Zhang, Y.-Y.; Zhao, Q. The inhibitory effects of carnosic acid on cervical cancer cells growth by promoting apoptosis via ROS-regulated signaling pathway. Biomed. Pharmacother. 2016, 82, 180–191. [Google Scholar] [CrossRef]
- Jing, Z.; Fei, W.; Zhou, J.; Zhang, L.; Chen, L.; Zhang, X.; Liang, X.; Xie, J.; Fang, Y.; Sui, X.; et al. Salvianolic acid B, a novel autophagy inducer, exerts antitumor activity as a single agent in colorectal cancer cells. Oncotarget 2016, 7, 61509–61519. [Google Scholar] [CrossRef]
- Mears, B.; Frank, S.; Nick, T.; Chia-Min, C.; Kyujoo, C.; Cynthia, C.; Melissa, N.; Hubert, S. Salvianolic Acid B Inhibits Growth of Cervical Cancer Cells In Vitro via Induction of Apoptosis through the Extrinsic Pathway. 2014. Available online: https://drum.lib.umd.edu/handle/1903/15532 (accessed on 20 April 2021).
- Yadav, N.; Parveen, S.; Banerjee, M. Potential of nano-phytochemicals in cervical cancer therapy. Clin. Chim. Acta 2020, 505, 60–72. [Google Scholar] [CrossRef]
- Boncler, M.; Golanski, J.; Lukasiak, M.; Redzynia, M.; Dastych, J.; Watala, C. A new approach for the assessment of the toxicity of polyphenol-rich compounds with the use of high content screening analysis. PLoS ONE 2017, 12, e0180022. [Google Scholar] [CrossRef]
- Martin, K.; Appel, C.L. Nutrition and Dietary Supplements Polyphenols as Dietary Supplements: A Double-Edged Sword. 2010. Available online: https://www.dovepress.com/ (accessed on 26 March 2021).
- Ruskovska, T.; Maksimova, V.; Milenkovic, D. Polyphenols in human nutrition: From thein vitroantioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability—An overview and perspective. Br. J. Nutr. 2020, 123, 241–254. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic Dietary Phytochemicals. NeuroMol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef]
- Bennetau-Pelissero, C.; Breton, B.; Bennetau, B.; Corraze, G.; Le Menn, F.; Davail-Cuisset, B.; Helou, C.; Kaushik, S. Effect of Genistein-Enriched Diets on the Endocrine Process of Gametogenesis and on Reproduction Efficiency of the Rainbow Trout Oncorhynchus mykiss. Gen. Comp. Endocrinol. 2001, 121, 173–187. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.-M.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Yang, C.S. Possible Controversy over Dietary Polyphenols: Benefits vs. Risks. Chem. Res. Toxicol. 2007, 20, 583–585. [Google Scholar] [CrossRef]
- Rojo, M.; Garrosa, M.; Jiménez, P.; Girbés, T.; Garcia-Recio, V.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Unexpected Toxicity of Green Tea Polyphenols in Combination with the Sambucus RIL Ebulin. Toxins 2020, 12, 542. [Google Scholar] [CrossRef]
- Riche, D.M.; McEwen, C.L.; Riche, K.D.; Sherman, J.J.; Wofford, M.R.; Deschamp, D.; Griswold, M. Analysis of Safety from a Human Clinical Trial with Pterostilbene. J. Toxicol. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.-Y.; He, K.-M.; Chen, J.-L.; Xu, Y.-M.; Lau, A.T.Y. Phytofabrication of Nanoparticles as Novel Drugs for Anticancer Applications. Molecules 2019, 24, 4246. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).