Abstract
Translational photopharmacological applications are limited through irradiation by light showing wavelengths within the bio-optical window. To achieve sufficient tissue penetration, using wavelengths >500 nm is mandatory. Nevertheless, the majority of photopharmacological compounds respond to irradiation with more energetic UV light, which shows only a minor depth of tissue penetration in the µm range. Thus, we became interested in UV light containing Cherenkov radiation (CR) induced as a by-product by clinically employed radionuclides labeling specific tissues. Therefore, CR may be applicable in novel photopharmacological approaches. To provide evidence for the hypothesis, we verified the clinically established radionuclides 68Ga and 90Y but not 18F in clinically used activities to be capable of generating CR in aqueous solutions. We then investigated whether the generated CR was able to photoactivate the caged kinase inhibitor cagedAZD5438 as a photoresponsive model system. Herein, 21% uncaging of the model system cagedAZD5438 occurred by incubation with 90Y, along with a non-specific compound decomposition for 68Ga and partly for 90Y. The findings suggest that the combination of a clinically employed radionuclide with an optimized photoresponsive agent could be beneficial for highly focused photopharmacological therapies.
1. Introduction
A recent strategy to overcome specificity issues of pharmacologically active compounds includes photopharmacological approaches, in which the biological activity of a compound can be regulated by the irradiation of light [1]. Using light-responsive molecules, temporal and spatial control and thus high precision in a therapeutic treatment could be achieved [2,3,4]. In line with this notion, Table S1 lists a selection of experimental photopharmacological applications that have been studied recently.
However, the majority of photoresponsive compounds react most effectively to irradiation with light showing wavelengths < 500 nm, typically showing a limiting penetration depth of only micrometers into biological tissue. Moreover, highly energetic UV light can cause harm to cells, resulting, among others, in apoptosis or DNA damage [5,6,7]. On the other hand, the light absorption of hemoglobin and water reaches a minimum between 650 and 900 nm, thus allowing for decent tissue penetration for relevant wavelengths and, as a consequence, for a sufficient translational bioavailability of irradiation. This wavelength range between 650 and 900 nm is commonly referred to as the bio-optical window, and photopharmacological research aims to develop compounds to be activated by irradiation within that range [8,9]. For therapeutically effective applications, the used wavelength of the irradiation should be adjusted ideally within the bio-optical window, and thus, a compound should be photoresponsive within this window, too. However, until today (as shown in Table S1), most photopharmacological approaches use lower wavelengths within the UV range [10,11,12,13,14,15,16,17,18,19,20]. Therefore, an alternative would be highly beneficial to circumvent this limited penetration depth of UV light for successful activation of photoresponsive compounds at deeper tissue levels.
In this context, we became interested in the usage of Cherenkov radiation (CR), which exhibits an emission spectrum in the near-UV range (250–600 nm) and hence covers the wavelength range of most of the photoresponsive compounds published so far (Table S1) [21,22]. Ran et al. already investigated the possibility of activation based on CR generated by 18F, and Nakamura et al. reported on CR by 18F-FDG for photoimmunotherapy [23]. Furthermore, CR has already been investigated more closely in the field of photodynamic therapy, for example, by Hart et al. for 90Y-induced CR [24,25,26,27].
CR can occur as a second form of radiation in addition to ionizing radiation generated from high-energy beta emitters, including clinically established radiopharmaceuticals. These are radiolabeled molecules designed to deliver therapeutic doses of ionizing radiation to specific tumor tissue areas within the body. At specific dose rates, they are used for diagnostics and radiotherapy, thus potentially offering highly interesting combinations for novel photopharmacological applications. CR is commonly known as the emergence of blue light emitted from high-energy radiation sources stored in water in a nuclear power plant. Furthermore, CR is the basic detection principle of a Cherenkov counter, which is a technique for the determination of beta emitting radionuclides used in biochemical assays (e.g., isotope 32P) [28,29]. In general, CR can be induced when a charged particle travels faster than light in a dielectric medium (e.g., water). The charged particle locally polarizes the medium molecules, generating excited electronic states. During their return to the ground state, the resulting energy is emitted by the system as CR. This CR typically occurs in an asymmetrical polarization event, the so-called “cone effect”, in which the resulting radiation waves can constructively superimpose each other. If the charged particles in the polarizable medium travel slower than the respective speed of light within the same medium, the resulting radiation waves cancel each other. Therefore, the threshold condition for the induction of CR depends on the refractive index of the polarizable medium as well as on the energy level of the charged particles [30]. The velocity in the respective medium v can be calculated using the following equation, where n is the refractive index of the medium and c is the speed of light in vacuum.
v = c/n
In radiopharmaceutical therapies, especially radionuclides that decay by beta-particle emission are commonly used. Beta decay of the nuclides results in the emission of high-energy electrons or positrons from the nucleus. These locally generated high-energy particles impact the surrounding tumor tissue to consequently kill tumor cells. Accordingly, we focused on the question of whether radiotherapeutical applications generating CR could be useful to activate photoresponsive compounds. Besides beta-emitting radionuclides, external beam radiation commonly used in the clinic for radiation tumor therapy is able to induce CR as well [31], whereby charged particles such as electrons are strongly accelerated in a linear particle accelerator for targeting tumor tissues. The resulting energy is also released in the form of photons, which can induce CR due to Compton scattering. In detail, a photon is scattered by a charged particle such as an electron, which leads to a transfer of energy to the rebounding particle. This energetic recoiling particle is able to induce CR [32,33,34,35].
In the present study, we investigated whether CR, generated by radionuclides or by external irradiation (see SI Section 2.8), could be useful to activate photoresponsive prodrug compounds. Thus, a locally and temporally controlled photopharmacological therapy without the need for external irradiation could be possible. Furthermore, a combination of photoresponsive chemotherapy and radiotherapy could achieve additive effects [10]. Additionally, the amount of necessary radiopharmaceutical activity could be reduced by such a combination, so that both patients and clinical staff would benefit from the concept as the medical applications would possibly be easier to manage. In the present study, from nuclides relevant for diagnostic and therapeutic purposes (Table 1), we took a selection, namely 18F, 68Ga, and 90Y, and aimed to verify whether these radioisotopes were able to produce quantifiable CR in an aqueous medium.

Table 1.
Parameters of selected radionuclides with clinical significance [36]. Every positron-emitting (ß+) nuclide results in electron capture (ec). This is another mode of beta decay in which an electron is captured by the atomic nucleus [37]. Iβ(abs) *: absolute β− or β+ intensity in %.
Additionally, we aimed to investigate the potential photocleavage of a caged compound as a photoresponsive model system, both for the selection of the nuclides and for photons and electrons from a clinically used linear particle accelerator (see SI Section 2.8). As the model compound, we chose the highly effective but somewhat unspecific protein kinase inhibitor AZD5438 caged with the well-established photoremovable protecting group 4,5-dimethoxy-2-nitrobenzyl (DMNB) [3,46,47,48] (Figure 1) to obtain a bioinactive prodrug. Protein kinases play an essential role in signal transduction and, in the case of their malfunction, lead to uncontrolled cell growth or reduced apoptosis. This often results in the development of cancer [49,50]. Cyclin-dependent kinases (CDKs) are especially fundamental for a functioning cell cycle and transcription regulation and are investigated as drug targets [51,52,53]. In line with this notion, AZD5438 has been developed as a potent inhibitor of CDKs with the following IC50 values: CDK1, 16 nM; CDK2, 6 nM; CDK9, 20 nM [54]. Furthermore, it is an orally bioavailable inhibitor which advanced into clinical studies but failed because of toxic side effects. Hence, the pharmacologically active compound AZD5438 and its inactive caged prodrug cagedAZD5438 may be suitable for highly specific photopharmacological applications, in that the temporal and spatial control of the drug’s activation can greatly decimate the proportion of toxic side effects.
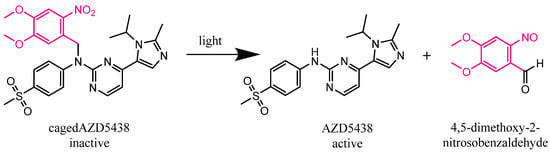
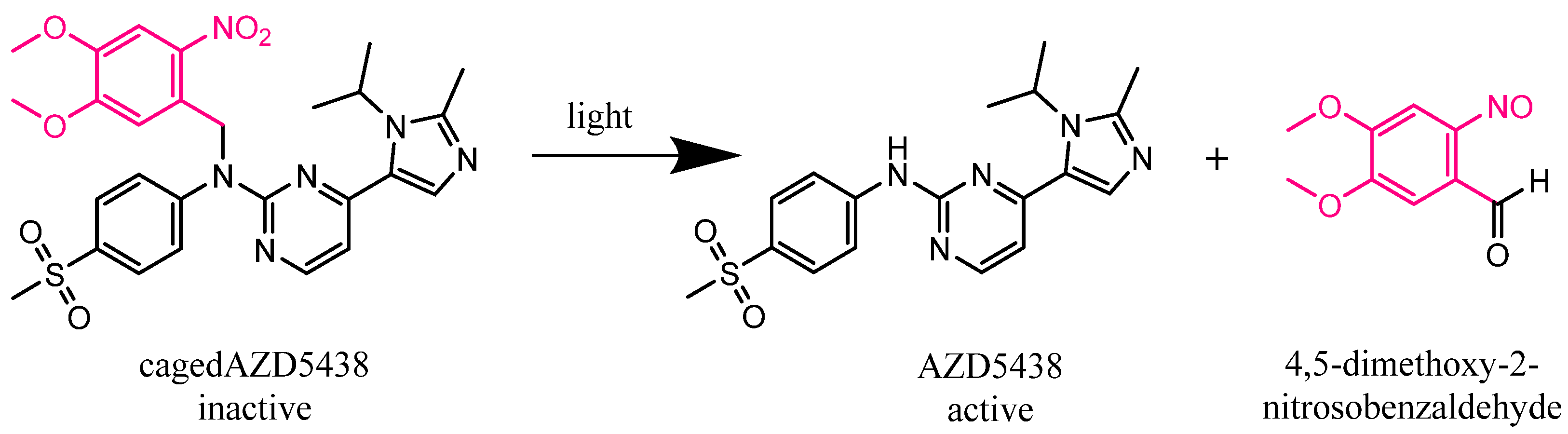
Figure 1.
Expected photolysis reaction of the inactive kinase inhibitor AZD5438 caged with the photoremovable protecting group 4,5-dimethoxy-2-nitrobenzyl (DMNB) (cagedAZD5438) to the active AZD5438 and the separated protection group according to Klán et al. [3].
2. Results and Discussion
2.1. Verification of CR Generated by the Decay of Nuclides in an Aqueous Solution
First, it was important to provide evidence for the generation of CR by the decay of the above-mentioned radionuclides in aqueous solutions. Among the typical read-out techniques for biochemical assays is a Cherenkov counter, which exploits exactly the principle of the resulting CR by the decay of radionuclides (e.g., 32P) [28]. However, such conventional detection methods could not be established for this study because of the obvious difficulties in handling the radioisotopes, including the long contamination times based on their slow decay. Therefore, when looking for alternative methods for the direct detection of CR, we came across a device called “kamiokanne” (KK). The KK is a simplified system based on the method of the “super-kamiokande” (SKK), which is located in Kamioka, Japan [55]. In fact, the SKK is being used to investigate secondary cosmic radiation. The simplified KK is composed of common thermos flasks with attached photomultiplier tubes (PMTs) and internal high-voltage supplies [56]. The KK is able to measure muons from secondary cosmic radiation, thus generating CR as electromagnetic radiation. The PMTs are able to detect this CR, and consequently, an electrical signal is generated as the read-out. Thus, we decided to test the KK for the detection of CR in our experiments (further details can be found in the SI). The adapted set-up of the KK in our lab is shown schematically in Figure 2a. Here, we investigated whether CR generated by the decay of radionuclides was detectable by the KK. From the multitude of possible radionuclides, the therapeutically and diagnostically used beta-emitting radionuclides 18F, 68Ga, and 90Y were chosen as a representative selection of beta emitters showing clinical significance (Table 1). As the key rationale for the selection of these radionuclides, it was important to focus on meaningful half-life values to allow sufficient repetitions of experiments within a reasonable period. Additionally, the decay energy of the respective radionuclides plays an important role. This energy is not constant, but a continuum spectrum is created in which the maximum emitted energy (Emax) and the average energy (Emean) are key parameters (Figure S8) [57,58,59,60].
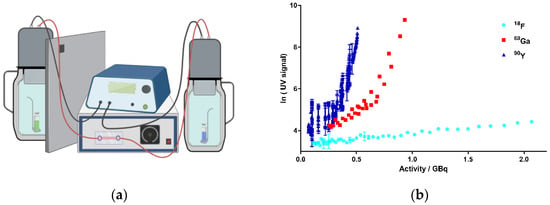
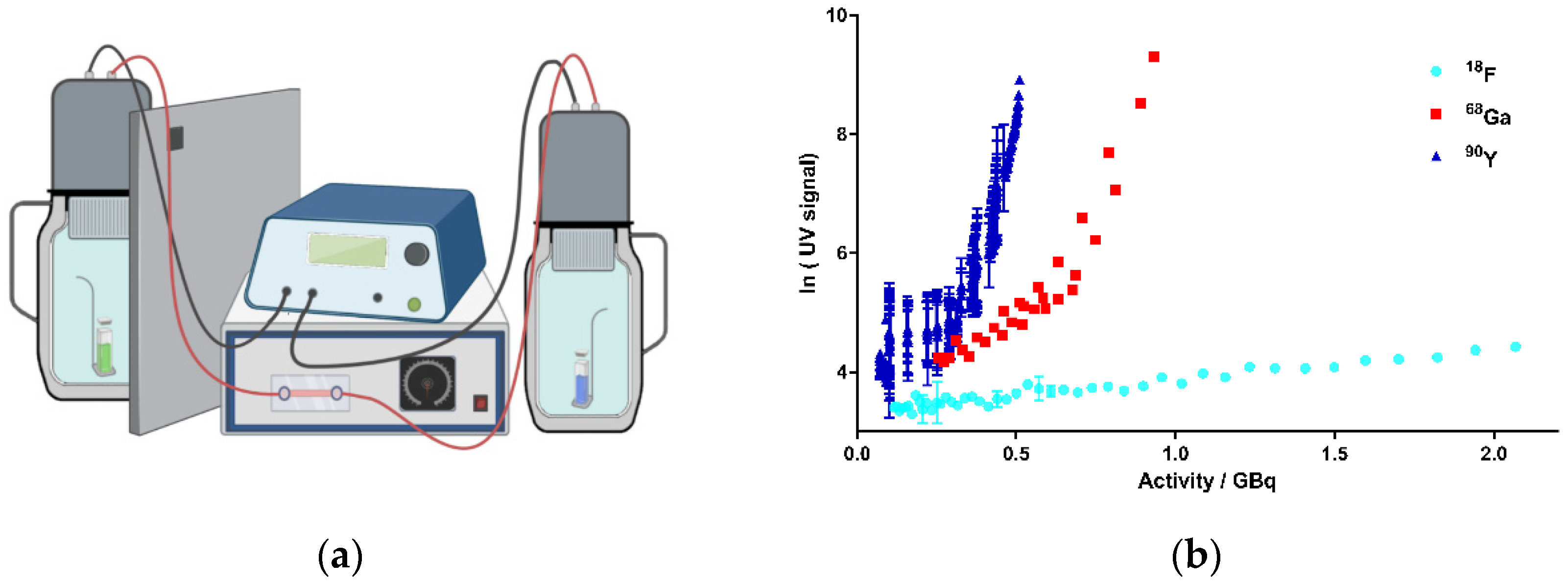
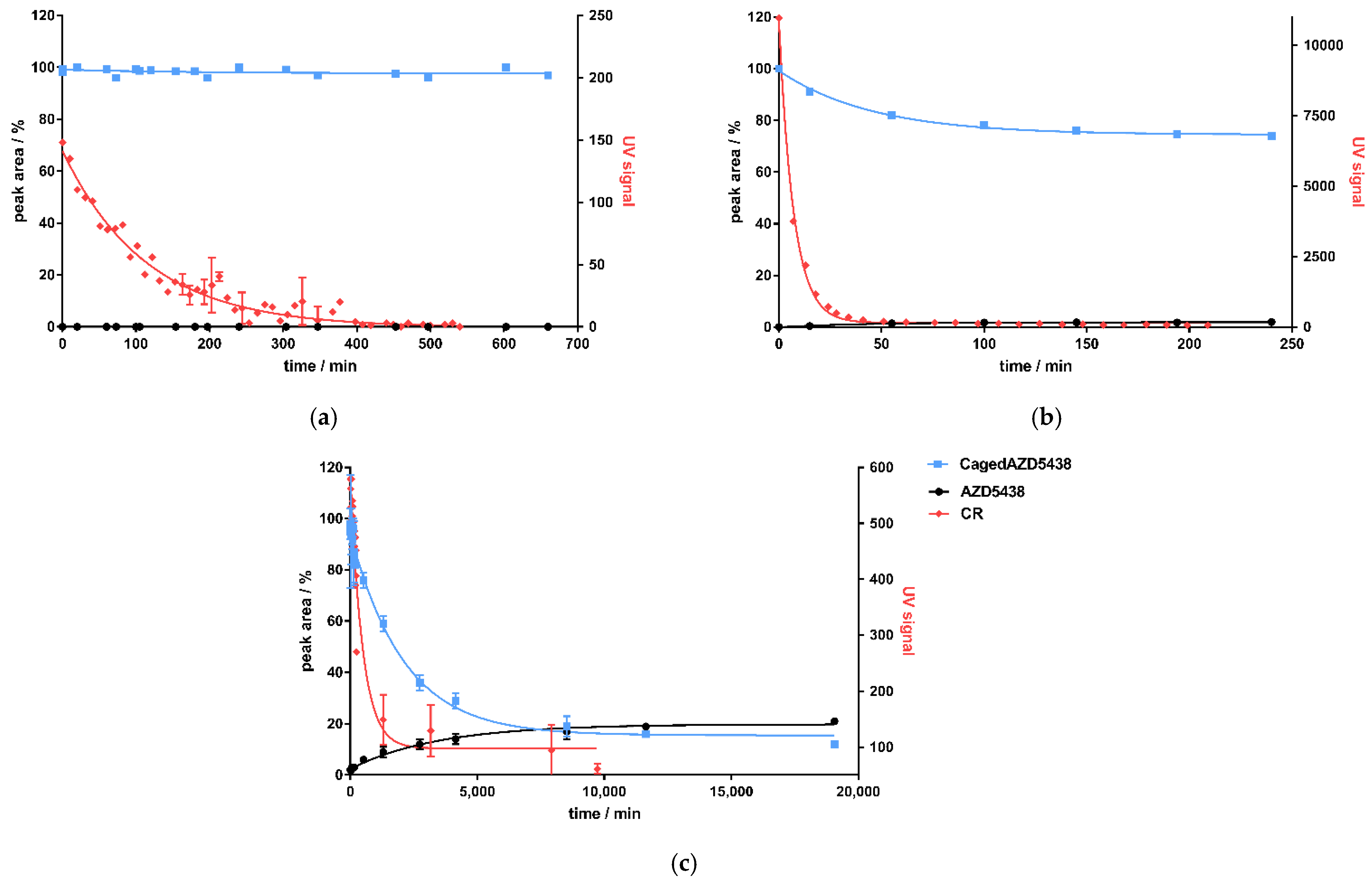
Figure 2.
Activity-dependent generation of CR by the decay of 18F, 68Ga, and 90Y in kamiokanne (KK) experiments. (a) Schematic test setup of KK experiments. Two thermos flasks separated by a lead wall were filled with water together with a holder for a sealed quartz cuvette containing the samples. The flasks were equipped with photomultiplier tubes (PMTs) and electronic devices to detect CR. The green cuvette represents a solution of the radionuclide sample, and the blue sample illustrates the negative control containing the solvent without radionuclides. On top of the thermos flasks, PMTs were attached to high-voltage supplies (figure created with BioRender.com). (b) Results from the KK experiments. The activities of the three radionuclides, 18F, 68Ga, and 90Y, are presented individually for each experiment in correlation to the resulting Cherenkov signal intensity (UV signal). The signal strength corresponds to the number of signals at 10-min intervals. Signals of cosmic radiation were used to determine the baseline (blank value). Each radionuclide was tested in three independent experiments; data represent mean ± SD.
A critical energy threshold value of 0.26 MeV was determined by Elrick et al. regarding the generation of CR in an aqueous solution [22]. In order to meet the requirements for potential translational purposes of this concept, we tested the radionuclides in energy ranges corresponding to their respective clinical applications. Thus, we performed the experiments in the activity range for 68Ga from 1.163 to 0.256 GBq, for 18F from 2.317 to 0.117 GBq, and for 90Y from 0.510 to 0.071 GBq. Figure 2b summarizes the results of the experiments. Based on the decay of these nuclides, activity-dependent generation of CR could be determined for all chosen nuclides. A significant increase in CR signals was observed for 68Ga and 90Y. For 68Ga, more activity was required compared to 90Y to achieve a comparable CR signal according to their respective decay energies. In contrast, only CR slightly above the blank value (negative control, CR from the secondary cosmic radiation) could be determined for 18F, although we used activities up to more than 2 GBq. The results were not unexpected, as the Emean emitted by 18F was 0.25 MeV and thus did not reach the threshold value for the formation of CR (Table 1) [22]. Taken together, we could establish a strong correlation between the nuclear decay of the selected nuclides and the resulting CR, suggesting 68Ga and 90Y to be suitable for photopharmacological applications. 18F produced CR as well, but to a much lesser extent, which might not be enough to photoactivate our model system cagedAZD5438.
2.2. Activation of a Caged Photoresponsive Compound by Cherenkov Radiation as Model System
Having demonstrated the generation of CR by the radionuclides 18F, 68Ga, and 90Y as a side-product, we next investigated whether this process could activate a photoresponsive compound. We selected the 4,5-dimethoxy-2-nitrobenzyl (DMNB) caged AZD5438 (cagedAZD5438) as a novel model compound based on the emission spectrum of the CR and the strong absorption of cagedAZD5438 within the range of 300–400 nm (Figure 3b). The DMNB group was already investigated by our group in connection with the central pharmacophore N-phenylpyrimidine-2-amine, a scaffold of many kinase inhibitors, including AZD5438 [61]. Molecular modeling of the X-ray-defined ligand complex of AZD5438 in CDK2 with cyclin E1 (pdb 4FKO) suggested the NH function to be suitable for caging. Herein, due to steric conflicts of the caging moiety, no plausible binding mode for cagedAZD5438 in CDK2 could be determined (see Figure S1) [62].
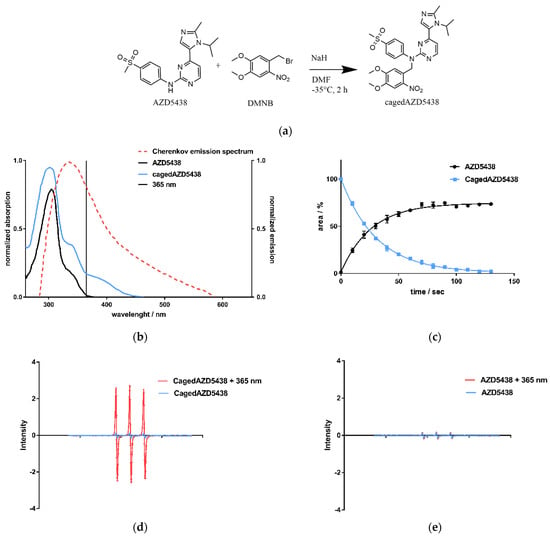
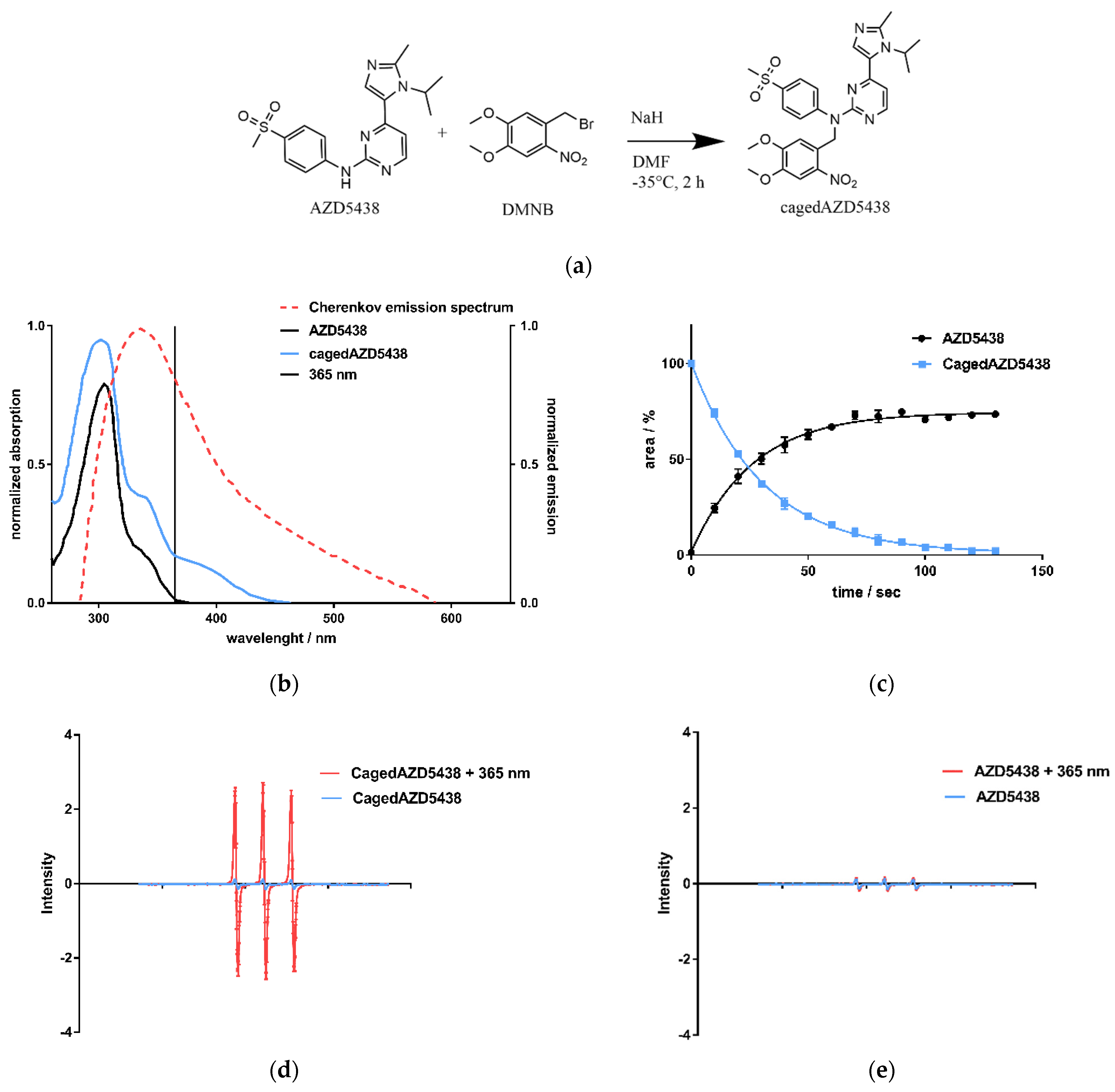
Figure 3.
(a) CagedAZD5438 was synthesized according to the general procedure for caging of N-phenylpyrimidin-2-amines with benzyl bromides [59]. In an SN2 reaction, benzyl bromide in dry DMF is added dropwise to AZD5438 at −35 °C under argon atmosphere (yield 55.6%, further details in SI). (b) UV/Vis spectra of AZD5438 and cagedAZD5438 with DMNB in aqueous medium with 20% DMSO as solubilizer. An overlay of the Cherenkov emission spectrum (dotted line) illustrates the potential usage for photoactivation, indicating 365 nm as a suitable wavelength for control experiments. Other UV/Vis spectra with different solutions are shown in the SI, Figure S7. (c) Photoactivation of cagedAZD5438 by irradiation with UV light at 365 nm; an aqueous compound solution containing 50 μM prodrug cagedAZD5438 (blue line) and 20% DMSO was irradiated at 365 nm, 37.5 mW, every 10 s and then analyzed by HPLC. Repeated experiments with the free inhibitor AZD5438 (Figure S9d) as a control were carried out. Each value is the mean ± SD of four independent experiments. (d) Electron spin resonance (ESR) spectra of the spin probe TMTH (1-hydroxy-4-isobutyramido-2,2,6,6-tetramethylpiperidine) incubated with cagedAZD5438 in DMSO without and with irradiation (365 nm, 100 s, 37.5 mW per well). (e) ESR spectra of a solution of AZD5438 with and without irradiation (365 nm, 100 s, 37.5 mW per well). As a positive control, the photosensitizer 1,4-naphthoquinone [63] in DMSO was tested under the same conditions. The sample solutions contained the spin probe TMTH (2.5 mM) and 50 μM cagedAZD5438 in 20% DMSO. Error bars indicate SD with N = 3.
We achieved the synthesis of cagedAZD5438 by the established general procedure for caging of N-phenylpyrimidin-2-amines with benzyl bromides under argon atmosphere and under light protection [59] (Figure 3a). First, we performed preliminary tests to determine the stability of cagedAZD5438 under different conditions (see SI Figures S4 and S5). For the photochemical characterization of cagedAZD5438, we tested the uncaging process upon irradiation. An aqueous solution of 50 µM cagedAZD5438 with 20% DMSO (to avoid solubility issues) was irradiated with light at 365 nm with 37.5 mW, and the samples were subsequently analyzed by HPLC (Figure 3c; for details of the photolysis experiments, see Figure S6). The inhibitor AZD5438 itself proved to be stable during UV irradiation (Figure S9d). In contrast, upon irradiation, cagedAZD5438 was uncaged to produce 75% AZD5438 after 100 s of irradiation. Using electron spin resonance spectroscopy (ESR) investigations, we could confirm that radicals were generated during UV irradiation of a solution of AZD5438. Consequently, a portion of AZD5438 was radically destroyed and thus not detected by the HPLC analysis (Figure 3c–e), in which a mixture of unidentified products occurred.
Next, we aimed to test the uncaging of cagedAZD5438 in vitro. For this purpose, we initially tested cagedAZD5438 in an enzymatic CDK2/cyclin E1 assay and in a cell proliferation assay under controlled light conditions. In the enzymatic CDK2/cyclin E1 assay, we determined AZD5438 to be highly active with an IC50 value of 2.8 nM, which is in the same range as the reference data (IC50 = 6 nM) [54]. In contrast, cagedAZD5438 proved to be much less active without irradiation (IC50 = 4798 nM). When irradiating the assay containing cagedAZD5438 with 365 nm, the biological activity on CDK2 could be restored, resulting in an IC50 value of 5.6 nM (Figure 4a) and achieving a photopharmacological factor of 850 (unirradiated vs. irradiated). Similar results were obtained in the cell proliferation assay using Panc89 cells with resazurin as a read-out. Here, cagedAZD5438 was determined to be biologically inactive with an IC50 value >10 μM (Figure 4b). Again, after irradiation with 365 nm, the biological potency of AZD5438 was fully restored, resulting in an IC50 value of 0.5 μM (reference AZD5438 IC50 = 0.8 μM). Taken together, in both the kinase and the cell assay, we could demonstrate a clear photoresponsive effect for cagedAZD5438, suggesting this compound to be a suitable model system for further experiments involving the radionuclides 18F, 68Ga, and 90Y and for particle accelerator assays (see SI Section 2.8).
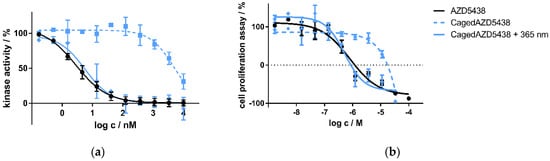
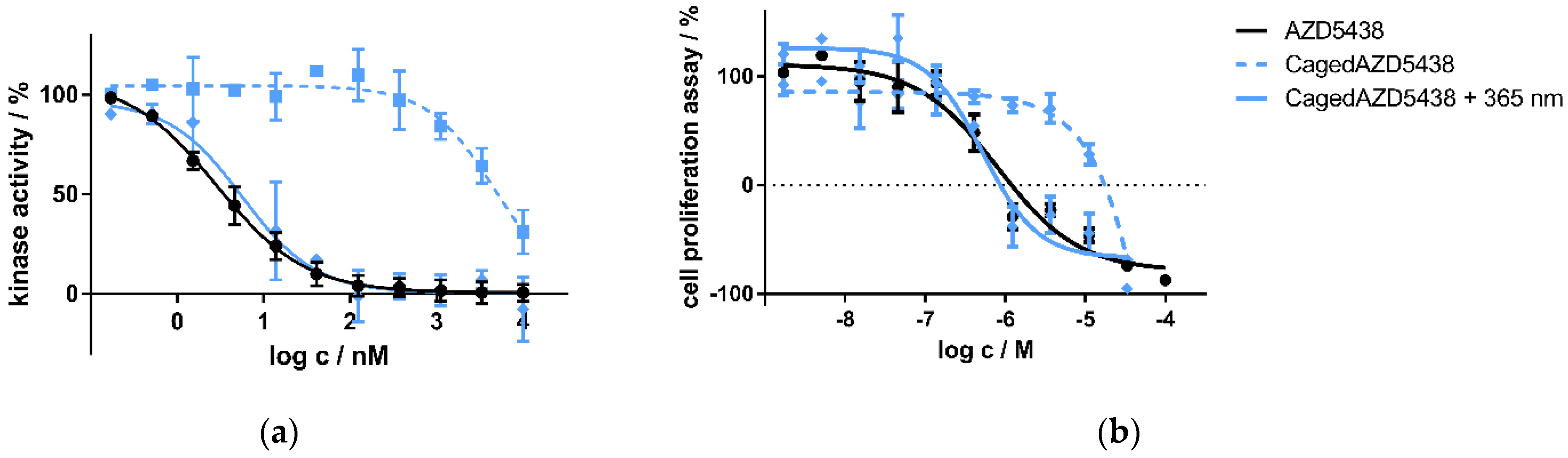
Figure 4.
(a) Kinase assay of caged and uncaged AZD5438 using the target kinase assay CDK2/cyclin E1 with and without UV irradiation. CDK2 in complex with cyclin E1 was incubated with the inhibitor AZD5438 as well as its caged prodrug cagedAZD5438 to determine kinase activity. In a second setup, 10 min after compound addition both assays were irradiated with UV light for two minutes (365 nm, 37.5 mW). Data represent mean ± SD from two independent experiments, each performed in triplicate. (b) Cell proliferation assay of the inhibitor AZD5438 and cagedAZD5438 with and without UV irradiation using Panc89 cells. UV irradiation was performed for 2 min, 365 nm, 37.5 mW. Cell proliferation was determined after 48 h of drug treatment. Data represent mean ± SEM from three independent experiments, each performed in quadruplicate.
2.3. 18Fluor
As discussed above, and in contrast to the other tested nuclides 68Ga and 90Y, in our settings, the CR signals generated by the decay of 18F were at the baseline level of cosmic ray signals in the KK experiments. However, 18F was already reported to be used for activating photoresponsive compounds in a photodynamic therapy approach using sensitizers to be activated by CR produced by 18F [24]. Furthermore, in 2012, Ran et al. showed that CR induced by 18F mediates uncaging. The authors demonstrated in a proof-of-concept study that a caged derivative of luciferin was uncaged by 18Fluorodeoxyglucose (18FDG, used for positron emission tomography (PET) in the clinic) in a mouse breast cancer model [23]. Conceptually, the decay of 18F showing 96.7% positron emission represents a borderline case with a maximum decay energy of 0.63 MeV and a mean energy of 0.25 MeV, which is very close to the threshold value of 0.26 MeV for the generation of CR [22,36]. However, we incubated cagedAZD5438 with 18F and 18FDG (20–300 MBq/mL) [12,13,61] for 24 h each, and both the caged and the uncaged species were quantified by HPLC analysis at intervals of 15 min (Table 2). Since cagedAZD5438 has a low solubility in water, we decided to use either DMSO or MeOH as the solvent. In line with the KK results showing no significant generation of CR, under the applied conditions, we observed no uncaging of cagedAZD5438 during incubation with the beta emitter 18F for up to 12 h (Figure 5a). In negative controls, the free inhibitor proved to be stable during irradiation (Figure S9a). In order to provide control experiments following the 24-h incubation time, we irradiated the 18F samples containing cagedAZD5438 with UV light of 365 nm. Here, strong photoactivation of cagedAZD5438 was demonstrated, suggesting the CR produced by the decay of 18F is not sufficient for the uncaging process.

Table 2.
Trials from cagedAZD5438 activation experiments using 18F and 18FDG as the radioactive source. As a solubilizer, either DMSO or MeOH was added. Each sample was analyzed by HPLC every 15 min for up to 24 h in two independent experiments. Following incubation with 18F and 18FDG showing no uncaging of cagedAZD5438, samples 1–5 were irradiated with UV light, yielding AZD5438 (positive controls).
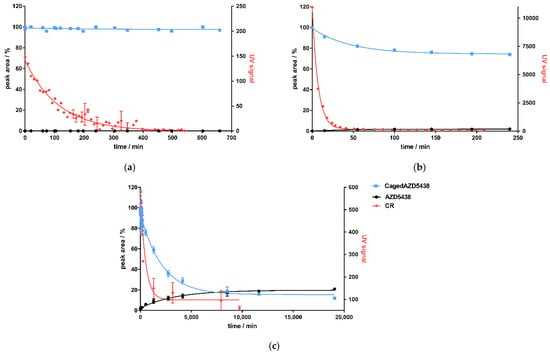
Figure 5.
Correlation of CR detected by the KK method as UV signal (red dots) and uncaging of cagedAZD5438 (blue line) by HPLC analysis to yield uncaged AZD5438 (black) for 3 isotopes: (a) 18Fluor, (b) 68Gallium, and (c) 90Yttrium. All curves are fitted with one-phase decay.
2.4. 68Gallium
Like 18F, 68Ga is a beta plus emitter showing 87.7% positron emission. However, the decay of 68Ga produced a significantly higher maximum decay energy of 1.9 MeV, and 68Ga had an Emean level of 0.84 MeV, considerably above the threshold of 0.26 MeV to generate CR. Thus, we incubated freshly prepared 68Ga samples ranging from 300 to 900 MBq with a solution of 50 µM cagedAZD5438 to investigate its photoactivation by HPLC (Table 3). Due to its short half-life, 68Ga was generated in situ in the lab and could be eluted directly from its parent isotope 68Ger using 1M HCl (68Ga generator).

Table 3.
Incubation of cagedAZD5438 with the beta plus emitter 68Ga under varying conditions (samples 1–6; in samples 3 and 4, the acidic pH was buffered with ammonium acetate and HEPES). Each sample was analyzed twice. As positive controls, after incubation with 68Ga, samples 1–6 were irradiated with UV light, showing uncaging of cagedAZD5438. As a negative control, a solution of the non-radioactive 69Ga was employed (sample 6). Incubating a solution of AZD5438 under the respective conditions used for samples 1–6 proved the inhibitor AZD5438 to be stable (Figure S9b).
Correlating to the detection of CR produced by 68Ga, the amount of cagedAZD5438 in the samples decreased to approximately 75%, reaching a plateau at 149 min (Figure 5b). A non-quantitative uncaging was already observed by the photochemical characterization of cagedAZD5438, yielding only ca. 75% AZD5438 (Figure 3c). However, during incubation of the samples with 68Ga, the concentration of AZD5438 did not increase correspondingly. In fact, the HPLC analysis showed a rather complex product mixture, suggesting an alternative degradation reaction by strong CR irradiation, by the emitted positrons, or by radical processes. Assays with a solution of AZD5438 incubated with 68Ga did not indicate any destruction (Figure S9b). In contrast, we detected a partial destruction of cagedAZD5438 in the UV experiments (Figure 3c), which, according to the ESR experiments (Figure 3d,e), belongs to radical processes. However, direct ESR experiments employing 68Ga were not possible due to the specific handling requirements of the radioactive samples. For the 68Ga experiments, although we could demonstrate production of CR, we observed no release of AZD5438 from cagedAZD5438. Instead, a 25% decrease in cagedAZD5438 was detected, reaching a plateau at 149.3 min for CR (produced by the 68Ga decay) and at 148.8 min for the decomposition of cagedAZD5438, showing a strong correlation (Figure 5b). Thus, these findings suggest an uncaging reaction mediated by the CR but also compound decomposition by positrons generated by the decay of 68Ga.
2.5. 90Yttrium
In contrast to 18F and 68Ga as positron emitters being used in radiopharmaceutical diagnostics, 90Y is involved in radiotherapy (Table 1). The radionuclide 90Y is a pure β-emitter possessing a decay energy of Emean = 2.28 MeV and a half-life time of 64 h. We aimed to investigate whether uncaging of cagedAZD5438 can be achieved by the decay of 90Y. Therefore, we incubated a 50 μM solution of cagedAZD5438 with a solution of 90Y showing 419 MBq containing 20% DMSO in a total volume of 1.25 mL (Table 4). As a negative control, we used the uncaged AZD5438 accordingly (Figure S9c), and as positive controls, samples of cagedAZD5438 were irradiated with UV light of 365 nm. The samples of cagedAZD5438 incubated with 90Y showed an uncaging reaction producing 21% AZD5438, reaching a plateau after 7 d (Figure 5c).

Table 4.
Activation of cagedAZD5438 upon incubation with the beta minus emitter 90Y (N = 3). As positive controls, samples of cagedAZD5438 were irradiated with UV light, proving quantitative uncaging.
In contrast to the experiments with 68Ga and 18F, a release of 21% AZD5438 resulted by incubating cagedAZD5438 with the β-emitter 90Y over a period of approx. 14 days (Figure 5c). However, besides the CR-mediated uncaging of cagedAZD5438 and similar to the 68Ga experiments (Figure 5b), a significant compound decomposition also occurred, which may be dependent on the β-decay of 90Y.
3. Conclusions
In this study, we verified experimentally using the KK method that the decay of the clinically applied beta emitters 68Ga and 90Y generates CR in an aqueous solution. A direct comparison of the main decay energies of the investigated nuclides showed that 18F with 0.25 MeV releases significantly less energy than 68Ga with 0.84 MeV and 90Y with 0.93 MeV. We next employed the novel photoresponsive CDK2 inhibitor prodrug cagedAZD5438 as a model system to investigate whether the CR was able to uncage the prodrug when incubated together with the radionuclides. In correlation to the minor CR produced by the β+ emitter 18F, no uncaging could be determined for cagedAZD5438. Incubation of cagedAZD5438 with the β+ emitter 68Ga partly resulted in a complex product mixture. Furthermore, incubation of cagedAZD5438 with the β-emitter 90Y yielded 21% AZD5438 but also indicated a compound decomposition towards a complex mixture. These findings suggest photoactivation of the photoresponsive model compound cagedAZD5438 by CR but also compound decomposition by the β+/β− irradiation produced by the decay of 68Ga and 90Y, respectively. Based on this proof-of-concept study, an optimized design of photoresponsive compounds could use CR for prodrug activation while providing stability against the β+/β− irradiation. Thus, CR induced by therapeutic radiopharmaceuticals such as 90Y could offer a possibility for a synergistic combination of radiotherapy with targeted photopharmacology in future applications.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22169010/s1.
Author Contributions
Conceptualization, C.P.; methodology, M.K. and A.D.; software, M.K.; validation, M.K., A.D., and T.R.; formal analysis, M.K., A.D., and T.R.; investigation, M.K., A.D., Y.Z., and T.R.; resources, C.P., U.L., M.K., A.D., and T.R.; writing—original draft preparation, M.K.; writing—review and editing, C.P. and M.Z.; visualization, M.K.; supervision, C.P., M.Z., and U.L.; project administration, M.K.; funding acquisition, C.P. and U.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deutsche Forschungsgemeinschaft (DFG), grant number PE1605_2_2. We acknowledge financial support by DFG within the funding programme “Open Access Publizieren”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Huggins, D.J.; Sherman, W.; Tidor, B. Rational Approaches to Improving Selectivity in Drug Design. J. Med. Chem. 2012, 55, 1424–1444. [Google Scholar] [CrossRef]
- Mayer, G.; Heckel, A. Biologically Active Molecules with a “Light Switch”. Angew. Chem. Int. Ed. 2006, 45, 4900–4921. [Google Scholar] [CrossRef] [PubMed]
- Klán, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2012, 113, 119–191. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Davies, G.C.R. Caged compounds: Photorelease technology for control of cellular chemistry and physiology. Nat. Methods 2007, 4, 619–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Gentile, M. Cell cycle arrest and apoptosis provoked by UV radiation-induced DNA damage are transcriptionally highly divergent responses. Nucleic Acids Res. 2003, 31, 4779–4790. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.G.; Bullen, A. Practical intravital two-photon microscopy for immunological research: Faster, brighter, deeper. Immunol. Cell Biol. 2010, 88, 438–444. [Google Scholar] [CrossRef]
- Sternberg, E.D.; Dolphin, D.; Brückner, C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Vomasta, D.; Högner, C.; Branda, N.R.; König, B. Regulation of human carbonic anhydrase I (hCAI) activity by using a photochromic inhibitor. Angew. Chem. Int. Ed. 2008, 47, 7644–7647. [Google Scholar] [CrossRef]
- Morckel, A.R.; Lusic, H.; Farzana, L.; Yoder, J.A.; Deiters, A.; Nascone-Yoder, N.M. A photoactivatable small-molecule inhibitor for light-controlled spatiotemporal regulation of Rho kinase in live embryos. Development 2012, 139, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Zindler, M.; Pinchuk, B.; Renn, C.; Horbert, R.; Döbber, A.; Peifer, C. Design, Synthesis, and Characterization of a Photoactivatable Caged Prodrug of Imatinib. ChemMedChem 2015, 10, 1335–1338. [Google Scholar] [CrossRef]
- Bliman, D.; Nilsson, J.R.; Kettunen, P.; Andréasson, J.; Grøtli, M. A Caged Ret Kinase Inhibitor and its Effect on Motoneuron Development in Zebrafish Embryos. Sci. Rep. 2015, 5, 13109. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.; Nilsson, J.R.; Solano, C.; Andréasson, J.; Grøtli, M. Design, Synthesis and Inhibitory Activity of Photoswitchable RET Kinase Inhibitors. Sci. Rep. 2015, 5, 9769. [Google Scholar] [CrossRef] [Green Version]
- Frank, J.A.; Yushchenko, D.A.; Hodson, D.J.; Lipstein, N.; Nagpal, J.; Rutter, G.A.; Rhee, J.-S.; Gottschalk, A.; Brose, N.; Schultz, C.; et al. Photoswitchable diacylglycerols enable optical control of protein kinase C. Nat. Chem. Biol. 2016, 12, 755–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.; Li, J.W.; Branda, N.R. Visible-Light-Triggered Activation of a Protein Kinase Inhibitor. ChemMedChem 2017, 12, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Rodat, T.; Heintze, L.; Weber, J.; Horbert, R.; Girreser, U.; Raeker, T.; Bußmann, L.; Kriegs, M.; Hartke, B.; et al. Axitinib: A Photoswitchable Approved Tyrosine Kinase Inhibitor. ChemMedChem 2018, 13, 2415–2426. [Google Scholar] [CrossRef]
- Goegan, B.; Terzi, F.; Bolze, F.; Cambridge, S.; Specht, A. Synthesis and Characterization of Photoactivatable Doxycycline Analogues Bearing Two-Photon-Sensitive Photoremovable Groups Suitable for Light-Induced Gene Expression. ChemBioChem 2018, 19, 1341–1348. [Google Scholar] [CrossRef]
- Hoorens, M.W.H.; Ourailidou, M.E.; Rodat, T.; van der Wouden, P.E.; Kobauri, P.; Kriegs, M.; Peifer, C.; Feringa, B.L.; Dekker, F.J.; Szymanski, W. Light-controlled inhibition of BRAFV600E kinase. Eur. J. Med. Chem. 2019, 179, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Trads, J.B.; Hüll, K.; Matsuura, B.S.; Laprell, L.; Fehrentz, T.; Görldt, N.; Kozek, K.A.; Weaver, C.D.; Klöcker, N.; Barber, D.M.; et al. Sign Inversion in Photopharmacology: Incorporation of Cyclic Azobenzenes in Photoswitchable Potassium Channel Blockers and Openers. Angew. Chem. Int. Ed. 2019, 58, 15421–15428. [Google Scholar] [CrossRef] [PubMed]
- Jelley, J.V. British Journal of Applied Physics Cerenkov radiation and its applications. Br. J. Appl. Phys. 1955, 6, 227. [Google Scholar] [CrossRef]
- Elrick, R.H.; Parker, R.P. The use of Cerenkov radiation in the measurement of β-emitting radionuclides. Int. J. Appl. Radiat. Isot. 1968, 19, 263–271. [Google Scholar] [CrossRef]
- Ran, C.; Zhang, Z.; Hooker, J.; Moore, A. In vivo photoactivation without "light": Use of Cherenkov radiation to overcome the penetration limit of light. Mol. Imaging Biol. 2012, 14, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Bulin, A.-L.; Truillet, C.; Chouikrat, R.; Lux, F.; Frochot, C.; Amans, D.; Ledoux, G.; Tillement, O.; Perriat, P.; Barberi-Heyob, M.; et al. X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. J. Phys. Chem. C 2013, 117, 21583–21589. [Google Scholar] [CrossRef]
- Lioret, V.; Bellaye, P.-S.; Arnould, C.; Collin, B.; Decréau, R.A. Dual Cherenkov Radiation-Induced Near-Infrared Luminescence Imaging and Photodynamic Therapy toward Tumor Resection. J. Med. Chem. 2020, 63, 9446–9456. [Google Scholar] [CrossRef] [PubMed]
- Cline, B.; Delahunty, I.; Xie, J. Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1541. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.B. Determination of specific activity of 32P-labeled compounds using Cerenkov counting. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1971, 12, 14–16. [Google Scholar]
- Al-Masri, M.S. Cerenkov counting technique. J. Radioanal. Nucl. Chem. Artic. 1996, 207, 205–213. [Google Scholar] [CrossRef]
- Rowińska, L.; Jaskólska, H.; Radwan, M. Aktivitätsbestimmung von Phosphor-32 mit Hilfe der Tscherenkow -Strahlung. Isot. Isot. Environ. Health Stud. 2008, 11, 223–225. [Google Scholar] [CrossRef]
- Axelsson, J.; Davis, S.C.; Gladstone, D.J.; Pogue, B.W. Cerenkov emission induced by external beam radiation stimulates molecular fluorescence. Med. Phys. 2011, 38, 4127–4132. [Google Scholar] [CrossRef] [Green Version]
- Compton, A.H. A Quantum Theory of the Scattering of X-rays by Light Elements. Phys. Rev. 1923, 21, 483–502. [Google Scholar] [CrossRef]
- Dinesh Mayani, D. Proton therapy for cancer treatment. J. Oncol. Pharm. Pract. 2010, 17, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Tanha, K.; Pashazadeh, A.M.; Pogue, B.W. Review of biomedical Čerenkov luminescence imaging applications. Biomed. Opt. Express 2015, 6, 3053. [Google Scholar] [CrossRef] [Green Version]
- Volkert, W.A.; Hoffman, T.J. Therapeutic Radiopharmaceuticals. Chem. Rev. 1999, 99, 2269–2292. [Google Scholar] [CrossRef]
- Livechart-Table of Nuclides-Nuclear Structure and Decay Data. Available online: https://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html#dcy1 (accessed on 20 May 2021).
- Montgomery, C.W. Electron capture. In Geochemistry; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 189–190. [Google Scholar]
- Baum, R.P. Nuklearmedizinische Diagnostik und Therapiekontrolle neuroendokriner Tumore (NET) mittels Rezeptor-PET/CT und Szintigraphie. Fortschr. Röntgenstr 2005, 177. [Google Scholar] [CrossRef]
- Krause, B.J.; Beyer, T.; Bockisch, A.; Delbeke, D.; Kotzerke, J.; Minkov, V.; Reiser, M.; Willich, N. FDG-PET/CT in der Onkologie. Leitlinie. Nukl. Nucl. Med. 2007, 46, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Biersack, H.-J.; Ezziddin, S.; Risse, J.; Bender, H.; Palmedo, H. Therapie mit Radioisotopen in der Onkologie. Palliative und kurative Ansätze. Schmerz 2005, 19, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Thieme. Nuklearmedizin: 122 Tabellen; Büll, U., Bares, R., Eds.; Thieme: Stuttgart, Germany, 2001. [Google Scholar]
- Haddad, T.; Fard-Esfahani, A.; Vali, R. A review of pediatric neuroendocrine tumors, their detection, and treatment by radioisotopes. Nucl. Med. Commun. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Bahrami-Samani, A.; Anvari, A.; Jalilian, A.R.; Shirvani-Arani, S.; Yousefnia, H.; Aghamiri, M.R.; Ghannadi-Maragheh, M. Production, Quality Control and Pharmacokinetic Studies of (177)Lu-EDTMP for Human Bone Pain Palliation Therapy Trials. Iran. J. Pharm. Res. 2012, 11, 137–144. [Google Scholar] [PubMed]
- Kessel, K.; Seifert, R.; Weckesser, M.; Roll, W.; Humberg, V.; Schlack, K.; Bögemann, M.; Bernemann, C.; Rahbar, K. Molecular analysis of circulating tumor cells of metastatic castration-resistant Prostate Cancer Patients receiving 177Lu-PSMA-617 Radioligand Therapy. Theranostics 2020, 10, 7645–7655. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Mödder, G. Radionuclide therapy of inflammatory joint diseases. Nucl. Med. Commun. 2002, 23, 829–831. [Google Scholar] [CrossRef]
- Bort, G.; Gallavardin, T.; Ogden, D.; Dalko, P.I. From One-Photon to Two-Photon Probes: “Caged” Compounds, Actuators, and Photoswitches. Angew. Chem. Int. Ed. 2013, 52, 4526–4537. [Google Scholar] [CrossRef]
- Corrie, J.E.T.; Furuta, T.; Givens, R.; Yousef, A.L.; Goeldner, M. Photoremovable Protecting Groups Used for the Caging of Biomolecules. In Dynamic Studies in Biology: Phototriggers, Photoswitches and Caged Biomolecules; Goeldner, M., Givens, R., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 1–94. [Google Scholar]
- Šolomek, T.; Mercier, S.; Bally, T.; Bochet, C.G. Photolysis of ortho-nitrobenzylic derivatives: The importance of the leaving group. Photochem. Photobiol. Sci. 2012, 11, 548. [Google Scholar] [CrossRef] [Green Version]
- Shchemelinin, I.; Sefc, L.; Necas, E. Protein kinases, their function and implication in cancer and other diseases. Folia Biol. 2006, 52, 81–100. [Google Scholar]
- Schwartz, P.A.; Murray, B.W. Protein kinase biochemistry and drug discovery. Bioorganic Chem. 2011, 39, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, P.M. Approved and Experimental Small-Molecule Oncology Kinase Inhibitor Drugs: A Mid-2016 Overview. Med. Res. Rev. 2017, 37, 314–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byth, K.F.; Thomas, A.; Hughes, G.; Forder, C.; McGregor, A.; Geh, C.; Oakes, S.; Green, C.; Walker, M.; Newcombe, N.; et al. AZD5438, a potent oral inhibitor of cyclin-dependent kinases 1, 2, and 9, leads to pharmacodynamic changes and potent antitumor effects in human tumor xenografts. Mol. Cancer Ther. 2009, 8, 1856–1866. [Google Scholar] [CrossRef] [Green Version]
- Super-Kamiokande Official Website. Available online: http://www-sk.icrr.u-tokyo.ac.jp/sk/index-e.html (accessed on 26 July 2021).
- Buschendorf, F. Messung der Kosmischen Strahlung—Myonenlebensdauer und-Rate; Gymnasium der Stadt Rahden: Rahden, Germany, 2012. [Google Scholar]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The luminescence of irradiated transparent media and the Čerenkov effect-I. The luminescence of aqueous solutions of radioactive isotopes. In Proceedings of the Royal Society of London—Series A: Mathematical and Physical Sciences Website; Royal Society: London, UK, 1997; Volume 216, pp. 90–102.
- Mougeot, X. Erratum: Reliability of usual assumptions in the calculation of β and ν spectra. Phys. Rev. C 2015, 92. [Google Scholar] [CrossRef] [Green Version]
- Mougeot, X. Reliability of usual assumptions in the calculation of β and ν spectra. Phys. Rev. C 2015, 91, 455. [Google Scholar] [CrossRef] [Green Version]
- Kirschner, S.; Döbber, A.; Krebs, M.; Witt, C.; Hartke, B.; Peifer, C. The Impact of Electronic Effects on Photolysis: A Model Study on the 4,5-Dimethoxy-2-nitrobenzyl Caged N-Phenylpyrimidine-2-amine Scaffold. ChemPhotoChem 2020, 4, 638–643. [Google Scholar] [CrossRef]
- Rodat, T.; Krebs, M.; Döbber, A.; Jansen, B.; Steffen-Heins, A.; Schwarz, K.; Peifer, C. Restricted suitability of BODIPY for caging in biological applications based on singlet oxygen generation. Photochem. Photobiol. Sci. 2020, 19, 1319–1325. [Google Scholar] [CrossRef]
- Alegrı́a, A.E.; Ferrer, A.; Santiago, G.; Sepúlveda, E.; Flores, W. Photochemistry of water-soluble quinones. Production of the hydroxyl radical, singlet oxygen and the superoxide ion. J. Photochem. Photobiol. A Chem. 1999, 127, 57–65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).