CD40 Cross-Linking Induces Migration of Renal Tumor Cell through Nuclear Factor of Activated T Cells (NFAT) Activation
Abstract
:1. Introduction
2. Results
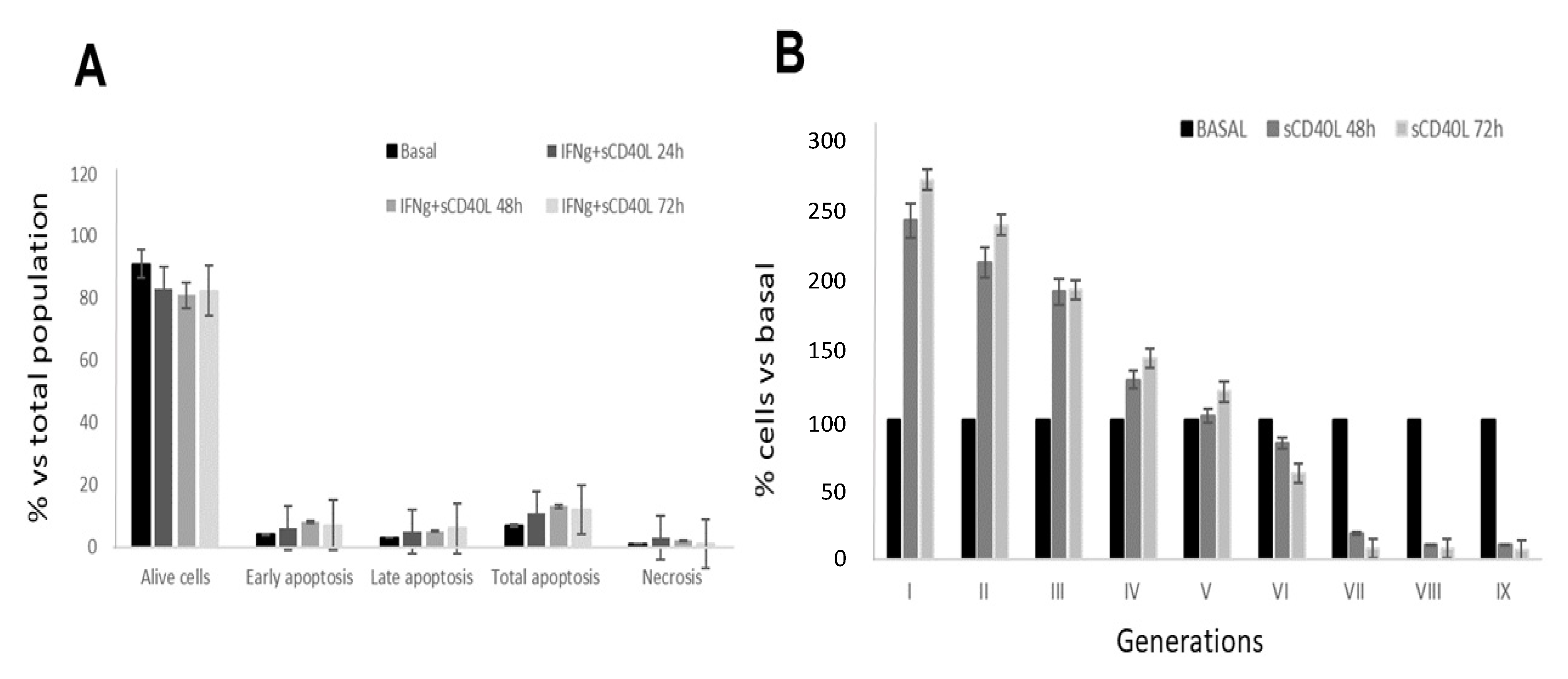
2.1. Effect of CD40 Ligation on Apoptosis and Cell Proliferation in RCC Cell Lines
2.2. CD40 Ligation Activates Different Signalling Pathways in RCC Cell Lines
2.3. RCC Cell Proliferation and Motility Induced by CD40 Ligation Depends on NFAT Activation
2.4. CD40 Ligation Promoted RCC Proliferation Acting on Cytoskeleton Organization
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Patients and Tissue Immunofluorescence
4.3. Cell Isolation and Short Term Cultures of Human Tumors
4.4. Flow Cytometry and Apoptosis Assay
4.5. CFDA-SE (5[6]-Carboxyfluorescein Diacetate Succinimidyl Ester) Cell Proliferation Assay
4.6. MTT Assay
4.7. Migration Assay
4.8. Western Blot Analysis
4.9. Transient Transfections and Luciferase Assay
4.10. Real Time PCR
4.11. Confocal Analysis
4.12. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawai, K.; Saijo, K.; Oikawa, T.; Ohno, T.; Akaza, H. Enhancement of T cell proliferative response against autologous cancer cells of a metastatic renal cell carcinoma patient after unexplained regression. Int. J. Urol. 2004, 11, 1130–1132. [Google Scholar] [CrossRef]
- Gigante, M.; Pontrelli, P.; Herr, W.; Gigante, M.; D’Avenia, M.; Zaza, G.; Cavalcanti, E.; Accetturo, M.; Lucarelli, G.; Carrieri, G.; et al. miR-29b and miR-198 overexpression in CD8+ T cells of renal cell carcinoma patients down-modulates JAK3 and MCL-1 leading to immune dysfunction. J. Transl. Med. 2016, 14, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussolati, B.; Russo, S.; Deambrosis, I.; Cantaluppi, V.; Volpe, A.; Ferrando, U.; Camussi, G. Expression of CD154 on renal cell carcinomas and effect on cell proliferation, motility and platelet-activating factor synthesis. Int. J. Cancer 2002, 100, 654–661. [Google Scholar] [CrossRef]
- Eliopoulos, A.G.; Young, L.S. The role of the CD40 pathway in the pathogenesis and treatment of cancer. Cur. Opin. Pharmacol. 2004, 4, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.T.; Podar, K.; Mitsiades, N.; Tai, Y.T.; Podar, K.; Mitsiades, N.; Lin, B.; Mitsiades, C.; Gupta, D.; Akiyama, M.; et al. CD40 induces human multiple myeloma cell migration via phosphatidylinositol 3-Kinase/Akt/NF-kB signaling. Blood 2003, 101, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Hiraoka, K.; Ishikawa, K.; Shoji, Y.; Noji, T.; Hontani, K.; Itoh, T.; Nakamura, T.; Tsuchikawa, T.; Shichinohe, T.; et al. CD40 Expression in Human Esophageal Squamous Cell Carcinoma Is Associated with Tumor Progression and Lymph Node Metastasis. Anticancer Res. 2016, 36, 4467–4475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prattichizzo, C.; Gigante, M.; Pontrelli, P.; Stella, A.; Rocchetti, M.T.; Gigante, M.; Maiorano, E.; Herr, W.; Battaglia, M.; Gesualdo, L.; et al. Establishment and characterization of a highly immunogenic human renal carcinoma cell line. Int. J. Oncol. 2016, 49, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Ibraheem, K.; Yhmed, A.M.A.; Qayyum, T.; Bryan, N.P.; Georgopoulos, N.T. CD40 induces renal cell carcinoma-specific differential regulation of TRAF proteins, ASK1 activation and JNK/p38-mediated, ROS-dependent mitochondrial apoptosis. Cell Death Discov. 2019, 5, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukocyte Biol. 2000, 67, 2–17. [Google Scholar] [CrossRef]
- Baxendale, A.J.; Dawson, C.W.; Stewart, S.E.; Mudaliar, V.; Reynolds, G.; Gordon, J.; Murray, P.G.; Young, L.S.; Eliopoulos, A.G. Constitutive activation of the CD40 pathway promotes cell transformation and neoplastic growth. Oncogene 2005, 24, 7913–7923. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Canel, M.; Serrels, A.; Frame, M.C.; Brunton, V.G. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J. Cell Sci. 2013, 126, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, Y.K.; Yu, J.; Shimoda, M.; Takada, Y. Integrin Binding to the Trimeric Interface of CD40L Plays a Critical Role in CD40/CD40L Signaling. J. Immunol. 2019, 203, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Engelman Mde, F.; Grande, R.M.; Naves, M.A.; de Franco, M.F.; de Paulo Castro Teixeira, V. Integrin-linked kinase (ILK) expression correlates with tumor severity in clear cell renal carcinoma. Pathol. Oncol. Res. 2013, 19, 27–33. [Google Scholar] [CrossRef]
- Liu, Y.; Shang, D. Transforming growth factor-β1 enhances proliferative and metastatic potential by up-regulating lymphoid enhancer-binding factor 1/integrin αMβ2 in human renal cell carcinoma. Mol. Cell Biochem. 2020, 465, 165–174. [Google Scholar] [CrossRef]
- Brenner, W.; Greber, I.; Gudejko-Thiel, J.; Brenner, W.; Greber, I.; Gudejko-Thiel, J.; Beitz, S.; Schneider, E.; Walenta, S.; Peters, K.; et al. Migration of renal carcinoma cells is dependent on protein kinase C delta via beta1 integrin and focal adhesion kinase. Int. J. Oncol. 2008, 32, 1125–1131. [Google Scholar]
- Mancini, M.; Toker, A. NFAT proteins: Emerging roles in cancer progression. Nat. Rev. Cancer 2009, 9, 810–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quang, C.T.; Leboucher, S.; Passaro, D.; Fuhrmann, L.; Nourieh, M.; Vincent-Salomon, A.; Ghysdael, J. The calcineurin/NFAT pathway is activated in diagnostic breast cancer cases and is essential to survival and metastasis of mammary cancer cells. Cell Death Dis. 2015, 6, e1658. [Google Scholar] [CrossRef] [PubMed]
- Jauliac, S.; López-Rodriguez, C.; Shaw, L.M.; Brown, L.F.; Rao, A.; Toker, A. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat. Cell Biol. 2002, 4, 540–544. [Google Scholar] [CrossRef]
- Pontrelli, P.; Ursi, M.; Ranieri, E.; Capobianco, C.; Schena, F.P.; Gesualdo, L.; Grandaliano, G. CD40L proinflammatory and profibrotic effects on proximal tubular epithelial cells: Role of NF B and lyn. J. Am. Soc. Nephrol. 2006, 17, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, S.; Redondo, J.M. Inhibitors of the calcineurin/NFAT pathway. Curr. Med. Chem. 2004, 11, 997–1007. [Google Scholar] [CrossRef]
- Lee, J.U.; Kim, L.K.; Choi, J.M. Revisiting the Concept of Targeting NFAT to Control T Cell Immunity and Autoimmune Diseases. Front. Immunol. 2018, 9, 2747. [Google Scholar] [CrossRef] [Green Version]
- LaVallee, T.M.; Prudovsky, I.A.; McMahon, G.A.; Hu, X.; Maciag, T. Activation of the MAP kinase pathway by FGF-1 correlates with cell proliferation induction, while activation of the Src pathway correlates with migration. J. Cell Biol. 1998, 141, 1647–1658. [Google Scholar] [CrossRef]
- Hillebrand, R.M.; Vogt, A.; Strassburg, C.P.; Gonzalez-Carmona, M.A.; Schmidt-Wolf IGH. Immune Check Point CD40-CD40L Activates Dendritic and Effector Cells Against Human Renal Carcinoma Cells. Anticancer Res. 2019, 39, 4643–4652. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Gregory Alvord, W.; Quiñones, O.A.; Stauffer, J.K.; Wiltrout, R.H. CD40 expression in renal cell carcinoma is associated with tumor apoptosis, CD8(+) T cell frequency and patient survival. Hum Immunol. 2014, 75, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, M.; Campbell, R.A.; Burkhardt, K.; Zhu, D.; Li, S.G.; Lee, H.J.; Wang, C.; Zeng, Z.; Gordon, M.S.; et al. Interference with nuclear factor kappa B and c-Jun NH2-terminal kinase signaling by TRAF6C small interfering RNA inhibits myeloma cell proliferation and enhances apoptosis. Oncogene 2006, 25, 6520–6527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Hwang, K.H.; Eom, M.; Kim, M.; Park, E.Y.; Jeong, Y.; Park, K.S.; Cha, S.K. WNK1 Promotes Renal Tumor Progression by Activating TRPC6-NFAT Pathway. FASEB J. 2019, 33, 8588–8599. [Google Scholar] [CrossRef]
- Küper, C.; Beck, F.X.; Neuhofer, W. NFAT5-mediated expression of S100A4 contributes to proliferation and migration of renal carcinoma cells. Front. Physiol. 2014, 5, 293. [Google Scholar] [CrossRef] [Green Version]
- Takashi Kawahara, T.; Kashiwagi, E.; Ide, H.; Li, Y.; Zheng, Y.; Ishiguro, H.; Miyamoto, H. The Role of NFATc1 in Prostate Cancer Progression: Cyclosporine A and Tacrolimus Inhibit Cell Proliferation, Migration, and Invasion. Prostate 2015, 75, 573–584. [Google Scholar] [CrossRef]
- Breuksch, I.; Prosinger, F.; Baehr, F.; Engelhardt, F.P.; Bauer, H.K.; Thüroff, J.W.; Heimes, A.S.; Hasenburg, A.; Prawitt, D.; Brenner, W. Integrin α5 triggers the metastatic potential in renal cell carcinoma. Oncotarget 2017, 8, 107530–107542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argentiero, A.; Solimando, A.G.; Krebs, M.; Leone, P.; Susca, N.; Brunetti, O.; Racanelli, V.; Vacca, A.; Silvestris, N. Anti-angiogenesis and Immunotherapy: Novel Paradigms to Envision Tailored Approaches in Renal Cell-Carcinoma. J. Clin. Med. 2020, 604, 1594. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.A.; Djureinovic, D.; Jessel, S.; Krykbaeva, I.; Zhang, L.; Jilaveanu, L.; Ralabate, A.; Johnson, B.; Levit, N.S.; Anderson, G.; et al. A phase I study of APX005M and cabiralizumab with/without nivolumab in patients with melanoma, kidney cancer or non-small cell lung cancer resistant to anti-PD-(L)1. Clin. Cancer Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Pandolfi, P.P. Giving blood: A new role for CD40 in tumorigenesis. J. Exp. Med. 2006, 203, 2409–2412. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Cappiello, V.; Fiore, N.; Pontrelli, P.; Gesualdo, L.; Schena, F.P.; Montinaro, V. CD40 ligand increases complement C3 secretion by proximal tubular epithelial cells. J. Am. Soc. Nephrol. 2005, 16, 2003–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvai, A.E.; Xu, L.; Korzus, E.; Brard, G.; Kalafus, D.; Mullen, T.M.; Rose, D.W.; Rosenfeld, M.G.; Glass, C.K. Nuclear integration of JAK/STAT and Ras/AP-1 signaling by CBP and p300. Proc. Natl. Acad. Sci. USA 1997, 94, 1074–1079. [Google Scholar] [CrossRef] [Green Version]
- Yano, O.; Kanellopoulos, J.; Kieran, M.; Le Bail, O.; Israël, A.; Kourilsky, P. Purification of KBF1, a common factor binding to both H-2 and beta 2-microglobulin enhancer. EMBO J. 1987, 6, 3317–3324. [Google Scholar] [CrossRef]
- Klatte, T.; Seligson, D.B.; Riggs, S.B.; Leppert, J.T.; Berkman, M.K.; Kleid, M.D.; Yu, H.; Kabbinavar, F.F.; Pantuck, A.J.; Belldegrun, A.S. Hypoxia-inducible factor 1 alpha in clear cell renal cell carcinoma. Clin. Cancer Res. 2007, 13, 7388–7393. [Google Scholar] [CrossRef] [Green Version]




| Cell Line | Basal |
|---|---|
| Name | % of Expression |
| PRCC4 | 3.8 |
| PRCC5 | 0.77 |
| PRCC6 | 2.2 |
| PRCC7 | 48.56 |
| PRCC11 | 64.47 |
| PRCC18 | 99.78 |
| PRCC20 | 19.15 |
| PRCC21 | 99.02 |
| RCC1851 | 49.03 |
| Mean ± SD | 42.97 ± 39.43 |
| Patient | Age/Sex | Stage | Grade | Adjuvant Treatment | Last Evaluation | Follow-Up (Months) |
|---|---|---|---|---|---|---|
| 1 | 63 M | T1N0M0 | 2 | / | NED | 55 |
| 2 | 55 M | T1N0M0 | 1 | / | NED | 67 |
| 3 | 74 M | T1bN0M0 | 2 | / | NED | 65 |
| 4 | 46 F | T1aN0M0 | 2 | / | NED | 52 |
| 5 | 76 F | T1N0M0 | 3 | / | NED | 39 |
| 6 | 53 F | T1bN0M0 | 3 | / | NED | 53 |
| 7 | 66 M | T1N0M0 | 3 | / | Mets, liver-chest | 32 |
| 8 | 45 M | T2N0M0 | 2 | TKI, RT, S | Mets, bone | 22 |
| 9 | 78 F | T3bN1M0 | 3 | / | Mets, lung-spleen | 62 |
| 10 | 53 M | T3aN0M0 | 3 | S, TKI | Mets, lung-spleen | 57 |
| 11 | 69 M | T3aN0M0 | 3 | S, TKI | Mets, lung-spleen | 41 |
| 12 | 62 M | T3aN0M0 | 1 | RT, S | Mets, bone | 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontrelli, P.; Gigante, M.; Spadaccino, F.; Netti, G.S.; Saldarelli, M.; Balducci, L.; Gigante, M.; Battaglia, M.; Storkus, W.J.; Castellano, G.; et al. CD40 Cross-Linking Induces Migration of Renal Tumor Cell through Nuclear Factor of Activated T Cells (NFAT) Activation. Int. J. Mol. Sci. 2021, 22, 8871. https://doi.org/10.3390/ijms22168871
Pontrelli P, Gigante M, Spadaccino F, Netti GS, Saldarelli M, Balducci L, Gigante M, Battaglia M, Storkus WJ, Castellano G, et al. CD40 Cross-Linking Induces Migration of Renal Tumor Cell through Nuclear Factor of Activated T Cells (NFAT) Activation. International Journal of Molecular Sciences. 2021; 22(16):8871. https://doi.org/10.3390/ijms22168871
Chicago/Turabian StylePontrelli, Paola, Margherita Gigante, Federica Spadaccino, Giuseppe Stefano Netti, Marilisa Saldarelli, Luigi Balducci, Maddalena Gigante, Michele Battaglia, Walter J. Storkus, Giuseppe Castellano, and et al. 2021. "CD40 Cross-Linking Induces Migration of Renal Tumor Cell through Nuclear Factor of Activated T Cells (NFAT) Activation" International Journal of Molecular Sciences 22, no. 16: 8871. https://doi.org/10.3390/ijms22168871







