Within-Host Adaptation of Staphylococcus aureus in a Bovine Mastitis Infection Is Associated with Increased Cytotoxicity
Abstract
1. Introduction
2. Results
2.1. Differences in the Surface Proteome between IN and HA
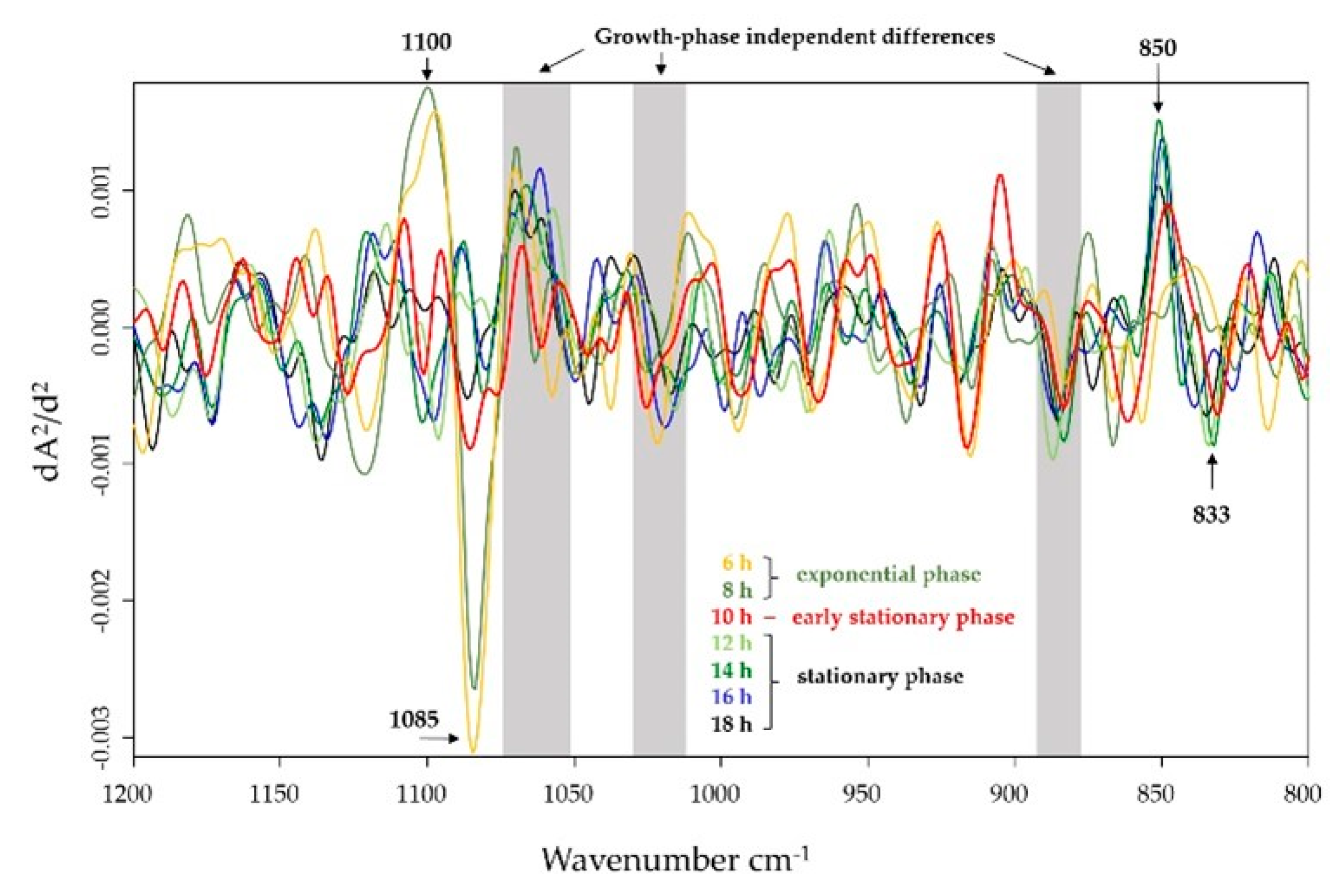
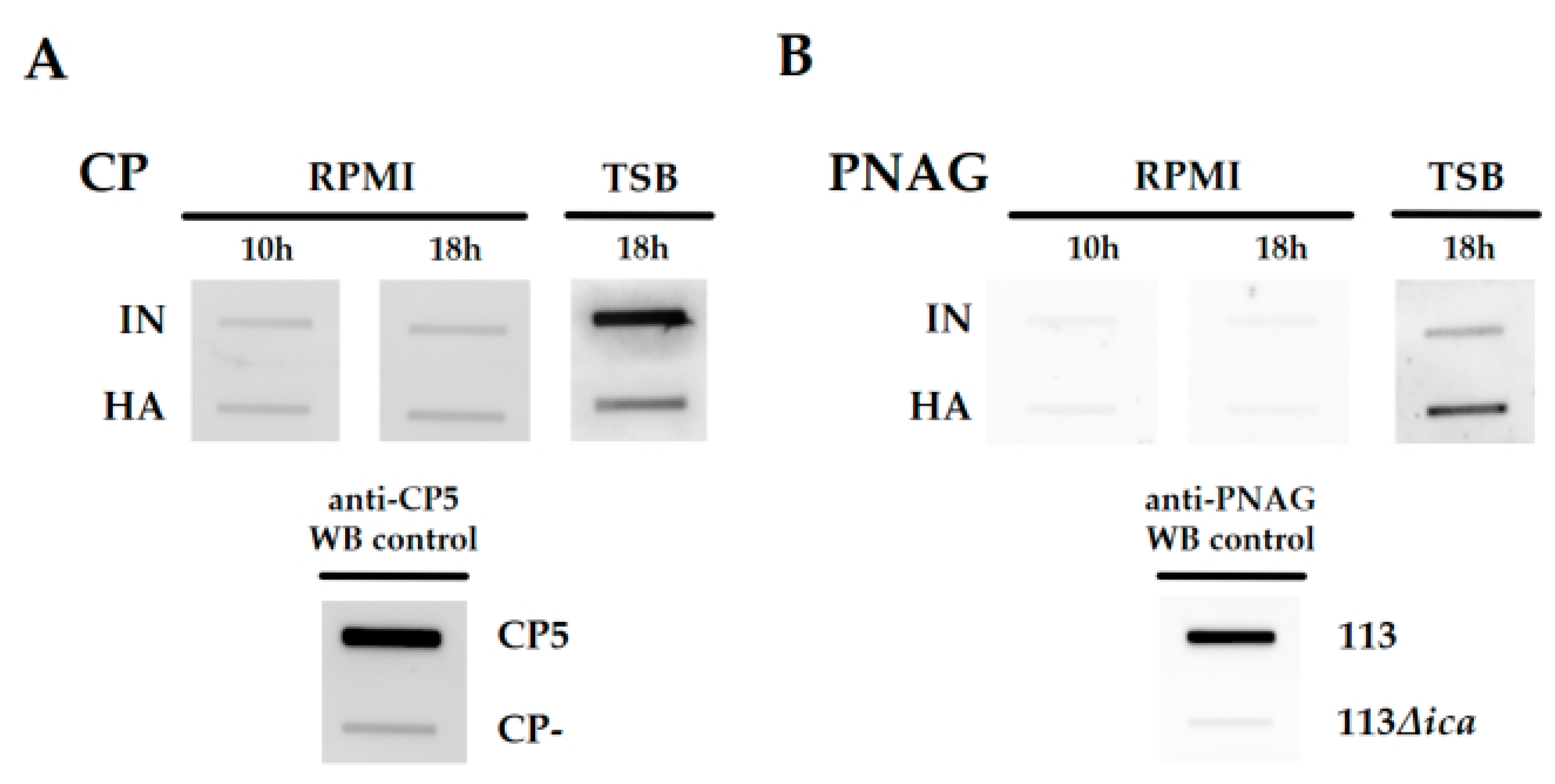
2.2. Differences in the Cell Envelope Glycopolymer Composition between IN and HA
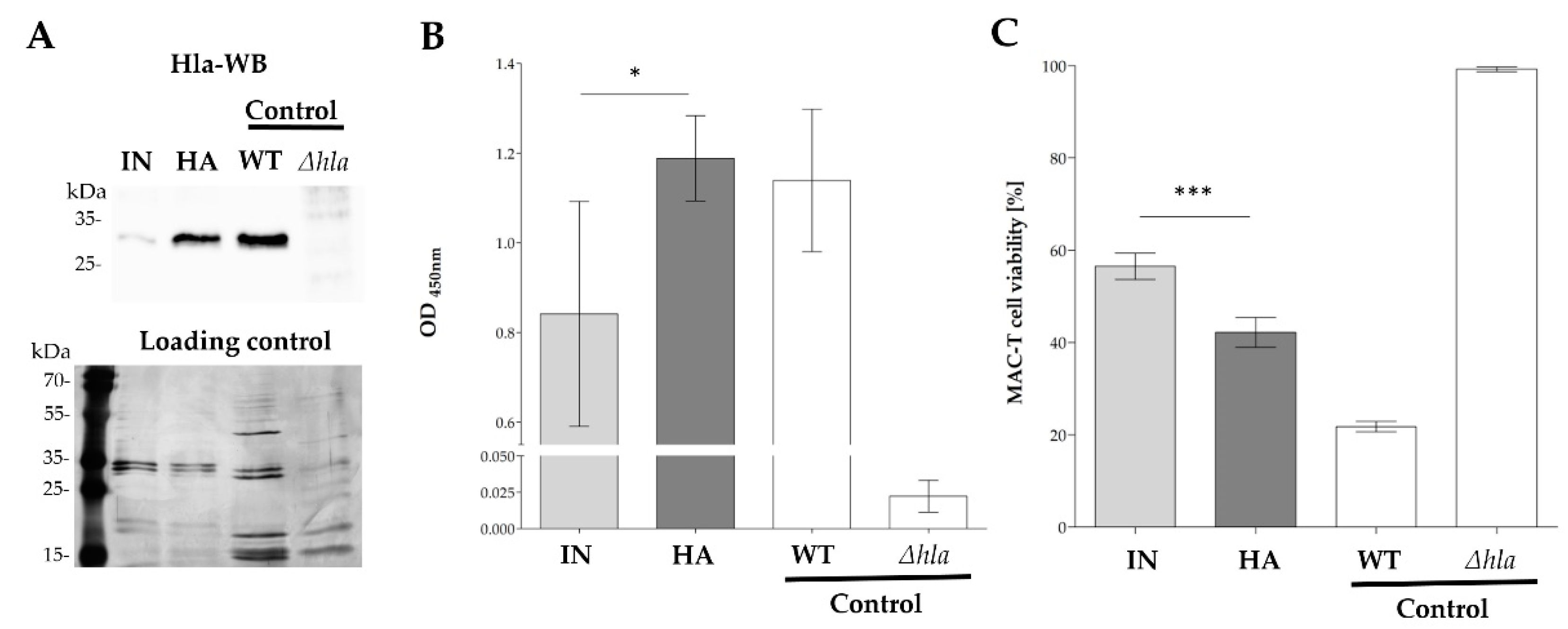
2.3. Increased Cytotoxicity of HA
3. Discussion
4. Materials and Methods
4.1. Bovine Isolates and Reference Strains
4.2. Bacterial Growth under the Infection-Relevant Conditions
4.3. MS-Based Surface Proteomics
4.4. FTIR Spectroscopic Measurement and Analysis
4.5. Immune-Based Detection of Glycopolymers and Hla
4.6. Biofilm Formation and PNAG-Production
4.7. Cytotoxicity and Hemolysis Assays
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barkema, H.W.; Schukken, Y.H.; Zadoks, R.N. Invited review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G.; Fitzgerald, J.R.; Watts, J.L.; Koop, G.; Middleton, J.R. Knowledge gaps and research priorities in Staphylococcus aureus mastitis control. Transbound. Emerg. Dis. 2018, 65, 149–165. [Google Scholar] [CrossRef]
- Melchior, M.B.; Vaarkamp, H.; Fink-Gremmels, J. Biofilms: A role in recurrent mastitis infections? Vet. J. 2006, 171, 398–407. [Google Scholar] [CrossRef]
- Hébert, A.; Sayasith, K.; Sénéchal, S.; Dubreuil, P.; Lagacé, J. Demonstration of intracellular Staphylococcus aureus in bovine mastitis alveolar cells and macrophages isolated from naturally infected cow milk. FEMS Microbiol. Lett. 2000, 193, 57–62. [Google Scholar] [CrossRef]
- Kusch, H.; Engelmann, S. Secrets of the secretome in Staphylococcus aureus. Int. J. Med. Microbiol. 2014, 304, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, P.; Pozzi, F.; Raschetti, M.; Bignoli, G.; Capra, E.; Graber, H.U.; Vezzoli, F.; Piccinini, R.; Bertasi, B.; Biffani, S. Genomic characteristics of Staphylococcus aureus strains associated with high within-herd prevalence of intramammary infections in dairy cows. J. Dairy Sci. 2015, 98, 6828–6838. [Google Scholar] [CrossRef] [PubMed]
- Grunert, T.; Stessl, B.; Wolf, F.; Sordelli, D.O.; Buzzola, F.R.; Ehling-Schulz, M. Distinct phenotypic traits of Staphylococcus aureus are associated with persistent, contagious bovine intramammary infections. Sci. Rep. 2018, 8, 15968. [Google Scholar] [CrossRef]
- Bardiau, M.; Detilleux, J.; Farnir, F.; Mainil, J.G.; Ote, I. Associations between properties linked with persistence in a collection of Staphylococcus aureus isolates from bovine mastitis. Vet. Microbiol. 2014, 169, 74–79. [Google Scholar] [CrossRef]
- Bardiau, M.; Caplin, J.; Detilleux, J.; Graber, H.; Moroni, P.; Taminiau, B.; Mainil, J.G. Existence of two groups of Staphylococcus aureus strains isolated from bovine mastitis based on biofilm formation, intracellular survival, capsular profile and agr-typing. Vet. Microbiol. 2016, 185, 1–6. [Google Scholar] [CrossRef]
- Le Maréchal, C.; Jan, G.; Even, S.; McCulloch, J.A.; Azevedo, V.; Thiéry, R.; Vautor, E.; Le Loir, Y. Development of serological proteome analysis of mastitis by Staphylococcus aureus in ewes. J. Microbiol. Methods 2009, 79, 131–136. [Google Scholar] [CrossRef]
- Le Maréchal, C.; Jardin, J.; Jan, G.; Even, S.; Pulido, C.; Guibert, J.M.; Hernandez, D.; François, P.; Schrenzel, J.; Demon, D.; et al. Staphylococcus aureus seroproteomes discriminate ruminant isolates causing mild or severe mastitis. Vet. Res. 2011, 15, 35. [Google Scholar] [CrossRef]
- Marbach, H.; Mayer, K.; Vogl, C.; Lee, J.Y.H.; Monk, I.R.; Sordelli, D.O.; Buzzola, F.R.; Ehling-Schulz, M.; Grunert, T. Within-host evolution of bovine Staphylococcus aureus selects for a SigB-deficient pathotype characterized by reduced virulence but enhanced proteolytic activity and biofilm formation. Sci. Rep. 2019, 9, 13479. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Lee, J.C. Structure and Function of Surface Polysaccharides of Staphylococcus aureus. Curr. Top. Microbiol. Immunol. 2015, 409, 57–93. [Google Scholar] [CrossRef]
- Grunert, T.; Wenning, M.; Barbagelata, M.S.; Fricker, M.; Sordelli, D.O.; Buzzola, F.R.; Ehling-Schulz, M. Rapid and reliable identification of Staphylococcus aureus capsular serotypes by means of artificial neural network-assisted Fourier transform infrared spectroscopy. J. Clin. Microbiol. 2013, 51, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Grunert, T.; Jovanovic, D.; Sirisarn, W.; Johler, S.; Weidenmaier, C.; Ehling-Schulz, M.; Xia, G. Analysis of Staphylococcus aureus wall teichoic acid glycoepitopes by Fourier Transform Infrared Spectroscopy provides novel insights into the staphylococcal glycocode. Sci. Rep. 2018, 8, 1889. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: London, UK, 2001. [Google Scholar]
- Kamnev, A.A. FTIR spectroscopic studies of bacterial cellular responses to environmental factors, plant-bacterial interactions and signalling. J. Spectrosc. 2008, 22, 83–95. [Google Scholar] [CrossRef]
- Naumann, D.; Barnickel, G.; Bradaczek, H.; Labischinski, H.; Giesbrecht, P. Infrared spectroscopy, a tool for probing bacterial peptidoglycan: Potentialities of infrared spectroscopy for cell wall analytical studies and rejection of models based on crystalline chitin. Eur. J. Biochem. 1982, 125, 505–515. [Google Scholar] [CrossRef]
- Bosch, A.; Serra, D.; Prieto, C.; Schmitt, J.; Naumann, D.; Yantorno, O. Characterization of Bordetella pertussis growing as biofilm by chemical analysis and FT-IR spectroscopy. Appl. Microbiol. Biotechnol. 2006, 71, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.L.; Chien, Y.T.; Bayer, A.S. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated sarA expression in Staphylococcus aureus. Infect. Immun. 1999, 67, 1331–1337. [Google Scholar] [CrossRef]
- Dastgheyb, S.; Parvizi, J.; Shapiro, I.M.; Hickok, N.J.; Otto, M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J. Infect. Dis. 2015, 211, 641–650. [Google Scholar] [CrossRef]
- Giachino, P.; Engelmann, S.; Bischoff, M. Sigma(B) activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 2001, 183, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Karlsson-Kanth, A.; Tegmark-Wisell, K.; Arvidson, S.; Oscarsson, J. Natural human isolates of Staphylococcus aureus selected for high production of proteases and alpha-hemolysin are sigmaB deficient. Int. J. Med. Microbiol. 2006, 296, 229–236. [Google Scholar] [CrossRef]
- Horsburgh, M.J.; Aish, J.L.; White, I.J.; Shaw, L.; Lithgow, J.K.; Foster, S.J. SigmaB modulates virulence determinant expression and stress resistance: Characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 2002, 184, 5457–5467. [Google Scholar] [CrossRef] [PubMed]
- McAdam, P.R.; Holmes, A.; Templeton, K.E.; Fitzgerald, J.R. Adaptive evolution of Staphylococcus aureus during chronic endobronchial infection of a cystic fibrosis patient. PLoS ONE 2011, 6, e24301. [Google Scholar] [CrossRef] [PubMed]
- Vytvytska, O.; Nagy, E.; Blüggel, M.; Meyer, H.E.; Kurzbauer, R.; Huber, L.A.; Klade, C.S. Identification of vaccine candidate antigens of Staphylococcus aureus by serological proteome analysis. Proteomics 2002, 2, 580–590. [Google Scholar] [CrossRef]
- Trivier, D.; Davril, M.; Houdret, N.; Courcol, R.J. Influence of iron depletion on growth kinetics, siderophore production, and protein expression of Staphylococcus aureus. FEMS Microbiol. Lett. 1995, 127, 195–199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baratéla, K.C.; Saridakis, H.O.; Gaziri, L.C.J.; Pelayo, J.S. Effects of medium composition, calcium, iron and oxygen on haemolysin production by Plesiomonas shigelloides isolated from water. J. Appl. Microbiol. 2001, 90, 482–487. [Google Scholar] [CrossRef]
- Ledala, N.; Zhang, B.; Seravalli, J.; Powers, R.; Somerville, G.A. Influence of iron and aeration on Staphylococcus aureus growth, metabolism, and transcription. J. Bacteriol. 2014, 196, 2178–2189. [Google Scholar] [CrossRef]
- Misra, N.; Pu, X.; Holt, D.N.; McGuire, M.A.; Tinker, J.K. Immunoproteomics to identify Staphylococcus aureus antigens expressed in bovine milk during mastitis. J. Dairy Sci. 2018, 101, 6296–6309. [Google Scholar] [CrossRef] [PubMed]
- Keen, P.M.; Waterman, A.E.; Bourne, F.J. Oxygen concentration in milk of healthy and mastitic cows and implications of low oxygen tension for the killing of Staphylococcus aureus by bovine neutrophils. J. Dairy Res. 1988, 55, 513–519. [Google Scholar] [CrossRef]
- Müller, M.; Reiß, S.; Schlüter, R.; Mäder, U.; Beyer, A.; Reiß, W.; Marles-Wright, J.; Lewis, R.J.; Pförtner, H.; Völker, U. Deletion of membrane-associated Asp 23 leads to upregulation of cell wall stress genes in Staphylococcus aureus. Mol. Microbiol. 2014, 93, 1259–1268. [Google Scholar] [CrossRef]
- Hempel, K.; Pané-Farré, J.; Otto, A.; Sievers, S.; Hecker, M.; Becher, D. Quantitative cell surface proteome profiling for SigB-dependent protein expression in the human pathogen Staphylococcus aureus via biotinylation approach. J. Proteome Res. 2010, 9, 1579–1590. [Google Scholar] [CrossRef]
- Maass, S.; Sievers, S.; Zühlke, D.; Kuzinski, J.; Sappa, P.K.; Muntel, J.; Hessling, B.; Bernhardt, J.; Sietmann, R.; Volker, U. Efficient, global-scale quantification of absolute protein amounts by integration of targeted mass spectrometry and two-dimensional gel-based proteomics. Anal. Chem. 2011, 83, 2677–2684. [Google Scholar] [CrossRef]
- Müller, M. Charakterisierung des Alkalischen Schock Proteins Asp23 in Staphylococcus aureus. Ph.D. Thesis, Ernst-Moritz-Arndt-University, Greifswald, Germany, 13 November 2014. [Google Scholar]
- Hemmadi, V.; Biswas, M. An overview of moonlighting proteins in Staphylococcus aureus infection. Arch. Microbiol. 2021, 203, 481–498. [Google Scholar] [CrossRef]
- Cui, L.; Lian, J.-Q.; Neoh, H.; Reyes, E.; Hiramatsu, K. DNA microarray-based identification of genes associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 3404–3413. [Google Scholar] [CrossRef]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus toxins and their molecular activity in infectious diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Abril, A.G.; Villa, T.G.; Barros-Velázquez, J.; Cañas, B.; Sánchez-Pérez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus exotoxins and their detection in the dairy industry and mastitis. Toxins 2020, 12, 537. [Google Scholar] [CrossRef]
- Berube, B.J.; Wardenburg, J.B. Staphylococcus aureus α-toxin: Nearly a century of intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.M.; Sturdevant, D.E.; Herron-Olson, L.; Otto, M.; Smyth, D.S.; Villaruz, A.E.; Kapur, V.; Hartigan, P.J.; Smyth, C.J.; Fitzgerald, J.R. Pathogenomic analysis of the common bovine Staphylococcus aureus clone (ET3): Emergence of a virulent subtype with potential risk to public health. J. Infect. Dis. 2008, 197, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Pöllath, C.; Siegmund, A.; Deinhardt-Emmer, S.; Hoerr, V.; Svensson, C.-M.; Figge, T.M.; Monecke, S.; Löffler, B. Clinical S. aureus Isolates Vary in Their Virulence to Promote Adaptation to the Host. Toxins 2019, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Smeltzer, M.S. Staphylococcus aureus Pathogenesis: The Importance of Reduced Cytotoxicity. Trends Microbiol. 2016, 9, 24. [Google Scholar] [CrossRef]
- Bramley, A.J.; Patel, A.H.; O’Reilly, M.; Foster, R.; Foster, T.J. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect. Immun. 1989, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Müller, E.; Büchler, J.; Stieber, B.; Ehricht, R. Staphylococcus aureus In Vitro Secretion of Alpha Toxin (hla) Correlates with the Affiliation to Clonal Complexes. PLoS ONE 2014, 9, e100427. [Google Scholar] [CrossRef]
- Capra, E.; Cremonesi, P.; Pietrelli, A.; Puccio, S.; Luini, M.; Stella, A.; Castiglioni, B. Genomic and transcriptomic comparison between Staphylococcus aureus strains associated with high and low within herd prevalence of intra-mammary infection. BMC Microbiol. 2017, 17, 21. [Google Scholar] [CrossRef]
- Graber, H.U.; Naskova, J.; Studer, E.; Kaufmann, T.; Kirchhofer, M.; Brechbühl, M.; Schaeren, W.; Steiner, A.; Fournier, C. Mastitis-related subtypes of bovine Staphylococcus aureus are characterized by different clinical properties. J. Dairy Sci. 2009, 92, 1442–1451. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Bischoff, M.; Lattar, S.M.; Llana, M.N.; Pförtner, H.; Niemann, S.; Geraci, J.; Van de Vyver, H.; Fraunholz, M.J.; Cheung, A.L.; et al. Sigma Factor SigB is crucial to mediate Staphylococcus aureus adaptation during chronic infections. PLoS Pathog. 2015, 11, e1004870. [Google Scholar] [CrossRef]
- Sadykov, M.R.; Mattes, T.A.; Luong, T.T.; Zhu, Y.; Day, S.R.; Sifri, C.D.; Lee, C.Y.; Somerville, G.A. Tricarboxylic Acid Cycle-Dependent Synthesis of Staphylococcus aureus Type 5 and 8 Capsular Polysaccharides. J. Bacteriol. 2010, 192, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Batte, J.L.; Sahukhal, G.S.; Elasri, O. MsaB and CodY Interact To Regulate Staphylococcus aureus. J. Bacteriol. 2018, 1–20. [Google Scholar] [CrossRef]
- Lin, M.H.; Shu, J.C.; Lin, L.P.; Chong, K.Y.; Cheng, Y.W.; Du, J.F.; Liu, S.T. Elucidating the crucial role of poly N-acetylglucosamine from Staphylococcus aureus in cellular adhesion and pathogenesis. PLoS ONE 2015, 10, e0124216. [Google Scholar] [CrossRef] [PubMed]
- Balwit, J.M.; Langevelde, P.V.; Vann, J.M.; Proctor, R.A. Gentamicin-Resistant Menadione And Hemin Auxotrophic Staphylococcus aureus Persist Within Cultured Endothelial Cells. J. Infect. Dis. 1994, 170, 1033–1037. [Google Scholar] [CrossRef]
- Watts, A.; Ke, D.; Wang, Q.; Pillay, A.; Nicholson-Weller, A.; Lee, J.C. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect. Immun. 2005, 73, 3502–3511. [Google Scholar] [CrossRef]
- Cramton, S.E.; Gerke, C.; Schnell, N.F.; Nichols, W.W.; Götz, F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 1999, 67, 5427–5433. [Google Scholar] [CrossRef]
- Johler, S.; Stephan, R.; Althaus, D.; Ehling-Schulz, M.; Grunert, T. High-resolution subtyping of Staphylococcus aureus strains by means of Fourier-transform infrared spectroscopy. Syst. Appl. Microbiol. 2016, 39, 189–194. [Google Scholar] [CrossRef]
- Tollersrud, T.; Kenny, K.; Reitz, A.J.; Lee, J.C. Genetic and serologic evaluation of capsule production by bovine mammary isolates of Staphylococcus aureus and other Staphylococcus spp. from Europe and the United States. J. Clin. Microbiol. 2000, 38, 2998–3003. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.P.; Barbagelata, M.S.; Gordiola, M.; Cheung, A.L.; Sordelli, D.O.; Buzzola, F.R. Salicylic acid diminishes Staphylococcus aureus capsular polysaccharide type 5 expression. Infect. Immun. 2010, 78, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Kropec, A.; Maira-Litran, T.; Jefferson, K.K.; Grout, M.; Cramton, S.E.; Götz, F.; Goldmann, D.A.; Pier, G.B. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect. Immun. 2005, 73, 6868–6876. [Google Scholar] [CrossRef]
- Grunert, T.; Leitner, N.R.; Marchetti-Deschmann, M.; Miller, I.; Wallner, B.; Radwan, M.; Vogl, C.; Kolbe, T.; Kratky, D.; Gemeiner, M. A comparative proteome analysis links tyrosine kinase 2 (Tyk2) to the regulation of cellular glucose and lipid metabolism in response to poly(I:C). J. Proteom. 2011, 74, 2866–2880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huynh, H.T.; Robitaille, G.; Turner, J.D. Establishment of bovine mammary epithelial cells (MAC-T): An in vitro model for bovine lactation. Exp. Cell Res. 1991, 197, 191–199. [Google Scholar] [CrossRef]
- Hirschhausen, N.; Block, D.; Bianconi, I.; Bragonzi, A.; Birtel, J.; Lee, J.C.; Dübbers, A.; Küster, P.; Kahl, J.; Peters, G. Extended Staphylococcus aureus persistence in cystic fibrosis is associated with bacterial adaptation. Int. J. Med. Microbiol. 2013, 303, 685–692. [Google Scholar] [CrossRef]
- Dreisbach, A.; Hempel, K.; Buist, G.; Hecker, M.; Becher, D.; van Dijl, J.M. Profiling the surfacome of Staphylococcus aureus. Proteomics 2010, 10, 3082–3096. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Roschitzki, B.; Riedel, K.; Eberl, L. Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J. Proteome Res. 2012, 11, 4906–4915. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Mehlan, H.; Bernhardt, J.; Hennig, A.; Michalik, S.; Surmann, K.; Pané-Farré, J.; Giese, A.; Weiss, S.; Backert, L.; et al. AureoWiki—The repository of the Staphylococcus aureus research and annotation community. Int. J. Med. Microbiol. 2018, 308, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Boekhorst, J.; Francke, C.; Siezen, R.J. LocateP: Genome-scale subcellular-location predictor for bacterial proteins. BMC Bioinform. 2008, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, K.; Kucklick, M.; Marbach, H.; Ehling-Schulz, M.; Engelmann, S.; Grunert, T. Within-Host Adaptation of Staphylococcus aureus in a Bovine Mastitis Infection Is Associated with Increased Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 8840. https://doi.org/10.3390/ijms22168840
Mayer K, Kucklick M, Marbach H, Ehling-Schulz M, Engelmann S, Grunert T. Within-Host Adaptation of Staphylococcus aureus in a Bovine Mastitis Infection Is Associated with Increased Cytotoxicity. International Journal of Molecular Sciences. 2021; 22(16):8840. https://doi.org/10.3390/ijms22168840
Chicago/Turabian StyleMayer, Katharina, Martin Kucklick, Helene Marbach, Monika Ehling-Schulz, Susanne Engelmann, and Tom Grunert. 2021. "Within-Host Adaptation of Staphylococcus aureus in a Bovine Mastitis Infection Is Associated with Increased Cytotoxicity" International Journal of Molecular Sciences 22, no. 16: 8840. https://doi.org/10.3390/ijms22168840
APA StyleMayer, K., Kucklick, M., Marbach, H., Ehling-Schulz, M., Engelmann, S., & Grunert, T. (2021). Within-Host Adaptation of Staphylococcus aureus in a Bovine Mastitis Infection Is Associated with Increased Cytotoxicity. International Journal of Molecular Sciences, 22(16), 8840. https://doi.org/10.3390/ijms22168840






