PPAR Gamma and Viral Infections of the Brain
Abstract
:1. Introduction
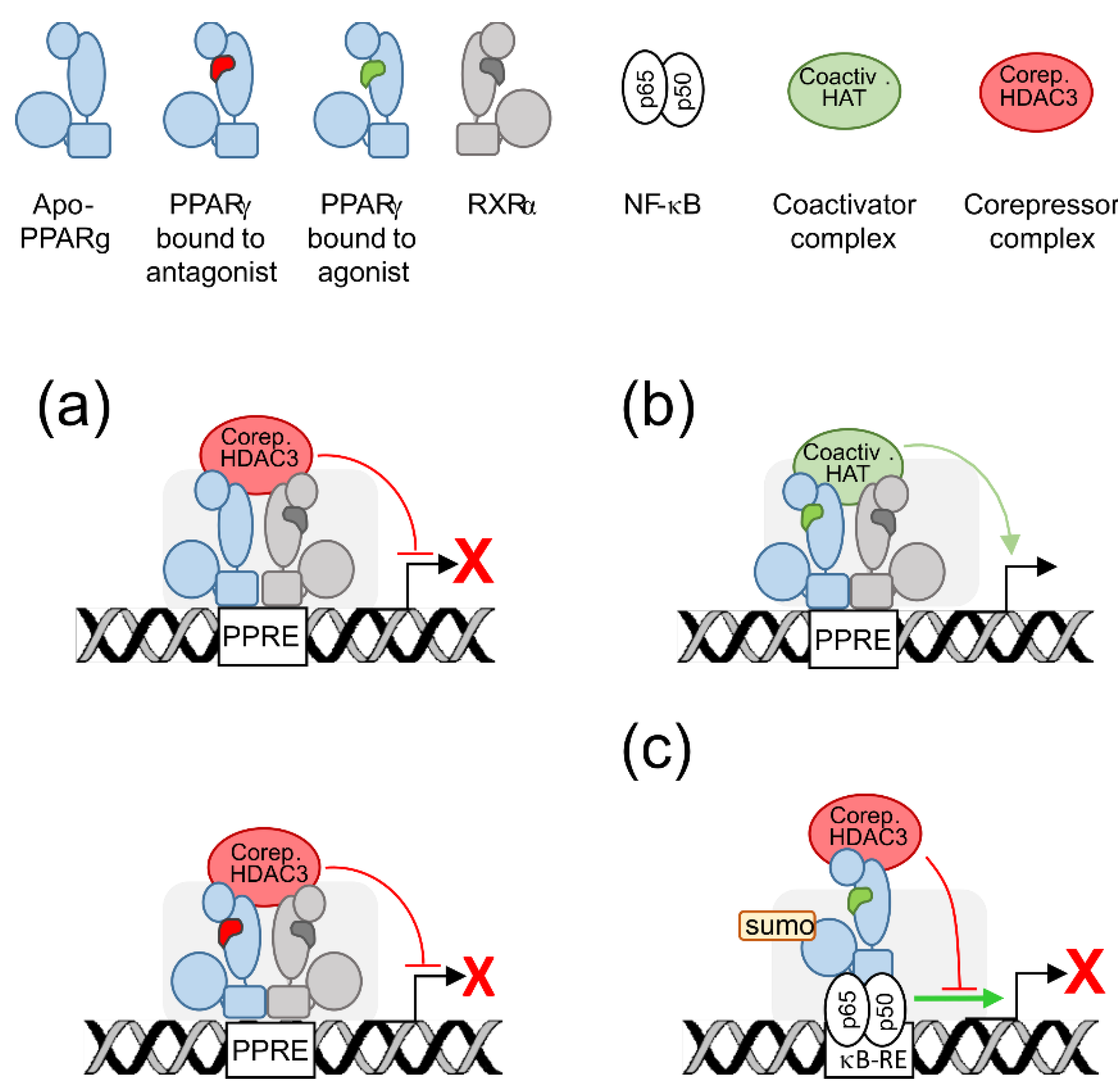
2. PPARγ Molecular Levers
3. PPARγ Expression in the Brain
4. PPARγ Responds to Specific Issues of the Brain Cell
4.1. Energy Supply, Oxidative Stress, and Mitochondria
4.2. Neuroinflammation
4.3. Neurogenesis
5. PPARγ in the Infected Adult or Developing Brain
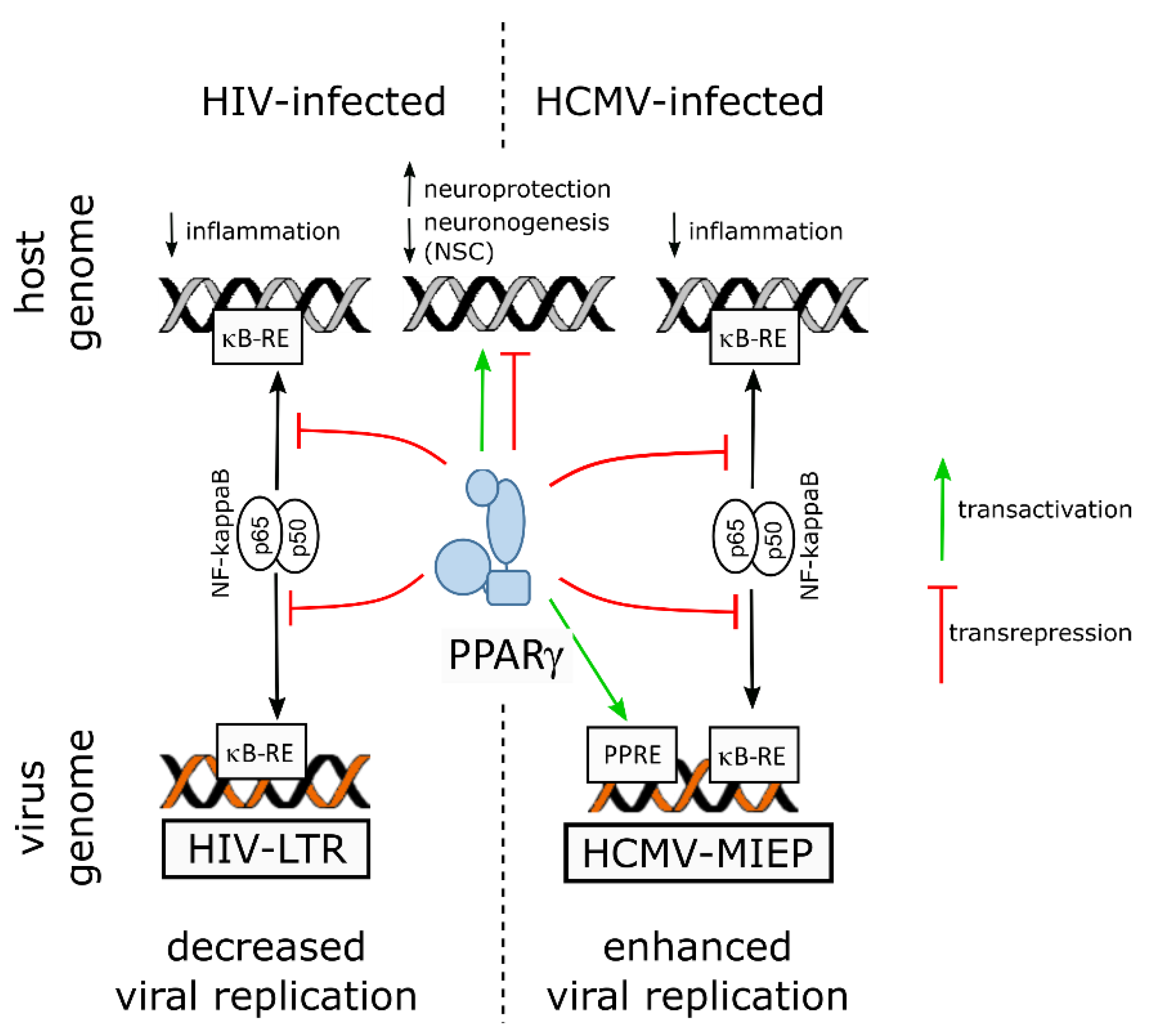
5.1. PPARγ, the Adult Brain and Human Immunodeficiency Virus 1
5.2. PPARγ, the Developing Brain and Zika Virus
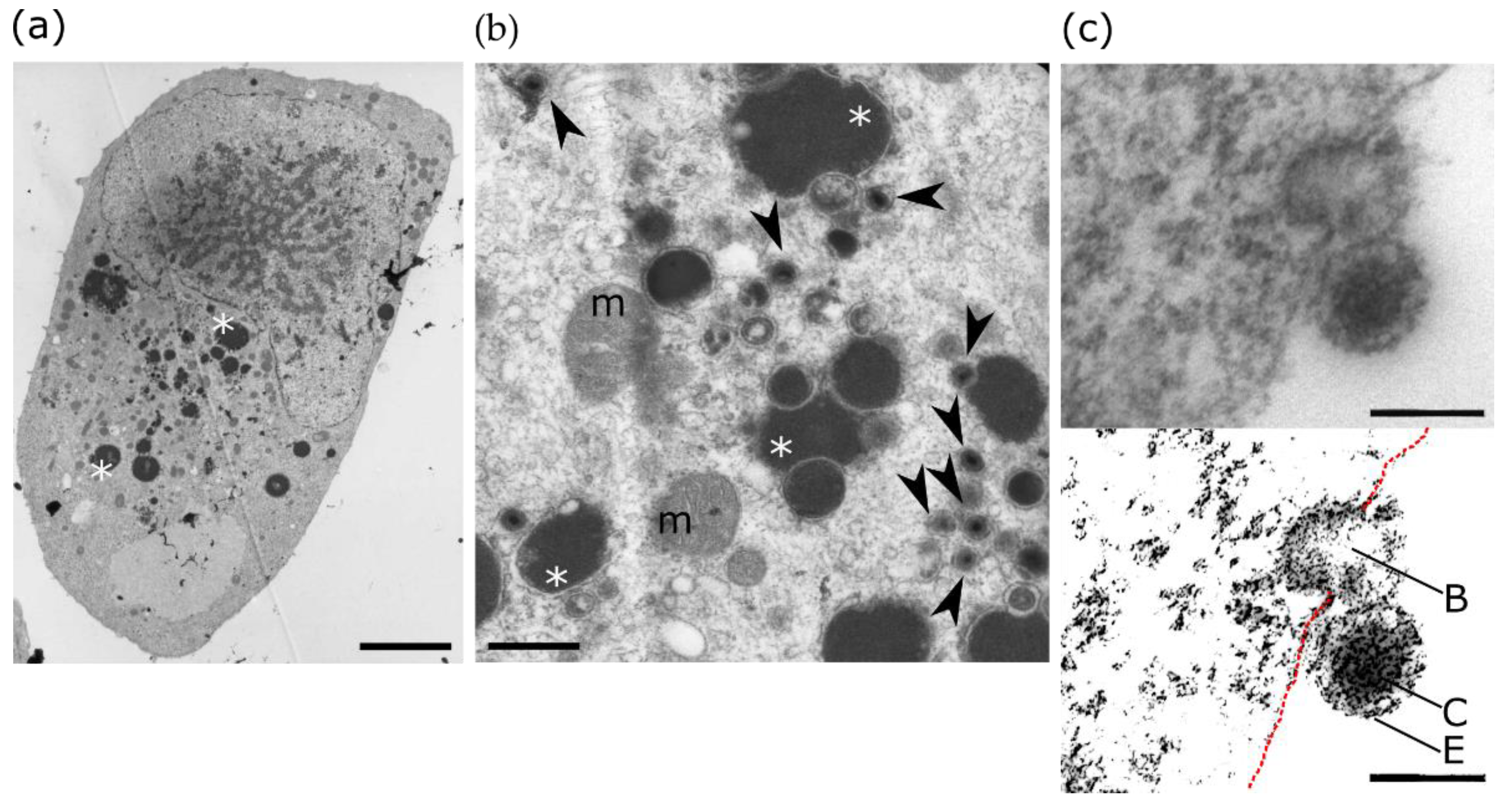
5.3. PPARγ, the Developing Brain and Human Cytomegalovirus
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Alvares, K.; Huang, Q.; Rao, M.S.; Reddy, J.K. Cloning of a New Member of the Peroxisome Proliferator-Activated Receptor Gene Family from Mouse Liver. J. Biol. Chem. 1993, 268, 26817–26820. [Google Scholar] [CrossRef]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef]
- Braissant, O.; Wahli, W. Differential Expression of Peroxisome Proliferator-Activated Receptor-α, -Î2, and -Î3 during Rat Embryonic Development. Endocrinology 1998, 139, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Nakajima, A.; Katayama, K.; Kudo, C.; Shibuya, A.; Kubota, N.; Terauchi, Y.; Tachibana, M.; Miyoshi, H.; Kamisaki, Y.; et al. Peroxisome Proliferator-Activated Receptor Gamma-Mediated Regulation of Neural Stem Cell Proliferation and Differentiation. J. Biol. Chem. 2006, 281, 12673–12681. [Google Scholar] [CrossRef] [Green Version]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Cai, W.; Yang, T.; Liu, H.; Han, L.; Zhang, K.; Hu, X.; Zhang, X.; Yin, K.J.; Gao, Y.; Bennett, M.V.L.; et al. Peroxisome Proliferator-Activated Receptor γ (PPARγ): A Master Gatekeeper in CNS Injury and Repair. Prog. Neurobiol. 2018, 163-164, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C.; Duchen, M.R. PPARγ as a Therapeutic Target to Rescue Mitochondrial Function in Neurological Disease. Free Radic. Biol. Med. 2016, 100, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Tufano, M.; Pinna, G. Is There a Future for PPARs in the Treatment of Neuropsychiatric Disorders? Molecules 2020, 25, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Lee, J.Y.H.; Tan, N.S. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int. J. Mol. Sci. 2019, 20, 5055. [Google Scholar] [CrossRef] [Green Version]
- Soung, A.; Klein, R.S. Viral Encephalitis and Neurologic Diseases: Focus on Astrocytes. Trends Mol. Med. 2018, 24, 950–962. [Google Scholar] [CrossRef]
- Tyler, K.L. Acute Viral Encephalitis. N. Engl. J. Med. 2018, 379, 557–566. [Google Scholar] [CrossRef]
- Cordeiro, C.N.; Tsimis, M.; Burd, I. Infections and Brain Development. Obstet. Gynecol. Surv. 2015, 70, 644–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiscott, J.; Kwon, H.; Génin, P. Hostile Takeovers: Viral Appropriation of the NF-ΚB Pathway. J. Clin. Investig. 2001, 107, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Weikum, E.R.; Liu, X.; Ortlund, E.A. The Nuclear Receptor Superfamily: A Structural Perspective. Protein Sci. 2018, 27, 1876–1892. [Google Scholar] [CrossRef]
- Shang, J.; Mosure, S.A.; Zheng, J.; Brust, R.; Bass, J.; Nichols, A.; Solt, L.A.; Griffin, P.R.; Kojetin, D.J. A Molecular Switch Regulating Transcriptional Repression and Activation of PPARγ. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Graves, R.A.; Budavari, A.I.; Erdjument-bromage, H.; Lui, M.; Hu, E.; Tempst, P.; Spiegelman, B.M. Adipocyte-Specific Transcription Factor ARF6 Is a Heterodimeric Complex of Two Nuclear Hormone Receptors, PPAR7 and RXRa. Nucleic Acids Res. 1994, 22, 5628–5634. [Google Scholar] [CrossRef] [Green Version]
- Lemay, D.G.; Hwang, D.H. Genome-Wide Identification of Peroxisome Proliferator Response Elements Using Integrated Computational Genomics. J. Lipid Res. 2006, 47, 1583–1587. [Google Scholar] [CrossRef] [Green Version]
- Wahli, W. A Gut Feeling of the PXR, PPAR and NF-kappaB Connection. J. Int. Med. 2008, 263, 613–619. [Google Scholar] [CrossRef]
- Pascual, G.; Fong, A.L.; Ogawa, S.; Gamliel, A.; Li, A.C.; Perissi, V.; Rose, D.W.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. A SUMOylation-Dependent Pathway Mediates Transrepression of Inflammatory Response Genes by PPAR-γ. Nature 2005, 437, 759–763. [Google Scholar] [CrossRef]
- Brunmeir, R.; Xu, F. Functional Regulation of PPARs through Post-Translational Modifications. Int. J. Mol. Sci. 2018, 19, 1738. [Google Scholar] [CrossRef] [Green Version]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty Acids, Eicosanoids and PPAR Gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.; Minghetti, L. PPAR-Gamma Agonists as Regulators of Microglial Activation and Brain Inflammation. Curr. Pharm. Des. 2007, 12, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Prashantha Kumar, B.R.; Kumar, A.P.; Jose, J.A.; Prabitha, P.; Yuvaraj, S.; Chipurupalli, S.; Jeyarani, V.; Manisha, C.; Banerjee, S.; Jeyabalan, J.B.; et al. Minutes of PPAR-γ Agonism and Neuroprotection. Neurochem. Int. 2020, 140, 104814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Hu, J.-P.; Yu, S.; Li, B.-K.; Cui, Y.; Ren, L.; Zhang, L.-D. Astragaloside IV, a Natural PPARγ Agonist, Reduces Aβ Production in Alzheimer’s Disease Through Inhibition of BACE1. Mol. Neurobiol. 2017, 54, 2939–2949. [Google Scholar] [CrossRef]
- Shi, L.; Lin, Q.; Li, X.; Nie, Y.; Sun, S.; Deng, X.; Wang, L.; Lu, J.; Tang, Y.; Luo, F. Alliin, a Garlic Organosulfur Compound, Ameliorates Gut Inflammation through MAPK-NF-ΚB/AP-1/STAT-1 Inactivation and PPAR-γ Activation. Mol. Nutr. Food Res. 2017, 61, 1601013. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.-N. Effects of Cannabidiol Interactions with Wnt/β-Catenin Pathway and PPARγ on Oxidative Stress and Neuroinflammation in Alzheimer’s Disease. Acta Biochim. Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makled, M.N.; Sharawy, M.H.; El-Awady, M.S. The Dual PPAR-α/γ Agonist Saroglitazar Ameliorates Thioacetamide-Induced Liver Fibrosis in Rats through Regulating Leptin. Naunyn. Schmiedebergs Arch. Pharmacol. 2019, 392, 1569–1576. [Google Scholar] [CrossRef]
- Boubia, B.; Poupardin, O.; Barth, M.; Binet, J.; Peralba, P.; Mounier, L.; Jacquier, E.; Gauthier, E.; Lepais, V.; Chatar, M.; et al. Design, Synthesis, and Evaluation of a Novel Series of Indole Sulfonamide Peroxisome Proliferator Activated Receptor (PPAR) α/γ/δ Triple Activators: Discovery of Lanifibranor, a New Antifibrotic Clinical Candidate. J. Med. Chem. 2018, 61, 2246–2265. [Google Scholar] [CrossRef]
- Bernardo, A.; Levi, G.; Minghetti, L. Role of the Peroxisome Proliferator-Activated Receptor-γ (PPAR-γ) and Its Natural Ligand 15-Deoxy-Δ(12,14)-Prostaglandin J2 in the Regulation of Microglial Functions. Eur. J. Neurosci. 2000, 12, 2215–2223. [Google Scholar] [CrossRef]
- Flores, J.J.; Klebe, D.; Rolland, W.B.; Lekic, T.; Krafft, P.R.; Zhang, J.H. PPARγ-Induced Upregulation of CD36 Enhances Hematoma Resolution and Attenuates Long-Term Neurological Deficits after Germinal Matrix Hemorrhage in Neonatal Rats. Neurobiol. Dis. 2016, 87, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Rolland, M.; Li, X.; Sellier, Y.; Martin, H.; Perez-Berezo, T.; Rauwel, B.; Benchoua, A.; Bessières, B.; Aziza, J.; Cenac, N.; et al. PPARγ Is Activated during Congenital Cytomegalovirus Infection and Inhibits Neuronogenesis from Human Neural Stem Cells. PLoS Pathog. 2016, 12, e1005547. [Google Scholar] [CrossRef] [Green Version]
- Hampel, J.K.; Brownrigg, L.M.; Vignarajah, D.; Croft, K.D.; Dharmarajan, A.M.; Bentel, J.M.; Puddey, I.B.; Yeap, B.B. Differential Modulation of Cell Cycle, Apoptosis and PPARgamma2 Gene Expression by PPARgamma Agonists Ciglitazone and 9-Hydroxyoctadecadienoic Acid in Monocytic Cells. Prostaglandins Leukot Essent. Fat. Acids 2006, 74, 283–293. [Google Scholar] [CrossRef]
- Negishi, M.; Shimizu, H.; Okada, S.; Kuwabara, A.; Okajima, F.; Mori, M. 9HODE Stimulates Cell Proliferation and Extracellular Matrix Synthesis in Human Mesangial Cells via PPARgamma. Exp. Biol. Med. 2004, 229, 1053–1060. [Google Scholar] [CrossRef]
- Brust, R.; Shang, J.; Fuhrmann, J.; Mosure, S.A.; Bass, J.; Cano, A.; Heidari, Z.; Chrisman, I.M.; Nemetchek, M.D.; Blayo, A.L.; et al. A Structural Mechanism for Directing Corepressor-Selective Inverse Agonism of PPARγ. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Toyota, Y.; Nomura, S.; Makishima, M.; Hashimoto, Y.; Ishikawa, M. Structure-Activity Relationships of Rosiglitazone for Peroxisome Proliferator-Activated Receptor Gamma Transrepression. Bioorgan. Med. Chem. Lett. 2017, 27, 2776–2780. [Google Scholar] [CrossRef]
- Moreno, S.; Farioli-Vecchioli, S.; CerÃ1, M.P. Immunolocalization of Peroxisome Proliferator-Activated Receptors and Retinoid x Receptors in the Adult Rat CNS. Neuroscience 2004, 123, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Warden, A.; Truitt, J.; Merriman, M.; Ponomareva, O.; Jameson, K.; Ferguson, L.B.; Mayfield, R.D.; Harris, R.A. Localization of PPAR Isotypes in the Adult Mouse and Human Brain. Sci. Rep. 2016, 6, 27618. [Google Scholar] [CrossRef]
- Gofflot, F.; Chartoire, N.; Vasseur, L.; Heikkinen, S.; Dembele, D.; Le Merrer, J.; Auwerx, J. Systematic Gene Expression Mapping Clusters Nuclear Receptors According to Their Function in the Brain. Cell 2007, 131, 405–418. [Google Scholar] [CrossRef]
- Kim, Y.; Vadodaria, K.C.; Lenkei, Z.; Kato, T.; Gage, F.H.; Marchetto, M.C.; Santos, R. Mitochondria, Metabolism, and Redox Mechanisms in Psychiatric Disorders. Antioxid. Redox Signal. 2019, 31, 275–317. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terman, A.; Kurz, T.; Navratil, M.; Arriaga, E.A.; Brunk, U.T. Mitochondrial Turnover and Aging of Long-Lived Postmitotic Cells: The Mitochondrial-Lysosomal Axis Theory of Aging. Antioxid. Redox Signal. 2010, 12, 503–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, M.C.; Nicol, C.J.; Cheng, Y.C.; Lin, K.H.; Yen, C.H.; Lin, C.H. Rosiglitazone Activation of PPARγ-Dependent Pathways Is Neuroprotective in Human Neural Stem Cells against Amyloid-Beta-Induced Mitochondrial Dysfunction and Oxidative Stress. Neurobiol. Aging 2016, 40, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Cheng, Y.C.; Lin, K.H.; Yen, C.H. PPARγ Regulates the Mitochondrial Dysfunction in Human Neural Stem Cells with Tumor Necrosis Factor Alpha. Neuroscience 2013, 229, 118–129. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [Green Version]
- Scarpulla, R.C. Metabolic Control of Mitochondrial Biogenesis through the PGC-1 Family Regulatory Network. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, I.; Chu, C.T.; Kaufman, B.A. The Mitochondrial Transcription Factor TFAM in Neurodegeneration: Emerging Evidence and Mechanisms. FEBS Lett. 2018, 592, 793–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, T.C. Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2) and Mitochondrial Dynamics/Mitophagy in Neurological Diseases. Antioxidants 2020, 9, 617. [Google Scholar] [CrossRef]
- Baghcheghi, Y.; Salmani, H.; Beheshti, F.; Shafei, M.N.; Sadeghnia, H.R.; Soukhtanloo, M.; Ebrahimzadeh Bideskan, A.; Hosseini, M. Effects of PPAR-γ Agonist, Pioglitazone on Brain Tissues Oxidative Damage and Learning and Memory Impairment in Juvenile Hypothyroid Rats. Int. J. Neurosci. 2019, 129, 1024–1038. [Google Scholar] [CrossRef] [PubMed]
- De Nuccio, C.; Bernardo, A.; Troiano, C.; Brignone, M.S.; Falchi, M.; Greco, A.; Rosini, M.; Basagni, F.; Lanni, C.; Serafini, M.M.; et al. Nrf2 and Ppar-γ Pathways in Oligodendrocyte Progenitors: Focus on Ros Protection, Mitochondrial Biogenesis and Promotion of Cell Differentiation. Int. J. Mol. Sci. 2020, 21, 7216. [Google Scholar] [CrossRef]
- Chang, J.S.; Ha, K. A Truncated PPAR Gamma 2 Localizes to Mitochondria and Regulates Mitochondrial Respiration in Brown Adipocytes. PLoS ONE 2018, 13, e0195007. [Google Scholar] [CrossRef]
- Jia, P.; Wu, X.; Pan, T.; Xu, S.; Hu, J.; Ding, X. Uncoupling Protein 1 Inhibits Mitochondrial Reactive Oxygen Species Generation and Alleviates Acute Kidney Injury. EBioMedicine 2019, 49, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Soliman, E.; Behairy, S.F.; El-maraghy, N.N.; Elshazly, S.M. PPAR-γ Agonist, Pioglitazone, Reduced Oxidative and Endoplasmic Reticulum Stress Associated with L-NAME-Induced Hypertension in Rats. Life Sci. 2019, 239, 117047. [Google Scholar] [CrossRef]
- Liu, Y.D.; Yu, S.L.; Wang, R.; Liu, J.N.; Jin, Y.S.; Li, Y.F.; An, R.H. Rosiglitazone Suppresses Calcium Oxalate Crystal Binding and Oxalate-Induced Oxidative Stress in Renal Epithelial Cells by Promoting PPAR- γ Activation and Subsequent Regulation of TGF- β 1 and HGF Expression. Oxid. Med. Cell. Longev. 2019, 2019, 4826525. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bi, X.; Zhang, Y.; Wang, Y.; Ding, W. Mitochondrial Dysfunction/NLRP3 Inflammasome Axis Contributes to Angiotensin II-Induced Skeletal Muscle Wasting via PPAR-γ. Lab. Investig. 2020, 100, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Q.; Zhou, J.-W. Neuroinflammation in the Central Nervous System: Symphony of Glial Cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Jiang, P.; Jiang, Q.; Yan, Y.; Hou, Z.; Luo, D. Propofol Ameliorates Neuropathic Pain and Neuroinflammation through PPAR γ Up-Regulation to Block Wnt/β-Catenin Pathway. Neurol. Res. 2021, 43, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Vallée, J.N.; Guillevin, R.; Lecarpentier, Y. Interactions Between the Canonical WNT/Beta-Catenin Pathway and PPAR Gamma on Neuroinflammation, Demyelination, and Remyelination in Multiple Sclerosis. Cell. Mol. Neurobiol. 2018, 38, 783–795. [Google Scholar] [CrossRef]
- Zhou, D.; Ji, L.; Chen, Y. TSPO Modulates IL-4-Induced Microglia/Macrophage M2 Polarization via PPAR-γ Pathway. J. Mol. Neurosci. 2020, 70, 542–549. [Google Scholar] [CrossRef]
- Abd El Fattah, M.A.; Abdelhamid, Y.A.; Elyamany, M.F.; Badary, O.A.; Heikal, O.A. Rice Bran Extract Protected against LPS-Induced Neuroinflammation in Mice through Targeting PPAR-γ Nuclear Receptor. Mol. Neurobiol. 2021, 58, 1504–1516. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Astakhova, A.A.; Goriainov, S.V.; Sergeeva, M.G. Comparison of PPAR Ligands as Modulators of Resolution of Inflammation, via Their Influence on Cytokines and Oxylipins Release in Astrocytes. Int. J. Mol. Sci. 2020, 21, 9577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, N.; Lu, L.; Lin, Q.; Li, L.; Dong, P.; Yang, B.; Li, D.; Fei, J. Pioglitazone Prevents Sevoflurane-induced Neuroinflammation and Cognitive Decline in a Rat Model of Chronic Intermittent Hypoxia by Upregulating Hippocampal PPAR-γ. Mol. Med. Rep. 2019, 49, 3815–3822. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, K.; Xiang, W.; Li, Y.; Hao, Y.; Guan, Y. Rosiglitazone Polarizes Microglia and Protects against Pilocarpine-Induced Status Epilepticus. CNS Neurosci. Ther. 2019, 25, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.; Sharma, B. A Selective Peroxisome Proliferator-Activated Receptor-γ Agonist Benefited Propionic Acid Induced Autism-like Behavioral Phenotypes in Rats by Attenuation of Neuroinflammation and Oxidative Stress. Chem. Biol. Interact. 2019, 311, 108758. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.M.F.; Bassani, T.B.; Cóppola-Segovia, V.; Moura, E.L.R.; Zanata, S.M.; Andreatini, R.; Vital, M.A.B.F. PPAR-γ Agonist Pioglitazone Reduces Microglial Proliferation and NF-ΚB Activation in the Substantia Nigra in the 6-Hydroxydopamine Model of Parkinson’s Disease. Pharmacol. Rep. 2019, 71, 556–564. [Google Scholar] [CrossRef]
- Justin, A.; Ashwini, P.; Jose, J.A.; Jeyarani, V.; Dhanabal, S.P.; Manisha, C.; Mandal, S.P.; Bhavimani, G.; Prabitha, P.; Yuvaraj, S.; et al. Two Rationally Identified Novel Glitazones Reversed the Behavioral Dysfunctions and Exhibited Neuroprotection Through Ameliorating Brain Cytokines and Oxy-Radicals in ICV-LPS Neuroinflammatory Rat Model. Front. Neurosci. 2020, 14, 530148. [Google Scholar] [CrossRef]
- Xu, W.; Lakshman, N.; Morshead, C.M. Building a Central Nervous System: The Neural Stem Cell Lineage Revealed. Neurogenesis 2017, 4, e1300037. [Google Scholar] [CrossRef] [Green Version]
- Stergiopoulos, A.; Politis, P.K. The Role of Nuclear Receptors in Controlling the Fine Balance between Proliferation and Differentiation of Neural Stem Cells. Arch. Biochem. Biophys. 2013, 534, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Gkikas, D.; Tsampoula, M.; Politis, P.K. Nuclear Receptors in Neural Stem/Progenitor Cell Homeostasis. Cell. Mol. Life Sci. 2017, 74, 4097–4120. [Google Scholar] [CrossRef]
- Knobloch, M.; Braun, S.M.G.; Zurkirchen, L.; Von Schoultz, C.; Zamboni, N.; Araúzo-Bravo, M.J.; Kovacs, W.J.; Karalay, Ö.; Suter, U.; MacHado, R.A.C.; et al. Metabolic Control of Adult Neural Stem Cell Activity by Fasn-Dependent Lipogenesis. Nature 2013, 493, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Khacho, M.; Harris, R.; Slack, R.S. Mitochondria as Central Regulators of Neural Stem Cell Fate and Cognitive Function. Nat. Rev. Neurosci. 2019, 20, 34–48. [Google Scholar] [CrossRef]
- Khacho, M.; Clark, A.; Svoboda, D.S.; Azzi, J.; MacLaurin, J.G.; Meghaizel, C.; Sesaki, H.; Lagace, D.C.; Germain, M.; Harper, M.E.; et al. Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell Stem Cell 2016, 19, 232–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santpere, G.; Telford, M.; Andrés-Benito, P.; Navarro, A.; Ferrer, I. The Presence of Human Herpesvirus 6 in the Brain in Health and Disease. Biomolecules 2020, 10, 1520. [Google Scholar] [CrossRef] [PubMed]
- Bétourné, A.; Szelechowski, M.; Thouard, A.; Abrial, E.; Jean, A.; Zaidi, F.; Foret, C.; Bonnaud, E.M.; Charlier, C.M.; Suberbielle, E.; et al. Hippocampal Expression of a Virus-Derived Protein Impairs Memory in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, 1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari Khaljiri, H.; Jamalkhah, M.; Amini Harandi, A.; Pakdaman, H.; Moradi, M.; Mowla, A. Comprehensive Review on Neuro-COVID-19 Pathophysiology and Clinical Consequences. Neurotox. Res. 2021, 1, 3. [Google Scholar] [CrossRef]
- Shaw, G.M.; Hunter, E. HIV Transmission. Cold Spring Harb. Perspect. Med. 2012, 2, a006965. [Google Scholar] [CrossRef]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV Infection. Nat. Rev. Dis. Primers 2015, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Cotto, B.; Natarajanseenivasan, K.; Langford, D. HIV-1 Infection Alters Energy Metabolism in the Brain: Contributions to HIV-Associated Neurocognitive Disorders. Prog. Neurobiol. 2019, 181, 101616. [Google Scholar] [CrossRef]
- Ko, A.; Kang, G.; Hattler, J.B.; Galadima, H.I.; Zhang, J.; Li, Q.; Kim, W.-K. Macrophages but Not Astrocytes Harbor HIV DNA in the Brains of HIV-1-Infected Aviremic Individuals on Suppressive Antiretroviral Therapy. J. Neuroimmune Pharmacol. 2019, 14, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Eggers, C.; Arendt, G.; Hahn, K.; Husstedt, I.W.; Maschke, M.; Neuen-Jacob, E.; Obermann, M.; Rosenkranz, T.; Schielke, E.; Straube, E. HIV-1-Associated Neurocognitive Disorder: Epidemiology, Pathogenesis, Diagnosis, and Treatment. J. Neurol. 2017, 264, 1715–1727. [Google Scholar] [CrossRef]
- Cenker, J.J.; Stultz, R.D.; McDonald, D. Brain Microglial Cells Are Highly Susceptible to HIV-1 Infection and Spread. AIDS Res. Hum. Retroviruses 2017, 33, 1155–1165. [Google Scholar] [CrossRef]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell. Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Lindl, K.A.; Marks, D.R.; Kolson, D.L.; Jordan-Sciutto, K.L. HIV-Associated Neurocognitive Disorder: Pathogenesis and Therapeutic Opportunities. J. Neuroimmune Pharmacol. 2010, 5, 294–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamik, M.K.; Asahchop, E.L.; Chan, W.F.; Zhu, Y.; Branton, W.G.; McKenzie, B.A.; Cohen, E.A.; Power, C. Insulin Treatment Prevents Neuroinflammation and Neuronal Injury with Restored Neurobehavioral Function in Models of HIV/AIDS Neurodegeneration. J. Neurosci. 2016, 36, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Omeragic, A.; Hoque, M.T.; Choi, U.; Bendayan, R. Peroxisome Proliferator-Activated Receptor-Gamma: Potential Molecular Therapeutic Target for HIV-1-Associated Brain Inflammation. J. Neuroinflamm. 2017, 14. [Google Scholar] [CrossRef]
- Potash, M.J.; Chao, W.; Bentsman, G.; Paris, N.; Saini, M.; Nitkiewicz, J.; Belem, P.; Sharer, L.; Brooks, A.I.; Volsky, D.J. A Mouse Model for Study of Systemic HIV-1 Infection, Antiviral Immune Responses, and Neuroinvasiveness. Proc. Natl. Acad. Sci. USA 2005, 102, 3760–3765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omeragic, A.; Kara-Yacoubian, N.; Kelschenbach, J.; Sahin, C.; Cummins, C.L.; Volsky, D.J.; Bendayan, R. Peroxisome Proliferator-Activated Receptor-Gamma Agonists Exhibit Anti-Inflammatory and Antiviral Effects in an EcoHIV Mouse Model. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Omeragic, A.; Saikali, M.F.; Currier, S.; Volsky, D.J.; Cummins, C.L.; Bendayan, R. Selective Peroxisome Proliferator-Activated Receptor-Gamma Modulator, INT131 Exhibits Anti-Inflammatory Effects in an EcoHIV Mouse Model. FASEB J. 2020, 34, 1996–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Eum, S.Y.; András, I.E.; Hennig, B.; Toborek, M. PPARα and PPARγ Attenuate HIV-induced Dysregulation of Tight Junction Proteins by Modulations of Matrix Metalloproteinase and Proteasome Activities. FASEB J. 2009, 23, 1596–1606. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Chen, L.; Zhang, B.; Park, M.; Toborek, M. PPAR Agonist-Mediated Protection against HIV Tat-Induced Cerebrovascular Toxicity Is Enhanced in MMP-9-Deficient Mice. J. Cereb. Blood Flow Metab. 2014, 34, 646–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, C.H.; Yeh, C.L.; Yeh, S.L.; Lin, E.S.; Wang, L.Y.; Wang, Y.H. Quercetin Metabolites Inhibit MMP-2 Expression in A549 Lung Cancer Cells by PPAR-γ Associated Mechanisms. J. Nutr. Biochem. 2016, 33, 45–53. [Google Scholar] [CrossRef]
- Shyu, L.-Y.; Chen, K.-M.; Lu, C.-Y.; Lai, S.-C. Regulation of Proinflammatory Enzymes by Peroxisome Proliferator–Activated Receptor Gamma in Astroglia Infected with Toxoplasma Gondii. J. Parasitol. 2020, 106, 564–571. [Google Scholar] [CrossRef]
- Philip, S.; Kundu, G.C. Osteopontin Induces Nuclear Factor ΚB-Mediated Promatrix Metalloproteinase-2 Activation through IκBα/IKK Signaling Pathways, and Curcumin (Diferulolylmethane) down-Regulates These Pathways. J. Biol. Chem. 2003, 278, 14487–14497. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Yang, Y.; Cui, Y.; Gao, J.; Wang, K.; Cui, J. Lipoxin A4 Methyl Ester Reduces Early Brain Injury by Inhibition of the Nuclear Factor Kappa B (NF-KB)-Dependent Matrix Metallopeptidase 9 (MMP-9) Pathway in a Rat Model of Intracerebral Hemorrhage. Med. Sci. Monit. 2019, 25, 1838–1847. [Google Scholar] [CrossRef]
- Potula, R.; Ramirez, S.H.; Knipe, B.; Leibhart, J.; Schall, K.; Heilman, D.; Morsey, B.; Mercer, A.; Papugani, A.; Dou, H.; et al. Peroxisome Proliferator-Activated Receptor-γ Activation Suppresses HIV-1 Replication in an Animal Model of Encephalitis. AIDS 2008, 22, 1539–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, K.M.; Song, H.; Ming, G.L. Pathophysiology and Mechanisms of Zika Virus Infection in the Nervous System. Annu. Rev. Neurosci. 2019, 42, 249–269. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Jacob, F.; Song, H.; Ming, G.L. Using Brain Organoids to Understand Zika Virus-Induced Microcephaly. Development 2017, 144, 952–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Hammack, C.; Ogden, S.C.; Wen, Z.; Qian, X.; Li, Y.; Yao, B.; Shin, J.; Zhang, F.; Lee, E.M.; et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 2016, 18, 587–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thulasi Raman, S.N.; Latreille, E.; Gao, J.; Zhang, W.; Wu, J.; Russell, M.S.; Walrond, L.; Cyr, T.; Lavoie, J.R.; Safronetz, D.; et al. Dysregulation of Ephrin Receptor and PPAR Signaling Pathways in Neural Progenitor Cells Infected by Zika Virus. Emerg. Microbes Infect. 2020, 9, 2046–2060. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Luong, Q.; Sharma, V.M.; Harberson, M.; Harper, B.; Colborn, A.; Berryman, D.E.; Jessen, N.; Jørgensen, J.O.L.; Kopchick, J.J.; et al. Growth Hormone Controls Lipolysis by Regulation of FSP27 Expression. J. Endocrinol. 2018, 239, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.N.M.; Kim, M.K.; Vo, V.T.A.; Choi, J.W.; Choi, J.H.; Kim, H.W.; Cha, S.K.; Park, K.S.; Jeong, Y. Inhibition of Oncogenic Src Induces FABP4-Mediated Lipolysis via PPARγ Activation Exerting Cancer Growth Suppression. EBioMedicine 2019, 41, 134–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, K.; Peng, Z.; Lin, W.; Kozar, R.A. Arginine Decreases Peroxisome Proliferator-Activated Receptor-γ Activity via c-Jun. Mol. Cell. Biochem. 2012, 362, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Triki, M.; Lapierre, M.; Cavailles, V.; Mokdad-Gargouri, R. Expression and Role of Nuclear Receptor Coregulators in Colorectal Cancer. World J. Gastroenterol. 2017, 23, 4480. [Google Scholar] [CrossRef]
- Cannon, M.J. Congenital Cytomegalovirus (CMV) Epidemiology and Awareness. J Clin Virol 2009, 46 (Suppl. 4), S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Cheeran, M.C.J.; Lokensgard, J.R.; Schleiss, M.R. Neuropathogenesis of Congenital Cytomegalovirus Infection: Disease Mechanisms and Prospects for Intervention. Clin Microbiol Rev 2009, 22, 99–126. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Byun, S.-H.; Kim, J.; Kwon, M.; Pleasure, S.J.; Ahn, J.-H.; Yoon, K. Human Cytomegalovirus IE2 Protein Disturbs Brain Development by the Dysregulation of Neural Stem Cell Maintenance and the Polarization of Migrating Neurons. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odeberg, J.; Wolmer, N.; Falci, S.; Westgren, M.; Seiger, A.; Söderberg-Nauclér, C. Human Cytomegalovirus Inhibits Neuronal Differentiation and Induces Apoptosis in Human Neural Precursor Cells. J. Virol. 2006, 80, 8929–8939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.H.; Hannemann, H.; Kulkarni, A.S.; Schwartz, P.H.; O’Dowd, J.M.; Fortunato, E.A. Human Cytomegalovirus Infection Causes Premature and Abnormal Differentiation of Human Neural Progenitor Cells. J. Virol. 2010, 84, 3528–3541. [Google Scholar] [CrossRef] [Green Version]
- Belzile, J.P.; Stark, T.J.; Yeo, G.W.; Spector, D.H. Human Cytomegalovirus Infection of Human Embryonic Stem Cell-Derived Primitive Neural Stem Cells Is Restricted at Several Steps but Leads to the Persistence of Viral DNA. J. Virol. 2014, 88, 4021–4039. [Google Scholar] [CrossRef] [Green Version]
- Odeberg, J.; Wolmer, N.; Falci, S.; Westgren, M.; Sundtröm, E.; Seiger, Å.; Söderberg-Nauclér, C. Late Human Cytomegalovirus (HCMV) Proteins Inhibit Differentiation of Human Neural Precursor Cells into Astrocytes. J. Neurosci. Res. 2007, 85, 583–593. [Google Scholar] [CrossRef]
- D’Aiuto, L.; Di Maio, R.; Heath, B.; Raimondi, G.; Milosevic, J.; Watson, A.M.; Bamne, M.; Parks, W.T.; Yang, L.; Lin, B.; et al. Human Induced Pluripotent Stem Cell-Derived Models to Investigate Human Cytomegalovirus Infection in Neural Cells. PLoS ONE 2012, 7, e49700. [Google Scholar] [CrossRef] [Green Version]
- Rolland, M.; Martin, H.; Bergamelli, M.; Sellier, Y.; Bessières, B.; Aziza, J.; Benchoua, A.; Leruez-Ville, M.; Gonzalez-Dunia, D.; Chavanas, S. Human Cytomegalovirus Infection Is Associated with Increased Expression of the Lissencephaly Gene PAFAH1B1 Encoding LIS1 in Neural Stem Cells and Congenitally Infected Brains. J. Pathol. 2021, 254, 92–102. [Google Scholar] [CrossRef]
- Leghmar, K.; Cenac, N.; Rolland, M.; Martin, H.; Rauwel, B.; Bertrand-Michel, J.; Le Faouder, P.; Bénard, M.; Casper, C.; Davrinche, C.; et al. Cytomegalovirus Infection Triggers the Secretion of the PPARgamma Agonists 15-Hydroxyeicosatetraenoic Acid (15-HETE) and 13-Hydroxyoctadecadienoic Acid (13-HODE) in Human Cytotrophoblasts and Placental Cultures. PLoS ONE 2015, 10, e0132627. [Google Scholar] [CrossRef] [PubMed]
- Stump, M.; Guo, D.F.; Lu, K.T.; Mukohda, M.; Cassell, M.D.; Norris, A.W.; Rahmouni, K.; Sigmund, C.D. Nervous System Expression of PPARγ and Mutant PPARγ Has Profound Effects on Metabolic Regulation and Brain Development. Endocrinology 2016, 157, 4266–4275. [Google Scholar] [CrossRef]
- Allal, C.; Buisson-Brenac, C.; Marion, V.; Claudel-Renard, C.; Faraut, T.; Dal Monte, P.; Streblow, D.; Record, M.; Davignon, J.L. Human Cytomegalovirus Carries a Cell-Derived Phospholipase A2 Required for Infectivity. J. Virol. 2004, 78, 7717–7726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelova, M.; Zwezdaryk, K.; Ferris, M.B.; Shan, B.; Morris, C.A.; Sullivan, D.E. Human Cytomegalovirus Infection Dysregulates the Canonical Wnt/β-Catenin Signaling Pathway. PLoS Pathog. 2012, 8, e1002959. [Google Scholar] [CrossRef] [Green Version]
- Rauwel, B.; Mariamé, B.; Martin, H.; Nielsen, R.; Allart, S.; Pipy, B.; Mandrup, S.; Devignes, M.D.; Evain-Brion, D.; Fournier, T.; et al. Activation of Peroxisome Proliferator-Activated Receptor Gamma by Human Cytomegalovirus for de Novo Replication Impairs Migration and Invasiveness of Cytotrophoblasts from Early Placentas. J. Virol. 2010, 84, 2946–2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffezzini, C.; Calvo-Garrido, J.; Wredenberg, A.; Freyer, C. Metabolic Regulation of Neurodifferentiation in the Adult Brain. Cell. Mol. Life Sci. 2020, 77, 2483–2496. [Google Scholar] [CrossRef] [Green Version]
- Munger, J.; Bajad, S.U.; Coller, H.A.; Shenk, T.; Rabinowitz, J.D. Dynamics of the Cellular Metabolome during Human Cytomegalovirus Infection. PLoS Pathog. 2006, 2, e132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamin, D.; Hadigan, C.; Lehrke, M.; Mazza, S.; Lazar, M.A.; Grinspoon, S. Resistin Levels in Human Immunodeficiency Virus-Infected Patients with Lipoatrophy Decrease in Response to Rosiglitazone. J. Clin. Endocrinol. Metab. 2005, 90, 3423–3426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Layrolle, P.; Payoux, P.; Chavanas, S. PPAR Gamma and Viral Infections of the Brain. Int. J. Mol. Sci. 2021, 22, 8876. https://doi.org/10.3390/ijms22168876
Layrolle P, Payoux P, Chavanas S. PPAR Gamma and Viral Infections of the Brain. International Journal of Molecular Sciences. 2021; 22(16):8876. https://doi.org/10.3390/ijms22168876
Chicago/Turabian StyleLayrolle, Pierre, Pierre Payoux, and Stéphane Chavanas. 2021. "PPAR Gamma and Viral Infections of the Brain" International Journal of Molecular Sciences 22, no. 16: 8876. https://doi.org/10.3390/ijms22168876
APA StyleLayrolle, P., Payoux, P., & Chavanas, S. (2021). PPAR Gamma and Viral Infections of the Brain. International Journal of Molecular Sciences, 22(16), 8876. https://doi.org/10.3390/ijms22168876







