Age-Dependent Chronic Lung Injury and Pulmonary Fibrosis following Single Exposure to Hydrochloric Acid
Abstract
:1. Introduction
2. Results
2.1. HCl Instillation Provokes Persistent Alveolar Inflammation
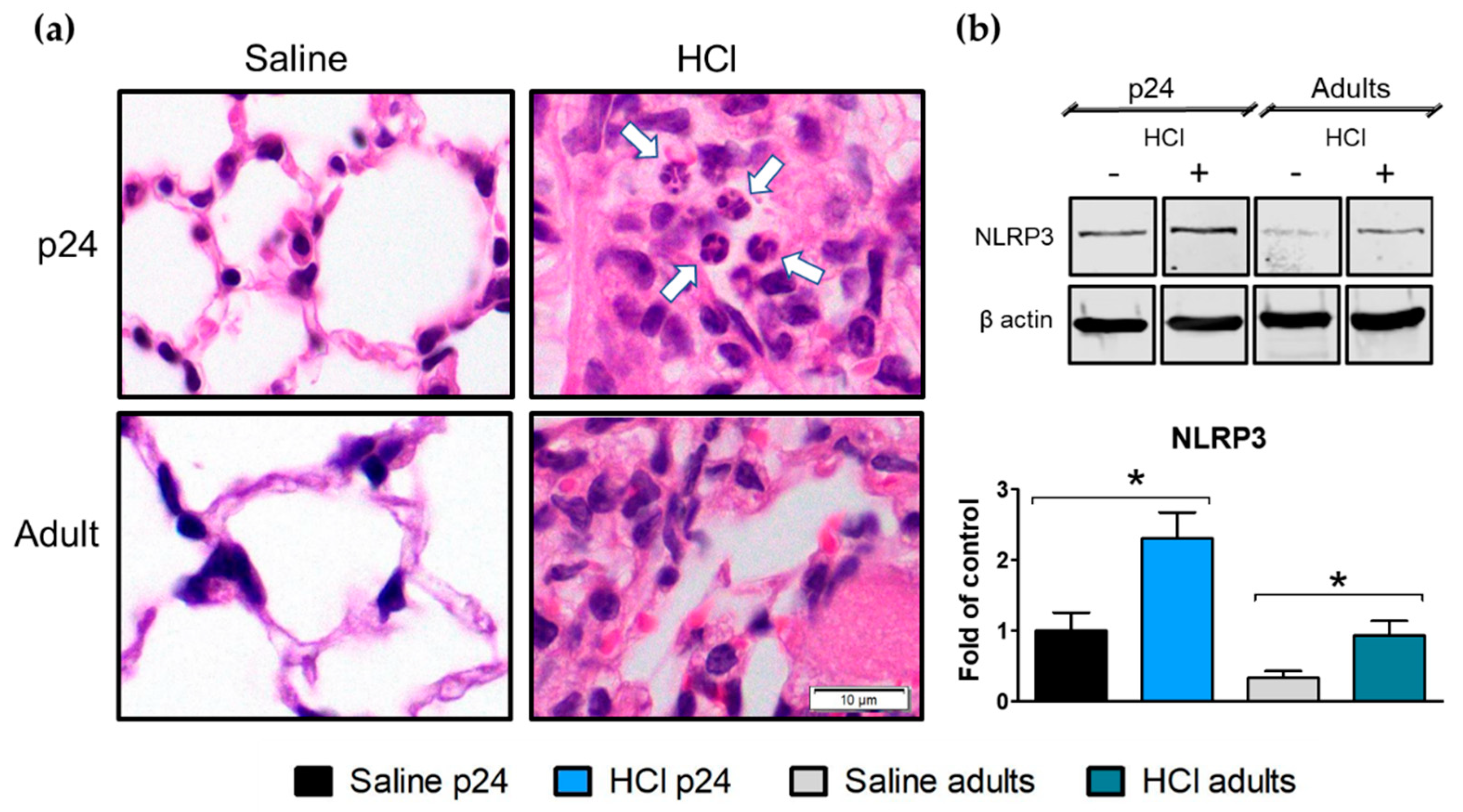
2.2. Histological Evidence of Inflammation and Age-Dependent Activation of NLRP3 Inflammasome
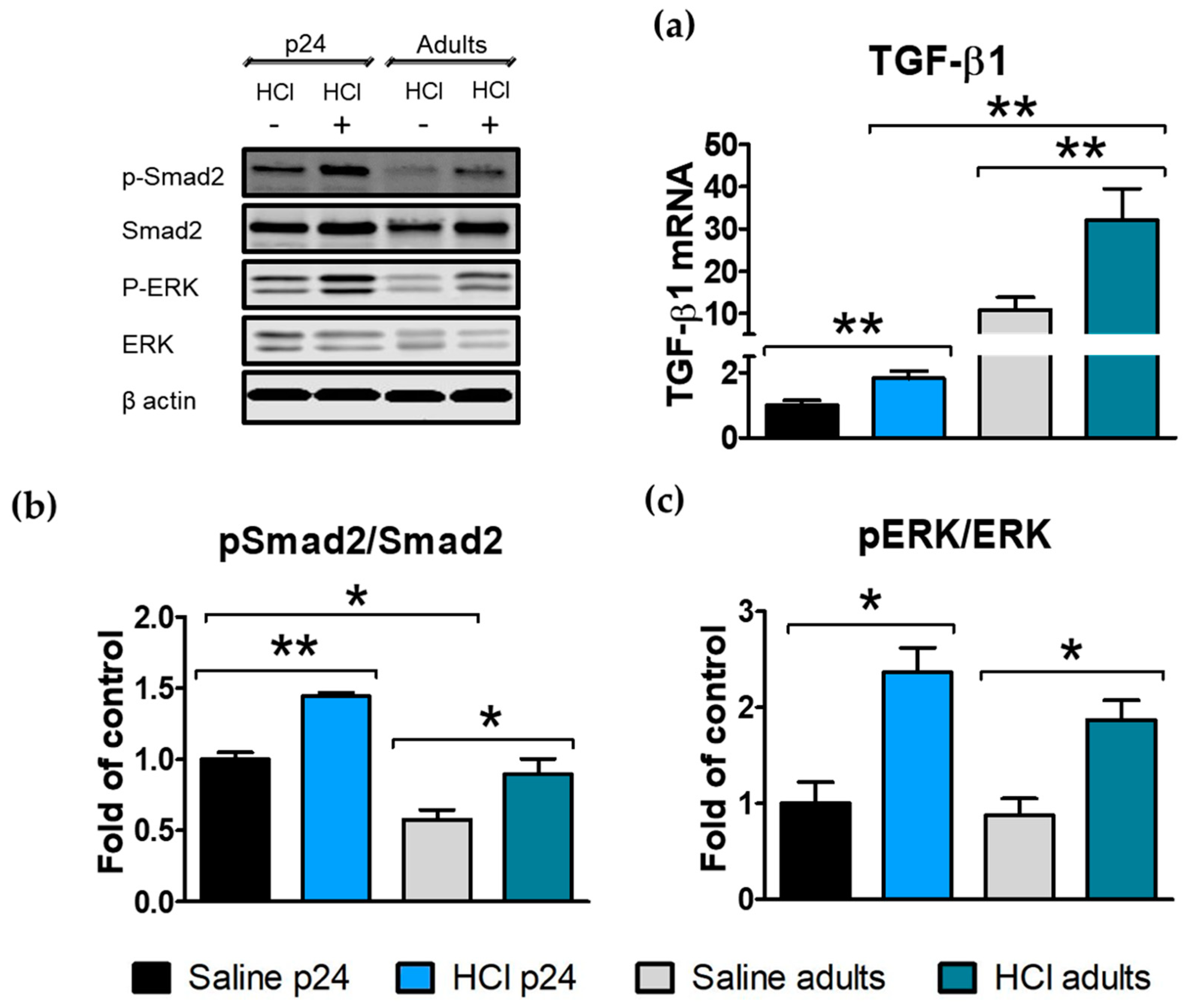
2.3. HCl Induces Age-Dependent Increases in TGF-β and Its Intracellular Signaling
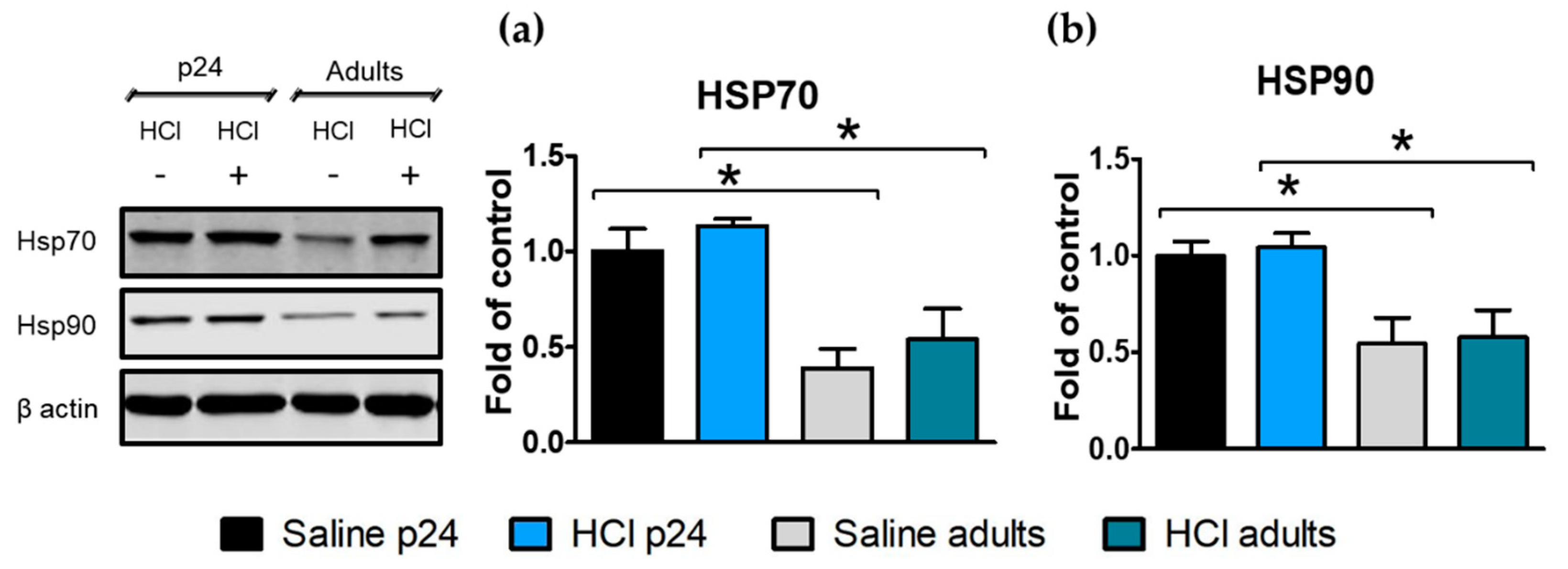
2.4. Age-Dependent Expression of Heat Shock Proteins in the Lung
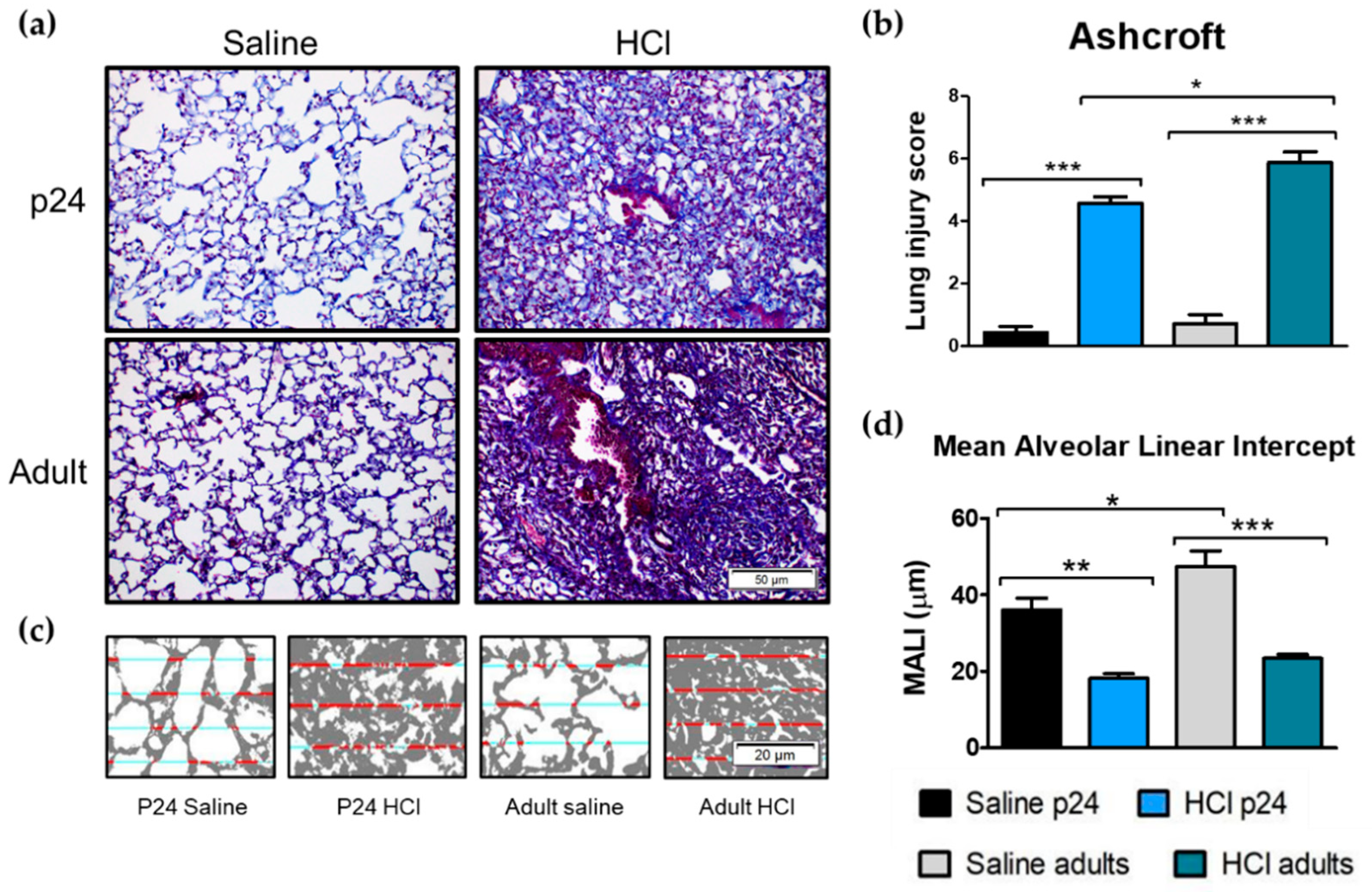
2.5. Age-Related Differences in Pulmonary Fibrosis, Ashcroft Score and Mean Alveolar Linear Intercept
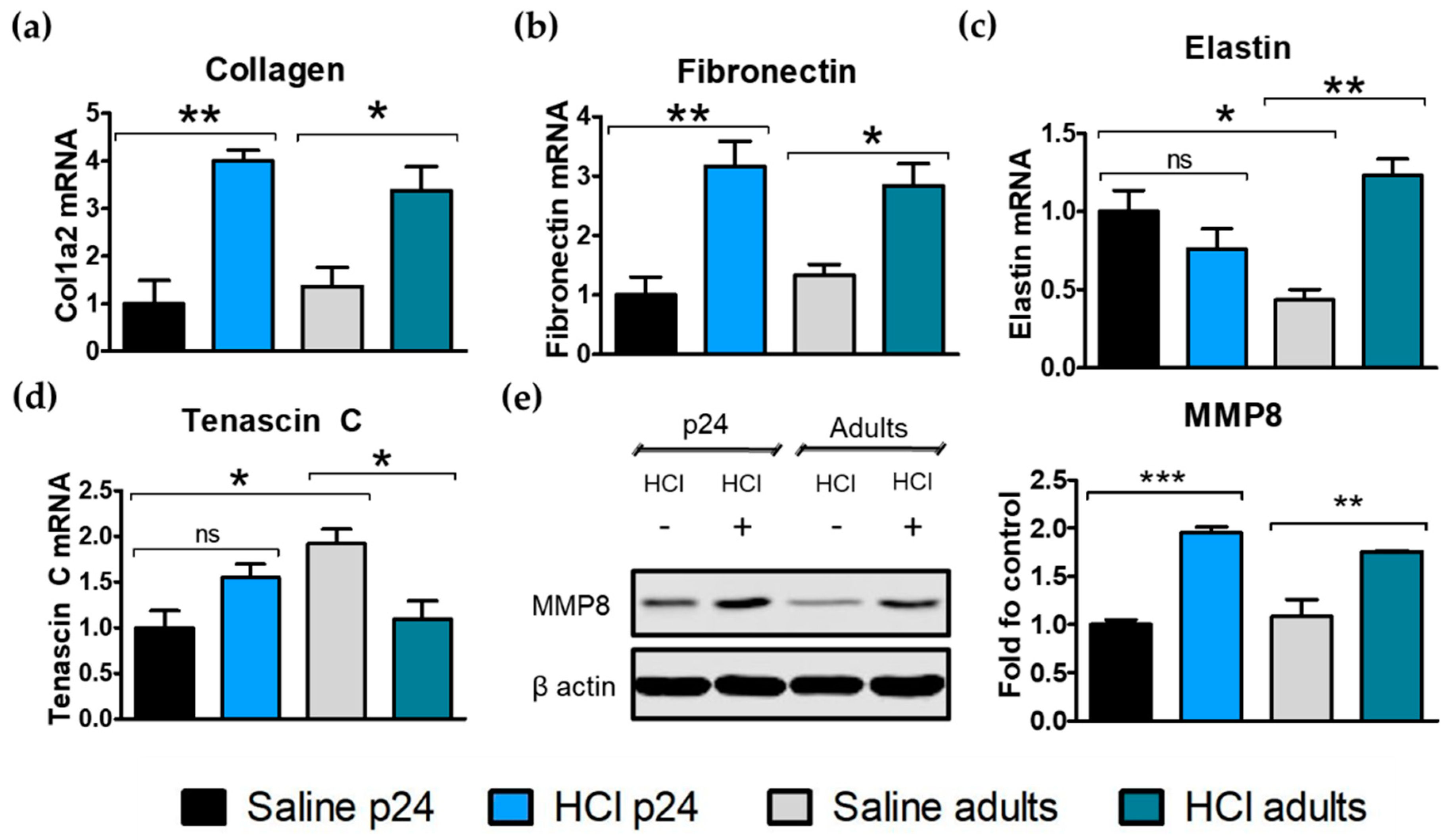
2.6. Age-Related Differences in Extracellular Matrix Protein Deposition
2.7. Lung Mechanics
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ethical Statement
4.3. Animals
4.4. HCl Dose Calculation and Translation to Adult and Pediatric Human Exposure
4.5. Bronchoalveolar Lavage Fluid (BALF) White Blood Cell Number and Total Protein Concentration
4.6. Histopathology and Lung Fibrosis Scoring
4.7. MeanAlveolar Linear Intercept
4.8. Tissue Collection
4.9. Western Blot Analysis
4.10. RNA Isolation and Quantitative Real-Time PCR (qPCR)
4.11. Lung Mechanics Measurements
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyce, S.H.; Simpson, K.A. Hydrochloric acid inhalation: Who needs admission? J. Accid. Emerg. Med. 1996, 13, 422–424. [Google Scholar] [CrossRef]
- U.S. Nuclear Regulatory Commission. Acute Toxicity of Hydrogen Chloride. Subcommittee on Rocket-Emission Toxicants. Available online: https://www.ncbi.nlm.nih.gov/books/NBK230426/ (accessed on June 2021).
- Davidson, J.T.; Rubin, S.; Eyal, Z.; Polliack, A. A comparison of the pulmonary response to the endotracheal instillation of 0.1 N hydrochloric acid and Hartmann’s solution in the rabbit. Br. J. Anaesth. 1974, 46, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amigoni, M.; Bellani, G.; Scanziani, M.; Masson, S.; Bertoli, E.; Radaelli, E.; Patroniti, N.; Di Lelio, A.; Pesenti, A.; Latini, R. Lung injury and recovery in a murine model of unilateral acid aspiration: Functional, biochemical, and morphologic characterization. Anesthesiology 2008, 108, 1037–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.V.; Wilson, M.R.; Takata, M. Resolution of acute lung injury and inflammation: A translational mouse model. Eur. Respir. J. 2012, 39, 1162. [Google Scholar] [CrossRef] [Green Version]
- Boulet, L.P. Increases in airway responsiveness following acute exposure to respiratory irritants. Reactive airway dysfunction syndrome or occupational asthma? Chest 1988, 94, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.M.; Weiss, M.A.; Bernstein, I.L. Reactive airways dysfunction syndrome (RADS). Persistent asthma syndrome after high level irritant exposures. Chest 1985, 88, 376–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deschamps, D.; Soler, P.; Rosenberg, N.; Baud, F.; Gervais, P. Persistent asthma after inhalation of a mixture of sodium hypochlorite and hydrochloric acid. Chest 1994, 105, 1895–1896. [Google Scholar] [CrossRef] [Green Version]
- Marinova, M.; Solopov, P.; Dimitropoulou, C.; Colunga Biancatelli, R.M.L.; Catravas, J.D. Acute exposure of mice to hydrochloric acid leads to the development of chronic lung injury and pulmonary fibrosis. Inhal. Toxicol. 2019, 31, 147–160. [Google Scholar] [CrossRef]
- Clark, K.A.; Chanda, D.; Balte, P.; Karmaus, W.J.; Cai, B.; Vena, J.; Lawson, A.B.; Mohr, L.C.; Gibson, J.J.; Svendsen, E.R. Respiratory symptoms and lung function 8–10 months after community exposure to chlorine gas: A public health intervention and cross-sectional analysis. BMC Public Health 2013, 13, 945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ATSDR. Medical managment Guidelines for Hydrogen Chloride. Agency for Toxic Substances and Disease Registry. 2014. Available online: https://wwwn.cdc.gov/TSP/MMG/MMGDetails.aspx?mmgid=758&toxid=147 (accessed on June 2021).
- Gorguner, M.; Akgun, M. Acute inhalation injury. Eurasian J. Med. 2010, 42, 28–35. [Google Scholar] [CrossRef]
- Sen, S. Pediatric inhalation injury. Burn. Trauma 2017, 5, 31. [Google Scholar] [CrossRef]
- Abrams, S.A. Chronic Pulmonary Insufficiency in Children and Its Effects on Growth and Development. J. Nutr. 2001, 131, 938S–941S. [Google Scholar] [CrossRef] [Green Version]
- Davis, P.B.; Kercsmar, C.M. Growth in Children with Chronic Lung Disease. N. Engl. J. Med. 2000, 342, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-C.; FitzSimmons, S.C.; Allen, D.B.; Kosorok, M.R.; Rosenstein, B.J.; Campbell, P.W.; Farrell, P.M. Risk of Persistent Growth Impairment after Alternate-Day Prednisone Treatment in Children with Cystic Fibrosis. N. Engl. J. Med. 2000, 342, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Solopov, P.; Colunga Biancatelli, R.M.; Dimitropoulou, C.; Catravas, J.D. Sex-Related Differences in Murine Models of Chemically Induced Pulmonary Fibrosis. Int. J. Mol. Sci. 2021, 22, 5909. [Google Scholar] [CrossRef]
- Marinova, M.; Solopov, P.; Dimitropoulou, C.; Colunga Biancatelli, R.M.L.; Catravas, J.D. Post-treatment with a heat shock protein 90 inhibitor prevents chronic lung injury and pulmonary fibrosis, following acute exposure of mice to HCl. Exp. Lung Res. 2020, 46, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Solopov, P.; Gregory, B.; Catravas, J.D. HSP90 Inhibition and Modulation of the Proteome: Therapeutical Implications for Idiopathic Pulmonary Fibrosis (IPF). Int. J. Mol. Sci 2020, 21, 5286. [Google Scholar] [CrossRef] [PubMed]
- Crowley, G.; Kwon, S.; Caraher, E.J.; Haider, S.H.; Lam, R.; Batra, P.; Melles, D.; Liu, M.; Nolan, A. Quantitative lung morphology: Semi-automated measurement of mean linear intercept. BMC Pulm. Med. 2019, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Burri, P.H. The postnatal growth of the rat lung III. Morphology. Anat. Rec. 1974, 180, 77–98. [Google Scholar] [CrossRef]
- Mund, S.I.; Stampanoni, M.; Schittny, J.C. Developmental alveolarization of the mouse lung. Dev. Dyn. 2008, 237, 2108–2116. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Solopov, P.; Gregory, B.; Catravas, J.D. The HSP90 Inhibitor, AUY-922, Protects and Repairs Human Lung Microvascular Endothelial Cells from Hydrochloric Acid-Induced Endothelial Barrier Dysfunction. Cells 2021, 10, 1489. [Google Scholar] [CrossRef]
- Jäger, B.; Seeliger, B.; Terwolbeck, O.; Warnecke, G.; Welte, T.; Müller, M.; Bode, C.; Prasse, A. The NLRP3-Inflammasome-Caspase-1 Pathway Is Upregulated in Idiopathic Pulmonary Fibrosis and Acute Exacerbations and Is Inducible by Apoptotic A549 Cells. Front. Immunol. 2021, 12, 642855. [Google Scholar] [CrossRef]
- Stout-Delgado, H.W.; Cho, S.J.; Chu, S.G.; Mitzel, D.N.; Villalba, J.; El-Chemaly, S.; Ryter, S.W.; Choi, A.M.K.; Rosas, I.O. Age-Dependent Susceptibility to Pulmonary Fibrosis Is Associated with NLRP3 Inflammasome Activation. Am. J. Respir. Cell Mol. Biol. 2016, 55, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Ghonime, M.G.; Shamaa, O.R.; Das, S.; Eldomany, R.A.; Fernandes-Alnemri, T.; Alnemri, E.S.; Gavrilin, M.A.; Wewers, M.D. Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J. Immunol. 2014, 192, 3881–3888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Fan, C.; Cai, D.; Zhang, Y.; Zuo, R.; Zhu, L.; Cao, Y.; Zhang, J.; Liu, C.; Chen, Y.; et al. Contribution of TGF-Beta-Mediated NLRP3-HMGB1 Activation to Tubulointerstitial Fibrosis in Rat With Angiotensin II-Induced Chronic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Artlett, C.M. The Role of the NLRP3 Inflammasome in Fibrosis. Open Rheumatol. J. 2012, 6, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Bonniaud, P.; Bellaye, P.-S.; Burgy, O.; Kolb, M. Heat shock protein: A hot topic in idiopathic pulmonary fibrosis. Eur. Respir. J. 2017, 49, 1602152. [Google Scholar] [CrossRef] [Green Version]
- Canella, A.; Welker, A.M.; Yoo, J.Y.; Xu, J.; Abas, F.S.; Kesanakurti, D.; Nagarajan, P.; Beattie, C.E.; Sulman, E.P.; Liu, J.; et al. Efficacy of Onalespib, a Long-Acting Second-Generation HSP90 Inhibitor, as a Single Agent and in Combination with Temozolomide against Malignant Gliomas. Clin. Cancer Res. 2017, 23, 6215. [Google Scholar] [CrossRef] [Green Version]
- Thangjam, G.S.; Birmpas, C.; Barabutis, N.; Gregory, B.W.; Clemens, M.A.; Newton, J.R.; Fulton, D.; Catravas, J.D. Hsp90 inhibition suppresses NF-κB transcriptional activation via Sirt-2 in human lung microvascular endothelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L964–L974. [Google Scholar] [CrossRef] [PubMed]
- Solopov, P.; Marinova, M.; Dimitropoulou, C.; Colunga Biancatelli, R.M.L.; Catravas, J.D. Development of chronic lung injury and pulmonary fibrosis in mice following acute exposure to nitrogen mustard. Inhal. Toxicol. 2020, 32, 141–154. [Google Scholar] [CrossRef]
- Solopov Pavel, B.R.M.L.C.; Margarita, M.; Christiana, D.; Catravas John, D. The HSP90 Inhibitor, AUY-922, Ameliorates the Development of Nitrogen Mustard-Induced Pulmonary Fibrosis and Lung Dysfunction in Mice. Int. J. Mol. Sci. 2020, 21, 4740. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.M.; Brown, I.R. Constitutive expression of heat shock proteins Hsp90, Hsc70, Hsp70 and Hsp60 in neural and non-neural tissues of the rat during postnatal development. Cell Stress Chaperones 1998, 3, 188–199. [Google Scholar] [CrossRef]
- Deutsch, G.H.; Young, L.R.; Deterding, R.R.; Fan, L.L.; Dell, S.D.; Bean, J.A.; Brody, A.S.; Nogee, L.M.; Trapnell, B.C.; Langston, C.; et al. Diffuse lung disease in young children: Application of a novel classification scheme. Am. J. Respir. Crit. Care Med. 2007, 176, 1120–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, N.; Sileo, C.; Thouvenin, G.; Berdah, L.; Delestrain, C.; Manali, E.; Papiris, S.; Léger, P.-L.; Pointe, H.D.L.; l’Hermine, A.C.; et al. Pulmonary Fibrosis in Children. J. Clin. Med. 2019, 8, 1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahluwalia, N.; Shea, B.S.; Tager, A.M. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am. J. Respir. Crit. Care Med. 2014, 190, 867–878. [Google Scholar] [CrossRef]
- Komi-Kuramochi, A.; Kawano, M.; Oda, Y.; Asada, M.; Suzuki, M.; Oki, J.; Imamura, T. Expression of fibroblast growth factors and their receptors during full-thickness skin wound healing in young and aged mice. J. Endocrinol. 2005, 186, 273–289. [Google Scholar] [CrossRef]
- Bartram, U.; Speer, C.P. The Role of Transforming Growth Factor β in Lung Development and Disease. Chest 2004, 125, 754–765. [Google Scholar] [CrossRef]
- Beers, M.F.; Solarin, K.O.; Guttentag, S.H.; Rosenbloom, J.; Kormilli, A.; Gonzales, L.W.; Ballard, P.L. TGF-beta1 inhibits surfactant component expression and epithelial cell maturation in cultured human fetal lung. Am. J. Physiol. 1998, 275, L950–L960. [Google Scholar]
- Whitsett, J.A.; Weaver, T.E.; Lieberman, M.A.; Clark, J.C.; Daugherty, C. Differential effects of epidermal growth factor and transforming growth factor-beta on synthesis of Mr = 35,000 surfactant-associated protein in fetal lung. J. Biol. Chem. 1987, 262, 7908–7913. [Google Scholar] [CrossRef]
- Zhou, L.; Dey, C.R.; Wert, S.E.; Whitsett, J.A. Arrested lung morphogenesis in transgenic mice bearing an SP-C-TGF-beta 1 chimeric gene. Dev. Biol. 1996, 175, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Serra, R.; Pelton, R.W.; Moses, H.L. TGF beta 1 inhibits branching morphogenesis and N-myc expression in lung bud organ cultures. Development 1994, 120, 2153–2161. [Google Scholar] [CrossRef]
- Jilling, T.; Ren, C.; Yee, A.; Aggarwal, S.; Halloran, B.; Ambalavanan, N.; Matalon, S. Exposure of neonatal mice to bromine impairs their alveolar development and lung function. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L137–L143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Wallis, C.; Van Winkle, L.S.; Wexler, A.S. Disruption of tracheobronchial airway growth following postnatal exposure to ozone and ultrafine particles. Inhal. Toxicol. 2011, 23, 520–531. [Google Scholar] [CrossRef]
- Mecham, R.P. Elastin in lung development and disease pathogenesis. Matrix Biol. 2018, 73, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Phipps, S.; Baines, K.J.; Oreo, K.M.; Gunawardhana, L.; Gibson, P.G. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur. Respir. J. 2014, 43, 1067. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.E. Inhaled efficacious dose translation from rodent to human: A retrospective analysis of clinical standards for respiratory diseases. Pharmacol. Ther. 2017, 178, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hickey, F.C. Noxious Gases and the Principles of Respiration Influencing Their Action. Second, revised edition (Henderson, Yandell; Haggard, Howard W.). J. Chem. Educ. 1943, 20, 259. [Google Scholar] [CrossRef] [Green Version]
- Forbes, B.; O’Lone, R.; Allen, P.P.; Cahn, A.; Clarke, C.; Collinge, M.; Dailey, L.A.; Donnelly, L.E.; Dybowski, J.; Hassall, D.; et al. Challenges for inhaled drug discovery and development: Induced alveolar macrophage responses. Adv. Drug Deliv. Rev. 2014, 71, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Snipes, M.B.; McClellan, R.O.; Mauderly, J.L.; Wolff, R.K. Retention patterns for inhaled particles in the lung: Comparisons between laboratory animals and humans for chronic exposures. Health Phys. 1989, 57 (Suppl. S1), 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veres-Székely, A.; Pap, D.; Sziksz, E.; Jávorszky, E.; Rokonay, R.; Lippai, R.; Tory, K.; Fekete, A.; Tulassay, T.; Szabó, A.J.; et al. Selective measurement of α smooth muscle actin: Why β-actin can not be used as a housekeeping gene when tissue fibrosis occurs. BMC Mol. Biol. 2017, 18, 12. [Google Scholar] [CrossRef] [Green Version]
- Tsao, P.-N.; Matsuoka, C.; Wei, S.-C.; Sato, A.; Sato, S.; Hasegawa, K.; Chen, H.-K.; Ling, T.-Y.; Mori, M.; Cardoso, W.V.; et al. Epithelial Notch signaling regulates lung alveolar morphogenesis and airway epithelial integrity. Proc. Natl. Acad. Sci. USA 2016, 113, 201511236. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colunga Biancatelli, R.M.L.; Solopov, P.; Dimitropoulou, C.; Catravas, J.D. Age-Dependent Chronic Lung Injury and Pulmonary Fibrosis following Single Exposure to Hydrochloric Acid. Int. J. Mol. Sci. 2021, 22, 8833. https://doi.org/10.3390/ijms22168833
Colunga Biancatelli RML, Solopov P, Dimitropoulou C, Catravas JD. Age-Dependent Chronic Lung Injury and Pulmonary Fibrosis following Single Exposure to Hydrochloric Acid. International Journal of Molecular Sciences. 2021; 22(16):8833. https://doi.org/10.3390/ijms22168833
Chicago/Turabian StyleColunga Biancatelli, Ruben M. L., Pavel Solopov, Christiana Dimitropoulou, and John D. Catravas. 2021. "Age-Dependent Chronic Lung Injury and Pulmonary Fibrosis following Single Exposure to Hydrochloric Acid" International Journal of Molecular Sciences 22, no. 16: 8833. https://doi.org/10.3390/ijms22168833
APA StyleColunga Biancatelli, R. M. L., Solopov, P., Dimitropoulou, C., & Catravas, J. D. (2021). Age-Dependent Chronic Lung Injury and Pulmonary Fibrosis following Single Exposure to Hydrochloric Acid. International Journal of Molecular Sciences, 22(16), 8833. https://doi.org/10.3390/ijms22168833







