The Role of Biomarkers in Atherothrombotic Stroke—A Systematic Review
Abstract
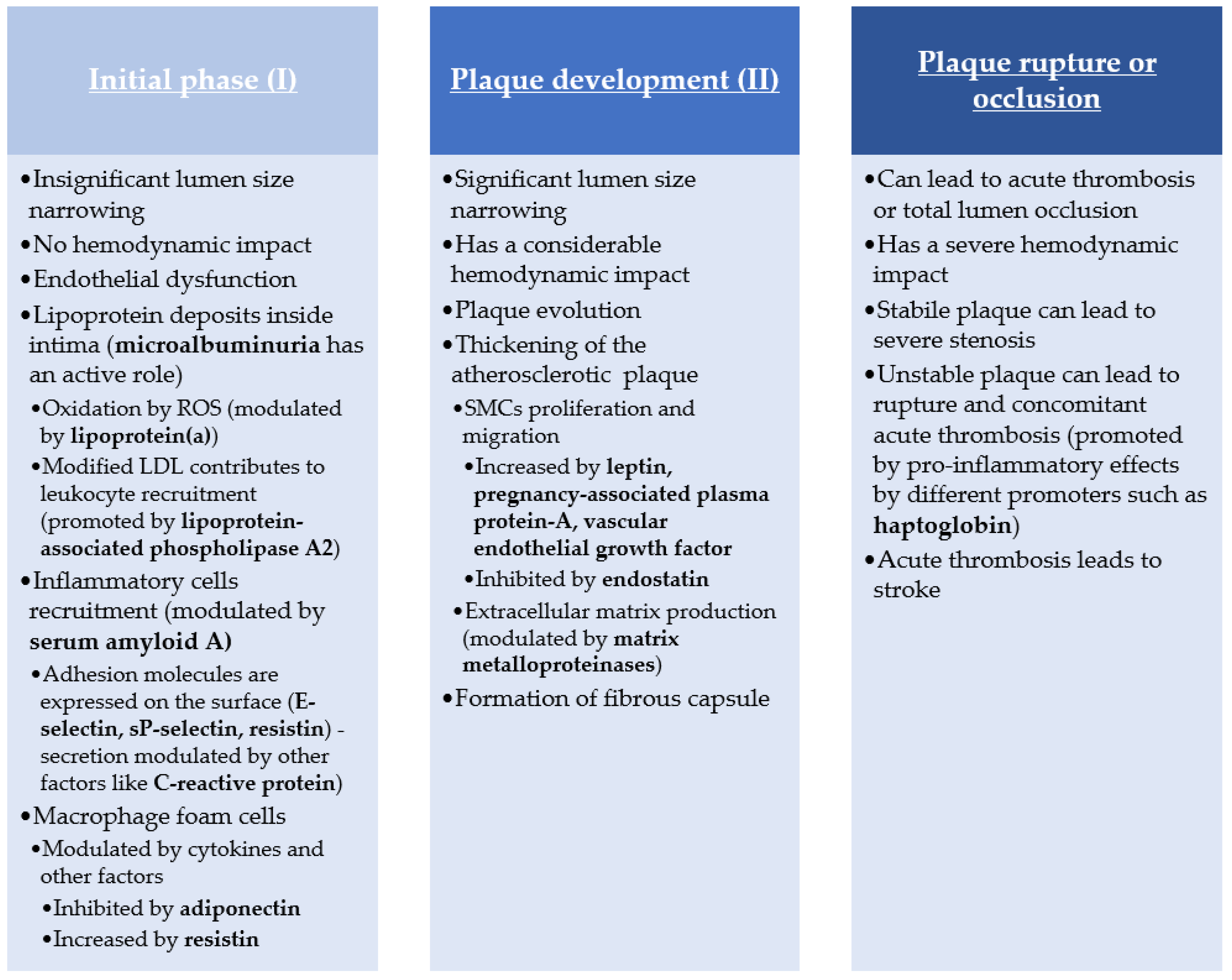
1. Introduction
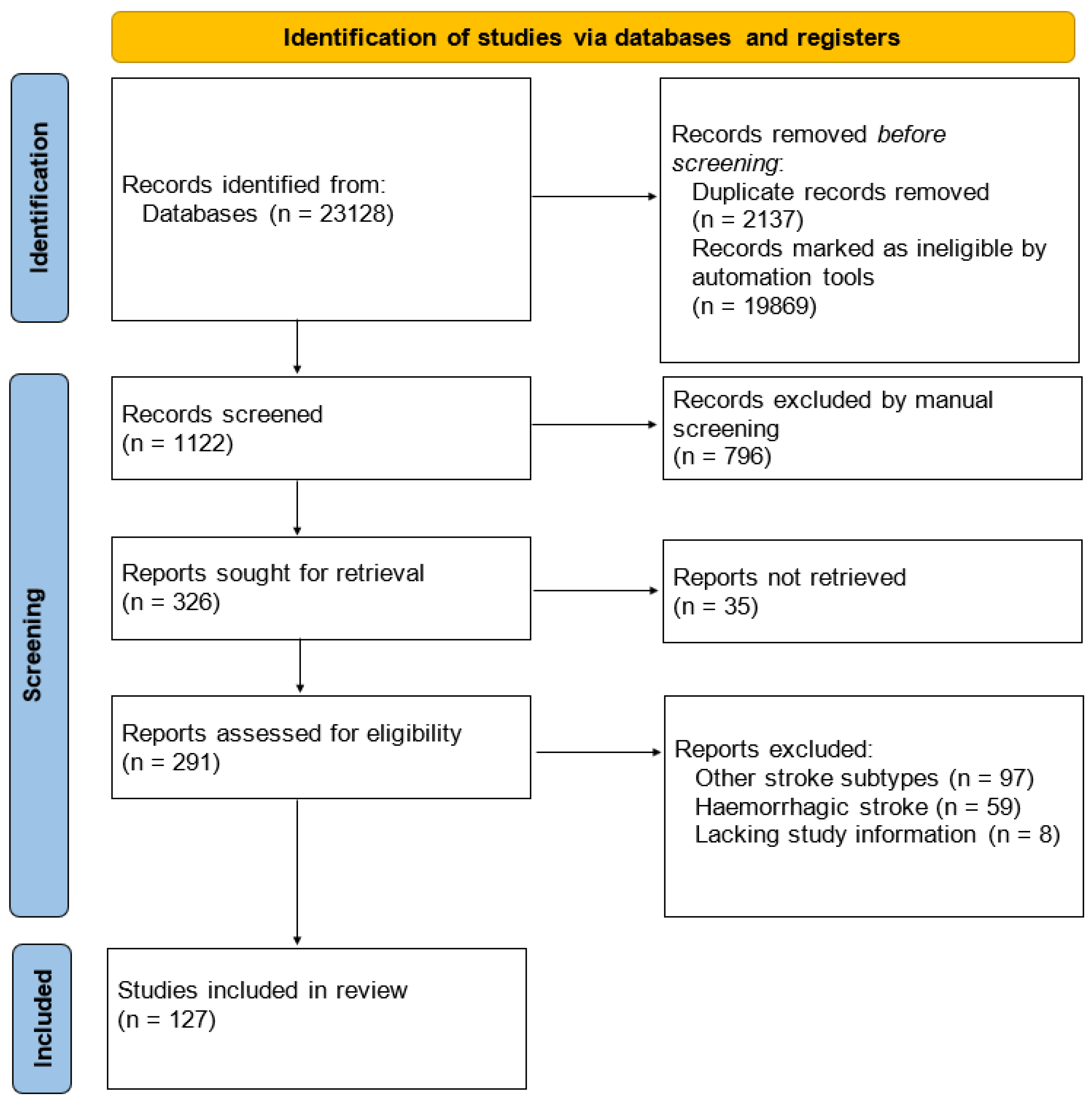
2. Materials and Methods
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Henderson, R.; Hobbie, J.; Landrigan, P.; Mattisoti, D.; Perera, F.; Pfttaer, E.; Silbergeld, E.; Wogan, G. Biological Markers in Environmental Health Research. Environ. Health Perspect. 1987, 7, 3–9. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T. Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Slikker, W. Biomarkers and Their Impact on Precision Medicine. Exp. Biol. Med. 2018, 243, 211. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S. Stroke in the Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Gisterå, A.; Hansson, G.K. The Immunology of Atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The Immune System in Atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic Mechanisms Following Ischemic Stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Bernardo-Castro, S.; Sousa, J.A.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of Blood–Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Front. Neurol. 2020, 11, 1605. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Montellano, F.A.; Ungethüm, K.; Ramiro, L.; Nacu, A.; Hellwig, S.; Fluri, F.; Whiteley, W.N.; Bustamante, A.; Montaner, J.; Heuschmann, P.U. Role of Blood-Based Biomarkers in Ischemic Stroke Prognosis. Stroke 2021, 52, 543–551. [Google Scholar] [CrossRef]
- Bennett, M.R.; Devarajan, P. Characteristics of an Ideal Biomarker of Kidney Diseases. In Biomarkers of Kidney Disease; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–24. [Google Scholar] [CrossRef]
- Soeki, T.; Sata, M. Inflammatory Biomarkers and Atherosclerosis. Int. Heart J. 2016, 57, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Wakugawa, Y.; Kiyohara, Y.; Tanizaki, Y.; Kubo, M.; Ninomiya, T.; Hata, J.; Doi, Y.; Okubo, K.; Oishi, Y.; Shikata, K.; et al. C-Reactive Protein and Risk of First-Ever Ischemic and Hemorrhagic Stroke in a General Japanese Population: The Hisayama Study. Stroke 2006, 37, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, W.; Jackson, C.; Lewis, S.; Lowe, G.; Rumley, A.; Sandercock, P.; Wardlaw, J.; Dennis, M.; Sudlow, C. Inflammatory Markers and Poor Outcome after Stroke: A Prospective Cohort Study and Systematic Review of Interleukin-6. PLoS Med. 2009, 6, e1000145. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Kral, B.G.; Blumenthal, R.S.; Fuster, V.; Campbell, C.Y.; Gluckman, T.J.; Lange, R.A.; Topol, E.J.; Willerson, J.T.; Desai, M.Y.; et al. The Use of High-Sensitivity Assays for C-Reactive Protein in Clinical Practice. Nat. Rev. Cardiol 2008, 5, 621–635. [Google Scholar] [CrossRef]
- Andersson, J.; Johansson, L.; Ladenvall, P.; Wiklund, P.-G.; Stegmayr, B.; Jern, C.; Boman, K. C-Reactive Protein Is a Determinant of First-Ever Stroke: Prospective Nested Case-Referent Study. Cereb. Dis. 2009, 27, 544–551. [Google Scholar] [CrossRef]
- Rost, N.S.; Wolf, P.A.; Kase, C.S.; Kelly-Hayes, M.; Silbershatz, H.; Massaro, J.M.; D’Agostino, R.B.; Franzblau, C.; Wilson, P.W.F. Plasma Concentration of C-Reactive Protein and Risk of Ischemic Stroke and Transient Ischemic Attack: The Framingham Study. Stroke 2001, 32, 2575–2579. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-K.; Yen, C.-J.; Chang, C.-H.; Kuo, C.-K.; Chen, J.-H.; Sorond, F. Relation of C-Reactive Protein to Stroke, Cognitive Disorders, and Depression in the General Population: Systematic Review and Meta-Analysis. Lancet Neurol. 2005, 4, 371–380. [Google Scholar] [CrossRef]
- Cheng, L.-S.; Tu, W.-J.; Shen, Y.; Zhang, L.-J.; Ji, K. Combination of High-Sensitivity C-Reactive Protein and Homocysteine Predicts the Post-Stroke Depression in Patients with Ischemic Stroke. Mol. Neurobiol. 2018, 55, 2952–2958. [Google Scholar] [CrossRef]
- Winbeck, K.; Poppert, H.; Etgen, T.; Conrad, B.; Sander, D. Prognostic Relevance of Early Serial C-Reactive Protein Measurements After First Ischemic Stroke. Stroke 2002, 33, 2459–2464. [Google Scholar] [CrossRef]
- Geng, H.-H.; Wang, X.-W.; Fu, R.-L.; Jing, M.-J.; Huang, L.-L.; Zhang, Q.; Wang, X.-X.; Wang, P.-X. The Relationship between C-Reactive Protein Level and Discharge Outcome in Patients with Acute Ischemic Stroke. IJERPH 2016, 13, 636. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Papa, F.; Bocola, V. C-Reactive Protein in Ischemic Stroke: An Independent Prognostic Factor. Stroke 2001, 32, 917–924. [Google Scholar] [CrossRef]
- Idicula, T.T.; Brogger, J.; Naess, H.; Waje-Andreassen, U.; Thomassen, L. Admission C—Reactive Protein after Acute Ischemic Stroke Is Associated with Stroke Severity and Mortality: The “Bergen Stroke Study”. BMC Neurol. 2009, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Shoaeb, M.A.; Shehata, M.A.; Taema, K.M.; Hammouda, M.A. CRP in Cerebrovascular Stroke: Prognostic Implications. Egypt. J. Crit. Care Med. 2014, 2, 43–52. [Google Scholar] [CrossRef]
- Muir, K.W.; Weir, C.J.; Alwan, W.; Squire, I.B.; Lees, K.R. C-Reactive Protein and Outcome after Ischemic Stroke. Stroke 1999, 30, 981–985. [Google Scholar] [CrossRef]
- Gussekloo, J.; Schaap, M.C.L.; Frölich, M.; Blauw, G.J.; Westendorp, R.G.J. C-Reactive Protein Is a Strong but Nonspecific Risk Factor of Fatal Stroke in Elderly Persons. ATVB 2000, 20, 1047–1051. [Google Scholar] [CrossRef]
- Krupinski, J.; Turu, M.M.; Slevin, M.; Martínez-González, J. Carotid Plaque, Stroke Pathogenesis, and CRP: Treatment of Ischemic Stroke. Curr. Treat. Opt. Cardio. Med. 2007, 9, 229–235. [Google Scholar] [CrossRef]
- Naifang, Y.; Zhenzhen, L.; Xuefeng, W.; Xiaoqian, X.; Wenman, W. Evaluation of Analytic and Clinical Performance of Thrombin–Antithrombin Complex and d-Dimer Assay in Prognosis of Acute Ischemic Stroke. Blood Coagul. Fibrinolysis 2020, 31, 303–309. [Google Scholar] [CrossRef]
- Fernandez-Cadenas, I.; Mendioroz, M.; Munuera, J.; Alvarez-Sabin, J.; Rovira, A.; Quiroga, A.; Corbeto, N.; Rubiera, M.; Delgado, P.; Rosell, A.; et al. Lower Concentrations of Thrombin-Antithrombin Complex (TAT) Correlate to Higher Recanalisation Rates among Ischaemic Stroke Patients Treated with t-PA. Thromb. Haemost. 2009, 102, 759–764. [Google Scholar] [CrossRef]
- Wu, G.; Quek, A.J.; Caradoc-Davies, T.T.; Ekkel, S.M.; Mazzitelli, B.; Whisstock, J.C.; Law, R.H.P. Structural Studies of Plasmin Inhibition. Biochem. Soc. Trans. 2019, 47, 541–557. [Google Scholar] [CrossRef]
- Lasierra, J.; Aza, M.J.; Viladés, E.; Poblet, S.; Barrao, F.; Bayon, E.; Gonzalez, J. Tissue Plasminogen Activator and Plasminogen Activator Inhibitor in Patients with Liver Cirrhosis. Fibrinolysis 1991, 5, 117–120. [Google Scholar] [CrossRef]
- Reed, G.L.; Houng, A.K.; Singh, S.; Wang, D. A2-Antiplasmin: New Insights and Opportunities for Ischemic Stroke. Semin. Thromb. Hemost. 2017, 43, 191–199. [Google Scholar] [CrossRef]
- Marti-Fabregas, J.; Borrell, M.; Cocho, D.; Belvis, R.; Castellanos, M.; Montaner, J.; Pagonabarraga, J.; Aleu, A.; Molina-Porcel, L.; Diaz-Manera, J.; et al. Hemostatic Markers of Recanalization in Patients with Ischemic Stroke Treated with Rt-PA. Neurology 2005, 65, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; De Mol, M.; Van Hoef, B.; Verstreken, M.; Collen, D. Depletion of Circulating A2-Antiplasmin by Intravenous Plasmin or Immunoneutralization Reduces Focal Cerebral Ischemic Injury in the Absence of Arterial Recanalization. Blood 2001, 97, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zan, X.; Xie, Z.; Li, Y.; Lin, S.; Li, H.; You, C. Association Between Plasminogen Activator Inhibitor-1 Genetic Polymorphisms and Stroke Susceptibility. Mol. Neurobiol. 2017, 54, 328–341. [Google Scholar] [CrossRef]
- Chen, R.; Yan, J.; Liu, P.; Wang, Z.; Wang, C. Plasminogen Activator Inhibitor Links Obesity and Thrombotic Cerebrovascular Diseases: The Roles of PAI-1 and Obesity on Stroke. Metab. Brain Dis. 2017, 32, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Dušanović Pjević, M.; Beslac Bumbaširevic, L.; Vojvodic, L.; Grk, M.; Maksimović, N.; Damnjanović, T.; Novaković, I.; Kačar, K.; Pesic, M.; Perovic, D.; et al. Analysis of the Association Between Polymorphisms within PAI-1 and ACE Genes and Ischemic Stroke Outcome After Rt-PA Therapy. J. Pharm. Pharm. Sci. 2019, 22, 142–149. [Google Scholar] [CrossRef]
- Szegedi, I.; Nagy, A.; Székely, E.G.; Czuriga-Kovács, K.R.; Sarkady, F.; Lánczi, L.I.; Berényi, E.; Csiba, L.; Bagoly, Z. PAI-1 5G/5G Genotype Is an Independent Risk of Intracranial Hemorrhage in Post-lysis Stroke Patients. Ann. Clin. Transl. Neurol. 2019, 6, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.-L.; Bishop, N.; Li, Z.; Cipolla, M.J. Inhibition of PAI (Plasminogen Activator Inhibitor)-1 Improves Brain Collateral Perfusion and Injury after Acute Ischemic Stroke in Aged Hypertensive Rats. Stroke 2018, 49, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Buring, J.E.; Rifai, N. Soluble P-Selectin and the Risk of Future Cardiovascular Events. Circulation 2001, 103, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Frenette, P.S.; Wagner, D.D. Adhesion Molecules. N. Engl. J. Med. 1996, 334, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.; Lip, G.; Blann, A. Platelet Morphology, Soluble P Selectin and Platelet P-Selectin in Acute Ischaemic Stroke: The West Birmingham Stroke Project. Thromb. Haemost. 2004, 92, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, W.; Bai, S. Association between Plasma Soluble P-Selectin Elements and Progressive Ischemic Stroke. Exp. Ther. Med. 2013, 5, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Pawelczyk, M.; Chmielewski, H.; Kaczorowska, B.; Przybyła, M.; Baj, Z. The Influence of Statin Therapy on Platelet Activity Markers in Hyperlipidemic Patients after Ischemic Stroke. Arch. Med Sci. AMS 2015, 1, 115–121. [Google Scholar] [CrossRef]
- Collins, T.; Williams, A.; Johnston, G.I.; Kim, J.; Eddy, R.; Shows, T.; Gimbrone, M.A.; Bevilacqua, M.P. Structure and Chromosomal Location of the Gene for Endothelial-Leukocyte Adhesion Molecule 1. J. Biol. Chem. 1991, 266, 2466–2473. [Google Scholar] [CrossRef]
- Huang, J.; Choudhri, T.F.; Winfree, C.J.; McTaggart, R.A.; Kiss, S.; Mocco, J.; Kim, L.J.; Protopsaltis, T.S.; Zhang, Y.; Pinsky, D.J.; et al. Postischemic Cerebrovascular E-Selectin Expression Mediates Tissue Injury in Murine Stroke. Stroke 2000, 31, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenberg, J.F.; Smeets, E.F.; Neefjes, J.J.; Shaffer, M.A.; Cinek, T.; Jeunhomme, T.M.; Ahern, T.J.; Buurman, W.A. E-Selectin and Intercellular Adhesion Molecule-1 Are Released by Activated Human Endothelial Cells in Vitro. Immunology 1992, 77, 543. [Google Scholar] [PubMed]
- Petrovic-Djergovic, D.; Goonewardena, S.N.; Pinsky, D.J. Inflammatory Disequilibrium in Stroke. Circ. Res. 2016, 119, 142–158. [Google Scholar] [CrossRef]
- Haidari, M.; Hajilooi, M.; Rafiei, A.R.; Rezaii, A.A.; Hoseinipanah, S.M. E-Selectin Genetic Variation as a Susceptibility Factor for Ischemic Stroke. Cereb. Dis. 2009, 28, 26–32. [Google Scholar] [CrossRef]
- Richard, S.; Lagerstedt, L.; Burkhard, P.R.; Debouverie, M.; Turck, N.; Sanchez, J.-C. E-Selectin and Vascular Cell Adhesion Molecule-1 as Biomarkers of 3-Month Outcome in Cerebrovascular Diseases. J. Inflamm. 2015, 12, 61. [Google Scholar] [CrossRef]
- Gairolla, J.; Kler, R.; Modi, M.; Khurana, D. Leptin and Adiponectin: Pathophysiological Role and Possible Therapeutic Target of Inflammation in Ischemic Stroke. Rev. Neurosci. 2017, 28, 295–306. [Google Scholar] [CrossRef]
- Gorgui, J.; Gasbarrino, K.; Georgakis, M.K.; Karalexi, M.A.; Nauche, B.; Petridou, E.T.; Daskalopoulou, S.S. Circulating Adiponectin Levels in Relation to Carotid Atherosclerotic Plaque Presence, Ischemic Stroke Risk, and Mortality: A Systematic Review and Meta-Analyses. Metabolism 2017, 69, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, N.; Susam, S.; Canpolat, O.; Belhan, O. The Emerging Role of Leptin, Adiponectin and Visfatin in Ischemic/Hemorrhagic Stroke. Br. J. Neurosurg. 2019, 33, 504–507. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Wang, Y.; Maimaitili, A.; Qin, H.; Dangmurenjiafu, G.; Wang, S. The Association between Serum Adiponectin and 3-Month Outcome after Ischemic Stroke. Cardiovasc. Diabetol. 2019, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, S.P.; Tsioulos, D.I.; Tsiakou, A.G.; Gratsias, Y.E.; Pefanis, A.V.; Mountokalakis, T.D. Plasma Adiponectin Levels and Five-Year Survival After First-Ever Ischemic Stroke. Stroke 2005, 36, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Scotece, M.; Gómez, R.; López, V.; Gómez-Reino, J.J.; Lago, F.; Gualillo, O. Adipokines: Biofactors from White Adipose Tissue. A Complex Hub among Inflammation, Metabolism, and Immunity. BioFactors 2011, 37, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Frodermann, V.; Rohde, D.; Courties, G.; Severe, N.; Schloss, M.J.; Amatullah, H.; McAlpine, C.S.; Cremer, S.; Hoyer, F.F.; Ji, F.; et al. Exercise Reduces Inflammatory Cell Production and Cardiovascular Inflammation via Instruction of Hematopoietic Progenitor Cells. Nat. Med. 2019, 25, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Whincup, P.H.; Lennon, L.; Sattar, N. Adiposity, Adipokines, and Risk of Incident Stroke in Older Men. Stroke 2013, 44, 3–8. [Google Scholar] [CrossRef]
- Jiménez, I.; Sobrino, T.; Rodríguez-Yáñez, M.; Pouso, M.; Cristobo, I.; Sabucedo, M.; Blanco, M.; Castellanos, M.; Leira, R.; Castillo, J. High Serum Levels of Leptin Are Associated with Post-Stroke Depression. Psychol. Med. 2009, 39, 1201. [Google Scholar] [CrossRef]
- Carbone, F.; Burger, F.; Roversi, G.; Tamborino, C.; Casetta, I.; Seraceni, S.; Trentini, A.; Padroni, M.; Bertolotto, M.; Dallegri, F.; et al. Leptin/Adiponectin Ratio Predicts Poststroke Neurological Outcome. Eur. J. Clin. Investig. 2015, 45, 1184–1191. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, Cardiovascular Diseases and Type 2 Diabetes Mellitus. Acta Pharm. Sin. 2018, 39, 1176–1188. [Google Scholar] [CrossRef]
- Behrouzifar, S.; Vakili, A.; Bandegi, A.R.; Kokhaei, P. Neuroprotective Nature of Adipokine Resistin in the Early Stages of Focal Cerebral Ischemia in a Stroke Mouse Model. Neurochem. Int. 2018, 114, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Osawa, H.; Doi, Y.; Makino, H.; Ninomiya, T.; Yonemoto, K.; Kawamura, R.; Hata, J.; Tanizaki, Y.; Iida, M.; Kiyohara, Y. Diabetes and Hypertension Markedly Increased the Risk of Ischemic Stroke Associated with High Serum Resistin Concentration in a General Japanese Population: The Hisayama Study. Cardiovasc. Diabetol. 2009, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Kaplan, R.C.; Wassertheil-Smoller, S.; Cushman, M.; Rohan, T.E.; McGinn, A.P.; Wang, T.; Strickler, H.D.; Scherer, P.E.; Mackey, R.; et al. Resistin, but Not Adiponectin and Leptin, Is Associated With the Risk of Ischemic Stroke Among Postmenopausal Women: Results From the Women’s Health Initiative. Stroke 2011, 42, 1813–1820. [Google Scholar] [CrossRef]
- Muse, E.D.; Feldman, D.I.; Blaha, M.J.; Dardari, Z.A.; Blumenthal, R.S.; Budoff, M.J.; Nasir, K.; Criqui, M.H.; Cushman, M.; McClelland, R.L.; et al. The Association of Resistin with Cardiovascular Disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2015, 239, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Nakashima, E.; Watarai, A.; Hamada, Y.; Naruse, K.; Kamiya, H.; Nakamura, N.; Kato, N.; Hamajima, N.; Sekido, Y.; et al. Polymorphism in Resistin Promoter Region at −420 Determines the Serum Resistin Levels and May Be a Risk Marker of Stroke in Japanese Type 2 Diabetic Patients. Diabetes Res. Clin. Pract. 2009, 84, 179–186. [Google Scholar] [CrossRef]
- Kochanowski, J.; Grudniak, M.; Baranowska-Bik, A.; Wolinska-Witort, E.; Kalisz, M.; Baranowska, B.; Bik, W. Resistin Levels in Women with Ischemic Stroke. Neuroendocrinol. Lett. 2012, 33, 603–607. [Google Scholar]
- Perovic, E.; Mrdjen, A.; Harapin, M.; Tesija Kuna, A.; Simundic, A.-M. Diagnostic and Prognostic Role of Resistin and Copeptin in Acute Ischemic Stroke. Top. Stroke Rehabil. 2017, 24, 614–618. [Google Scholar] [CrossRef]
- Dong, X.-L.; Xu, S.-J.; Zhang, L.; Zhang, X.-Q.; Liu, T.; Gao, Q.-Y.; Qian, Q.-Q.; Sun, B.-L.; Yang, M.-F. Serum Resistin Levels May Contribute to an Increased Risk of Acute Cerebral Infarction. Mol. Neurobiol. 2017, 54, 1919–1926. [Google Scholar] [CrossRef]
- Efstathiou, S.P.; Tsiakou, A.G.; Tsioulos, D.I.; Panagiotou, T.N.; Pefanis, A.V.; Achimastos, A.D.; Mountokalakis, T.D. Prognostic Significance of Plasma Resistin Levels in Patients with Atherothrombotic Ischemic Stroke. Clin. Chim. Acta 2007, 378, 78–85. [Google Scholar] [CrossRef]
- Adam, S.S.; Key, N.S.; Greenberg, C.S. D-Dimer Antigen: Current Concepts and Future Prospects. Blood 2009, 113, 2878–2887. [Google Scholar] [CrossRef]
- Huţanu, A.; Iancu, M.; Bălaşa, R.; Maier, S.; Dobreanu, M. Predicting Functional Outcome of Ischemic Stroke Patients in Romania Based on Plasma CRP, STNFR-1, D-Dimers, NGAL and NSE Measured Using a Biochip Array. Acta Pharm. Sin. 2018, 39, 1228–1236. [Google Scholar] [CrossRef]
- Ohara, T.; Farhoudi, M.; Bang, O.Y.; Koga, M.; Demchuk, A.M. The Emerging Value of Serum D-Dimer Measurement in the Work-up and Management of Ischemic Stroke. Int. J. Stroke 2020, 15, 122–131. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Y.; Shan, B.; He, M.; Ren, Q.; Zeng, Y.; Liu, Z.; Liu, H.; Xu, J. Elevated Level of D-Dimer Increases the Risk of Stroke. Oncotarget 2018, 9, 2208–2219. [Google Scholar] [CrossRef]
- Barber, M.; Langhorne, P.; Rumley, A.; Lowe, G.D.O.; Stott, D.J. Hemostatic Function and Progressing Ischemic Stroke: D-Dimer Predicts Early Clinical Progression. Stroke 2004, 35, 1421–1425. [Google Scholar] [CrossRef]
- Haapaniemi, E.; Tatlisumak, T. Is D-Dimer Helpful in Evaluating Stroke Patients? A Systematic Review. Acta Neurol. Scand. 2009, 119, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-J.; Chen, C.-H.; Yeh, S.-J.; Tsai, L.-K.; Tang, S.-C.; Jeng, J.-S. High Plasma D-Dimer Indicates Unfavorable Outcome of Acute Ischemic Stroke Patients Receiving Intravenous Thrombolysis. Cereb. Dis. 2016, 42, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M. Lehninger Principles of Biochemistry, 3rd edition; By David L., Nelson and Michael, M. Cox. Chem. Educ. 2001, 6, 69–70. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Li, P.; Li, J.; Bao, H.; Zhang, Y.; Wang, B.; Sun, N.; Wang, J.; He, M.; et al. Association between Percent Decline in Serum Total Homocysteine and Risk of First Stroke. Neurology 2017, 89, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Moake, J.L. Hyperhomocysteinemia-Hematology and Oncology. Available online: https://www.msdmanuals.com/professional/hematology-and-oncology/thrombotic-disorders/hyperhomocysteinemia (accessed on 11 July 2021).
- Miles, L.M.; Allen, E.; Mills, K.; Clarke, R.; Uauy, R.; Dangour, A.D. Vitamin B-12 Status and Neurologic Function in Older People: A Cross-Sectional Analysis of Baseline Trial Data from the Older People and Enhanced Neurological Function (OPEN) Study. Am. J. Clin. Nutr. 2016, 104, 790–796. [Google Scholar] [CrossRef]
- Harris, S.; Rasyid, A.; Kurniawan, M.; Mesiano, T.; Hidayat, R. Association of High Blood Homocysteine and Risk of Increased Severity of Ischemic Stroke Events. Int. J. Angiol. 2019, 28, 034–038. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, Y.; Zhang, S.; Tie, T.; Cheng, Y.; Su, X.; Man, Z.; Hou, J.; Sun, L.; Tian, M.; et al. The Association between Homocysteine and Ischemic Stroke Subtypes in Chinese: A Meta-Analysis. Medicine 2020, 99, e19467. [Google Scholar] [CrossRef]
- Yan, L.; Li-Li, C.; Lin, L.; Qin-De, Q. Serum Levels of Homocysteine at Admission Are Associated with Post-Stroke Depression in Acute Ischemic Stroke. Neurol. Sci. 2017, 38, 811–817. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, S.; Guan, Y.; Zhang, M.; Lu, H.; Yue, W.; Zhang, B.; Li, M.; Xue, J.; Ji, Y. Changes in Total Homocysteine Levels after Acute Stroke and Recurrence of Stroke. Sci. Rep. 2018, 8, 6993. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, K.; Chen, J.; Liao, Y.; Qin, Q.; Ma, A.; Wang, D.; Zhu, Z.; Wang, Y.; Hui, R. High Plasma Homocysteine Levels Contribute to the Risk of Stroke Recurrence and All-Cause Mortality in a Large Prospective Stroke Population. Clin. Sci. 2010, 118, 187–194. [Google Scholar] [CrossRef]
- Qin, Z.; Tang, L.; Huang, Q.; Chen, Y.; Zhong, W.; Tang, X. A Systematic Review of the Correlation between Serum Asymmetric Dimethylarginine, Carotid Atherosclerosis and Ischaemic Stroke. Eur. J. Clin. Investig. 2021, 51, e13558. [Google Scholar] [CrossRef]
- Worthmann, H.; Chen, S.; Martens-Lobenhoffer, J.; Li, N.; Deb, M.; Tryc, A.B.; Goldbecker, A.; Dong, Q.; Kielstein, J.T.; Bode-Böger, S.M.; et al. High Plasma Dimethylarginine Levels Are Associated with Adverse Clinical Outcome After Stroke. JAT 2011, 18, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, N.; Deb-Chatterji, M.; Dong, Q.; Kielstein, J.; Weissenborn, K.; Worthmann, H. Asymmetric Dimethyarginine as Marker and Mediator in Ischemic Stroke. IJMS 2012, 13, 15983–16004. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Ueda, M.; Katsura, K.; Otsuka, T.; Abe, A.; Nagayama, H.; Katayama, Y. Asymmetric Dimethylarginine (ADMA) as a Possible Risk Marker for Ischemic Stroke. J. Neurol. Sci. 2010, 290, 12–15. [Google Scholar] [CrossRef]
- Ercan, M.; Mungan, S.; Güzel, I.; Celik, H.; Bal, C.; Abusoglu, S.; Akbulut, D.; Firat Oguz, E.; MEric Yilmaz, F. Serum Asymmetric Dimethylarginine and Nitric Oxide Levelsin Turkish Patients with Acute Ischemic Stroke. Adv. Clin. Exp. Med. 2018, 28, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Mamatha, S.N.; Nagaraja, D.; Philip, M.; Christopher, R. Asymmetric Dimethylarginine as a Risk Marker for Early-Onset Ischemic Stroke in Indian Population. Clin. Chim. Acta 2011, 412, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Otsuka, T.; Ueda, M.; Inagaki, H.; Muraga, K.; Abe, A.; Kawada, T.; Katayama, Y. Asymmetric Dimethylarginine Is Related to the Predicted Stroke Risk in Middle-Aged Japanese Men. J. Neurol. Sci. 2014, 338, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Worthmann, H.; Martens-Lobenhoffer, J.; Joumaah, M.; Li, N.; Lichtinghagen, R.; Hecker, H.; Kielstein, J.T.; Ehrenreich, H.; Bode-Böger, S.M.; Weissenborn, K. Asymmetric Dimethylarginine in Response to Recombinant Tissue-Type Plasminogen Activator and Erythropoietin in Acute Stroke. Stroke 2013, 44, 2128–2133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xia, X.-S.; Li, X.; Wang, L.; Wang, J.-Z.; Ma, J.-P.; Wu, C.-J. Supplementation of Folic Acid and Vitamin B12 Reduces Plasma Levels of Asymmetric Dimethylarginine in Patients with Acute Ischemic Stroke. J. Clin. Neurosci. 2014, 21, 1586–1590. [Google Scholar] [CrossRef]
- Zhang, J.; Du, R.; Peng, K.; Wu, X.; Hu, C.; Li, M.; Xu, Y.; Xu, M.; Wang, S.; Bi, Y.; et al. Serum Lipoprotein (a) Is Associated with Increased Risk of Stroke in Chinese Adults: A Prospective Study. Atherosclerosis 2019, 289, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.-A. Prognostic Value of Serum Lipoprotein(a) Levels in Patients with Acute Ischemic Stroke. Neuroreport 2014, 25, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Smolders, B.; Lemmens, R.; Thijs, V. Lipoprotein (a) and Stroke: A Meta-Analysis of Observational Studies. Stroke 2007, 38, 1959–1966. [Google Scholar] [CrossRef]
- Gaw, A. Lipoprotein(a) and Risk of Myocardial Infarction. JAMA 1994, 271, 1077. [Google Scholar] [CrossRef]
- Nave, A.H.; Lange, K.S.; Leonards, C.O.; Siegerink, B.; Doehner, W.; Landmesser, U.; Steinhagen-Thiessen, E.; Endres, M.; Ebinger, M. Lipoprotein (a) as a Risk Factor for Ischemic Stroke: A Meta-Analysis. Atherosclerosis 2015, 242, 496–503. [Google Scholar] [CrossRef]
- Langsted, A.; Nordestgaard, B.G.; Kamstrup, P.R. Elevated Lipoprotein(a) and Risk of Ischemic Stroke. J. Am. Coll. Cardiol. 2019, 74, 54–66. [Google Scholar] [CrossRef]
- Lange, K.S.; Nave, A.H.; Liman, T.G.; Grittner, U.; Endres, M.; Ebinger, M. Lipoprotein(a) Levels and Recurrent Vascular Events After First Ischemic Stroke. Stroke 2017, 48, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Gordts, P.L.S.M.; Nora, C.; Yeang, C.; Witztum, J.L. Statin Therapy Increases Lipoprotein(a) Levels. Eur. Heart J. 2020, 41, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- He, P.-P.; Jiang, T.; OuYang, X.-P.; Liang, Y.-Q.; Zou, J.-Q.; Wang, Y.; Shen, Q.-Q.; Liao, L.; Zheng, X.-L. Lipoprotein Lipase: Biosynthesis, Regulatory Factors, and Its Role in Atherosclerosis and Other Diseases. Clin. Chim. Acta 2018, 480, 126–137. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, J.; Deng, W.-S.; Sun, P. Association between Lipoprotein Lipase Polymorphism and the Risk of Stroke: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 2570–2578. [Google Scholar] [CrossRef]
- Nejati, M.; Atlasi, M.A.; Karimian, M.; Nikzad, H.; Azami, A. Lipoprotein Lipase Gene Polymorphisms as Risk Factors for Stroke: A Computational and Meta-Analysis. Iran. J. Basic Med. Sci. 2018, 21, 701. [Google Scholar] [CrossRef]
- Shimo-Nakanishi, Y.; Urabe, T.; Hattori, N.; Watanabe, Y.; Nagao, T.; Yokochi, M.; Hamamoto, M.; Mizuno, Y. Polymorphism of the Lipoprotein Lipase Gene and Risk of Atherothrombotic Cerebral Infarction in the Japanese. Stroke 2001, 32, 1481–1486. [Google Scholar] [CrossRef]
- Lipoprotein-Associated Phospholipase A2 and Risk of Coronary Disease, Stroke, and Mortality: Collaborative Analysis of 32 Prospective Studies. Lancet 2010, 375, 1536–1544. [CrossRef]
- Delgado, P.; Chacón, P.; Penalba, A.; Pelegri, D.; García-Berrocoso, T.; Giralt, D.; Santamarina, E.; Ribó, M.; Maisterra, O.; Alvarez-Sabín, J.; et al. Lipoprotein-Associated Phospholipase A2 Activity Is Associated with Large-Artery Atherosclerotic Etiology and Recurrent Stroke in TIA Patients. Cereb. Dis. 2012, 33, 150–158. [Google Scholar] [CrossRef]
- Massot, A.; Pelegri, D.; Penalba, A.; Arenillas, J.; Boada, C.; Giralt, D.; Ribó, M.; Molina, C.A.; Rosell, A.; Alvarez-Sabín, J.; et al. Lipoprotein-Associated Phospholipase A2 Testing Usefulness among Patients with Symptomatic Intracranial Atherosclerotic Disease. Atherosclerosis 2011, 218, 181–187. [Google Scholar] [CrossRef]
- Tian, Y.; Jia, H.; Li, S.; Wu, Y.; Guo, L.; Tan, G.; Li, B. The Associations of Stroke, Transient Ischemic Attack, and/or Stroke-Related Recurrent Vascular Events with Lipoprotein-Associated Phospholipase A2: A Systematic Review and Meta-Analysis. Medicine 2017, 96, e9413. [Google Scholar] [CrossRef]
- Hu, G.; Liu, D.; Tong, H.; Huang, W.; Hu, Y.; Huang, Y. Lipoprotein-Associated Phospholipase A2 Activity and Mass as Independent Risk Factor of Stroke: A Meta-Analysis. BioMed Res. Int. 2019, 2019, 8642784. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ke, Z.; Zhao, Y.; Cai, Z. The Elevated Lipoprotein-Associated Phospholipase A2 Activity Is Associated with the Occurrence and Recurrence of Acute Cerebral Infarction. Neuroreport 2017, 28, 325–330. [Google Scholar] [CrossRef]
- Katan, M.; Moon, Y.P.; Paik, M.C.; Wolfert, R.L.; Sacco, R.L.; Elkind, M.S.V. Lipoprotein-Associated Phospholipase A2 Is Associated with Atherosclerotic Stroke Risk: The Northern Manhattan Study. PLoS ONE 2014, 9, e83393. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhong, C.; Bu, X.; Xu, T.; Wang, A.; Peng, Y.; Xu, T.; Wang, J.; Peng, H.; Li, Q.; et al. Prognostic Value of Lipoprotein-Associated Phospholipase A2 Mass for All-Cause Mortality and Vascular Events within One Year after Acute Ischemic Stroke. Atherosclerosis 2017, 266, 1–7. [Google Scholar] [CrossRef]
- Andersen, C.B.F.; Stødkilde, K.; Sæderup, K.L.; Kuhlee, A.; Raunser, S.; Graversen, J.H.; Moestrup, S.K. Haptoglobin. Antioxid. Redox Signal. 2017, 26, 814–831. [Google Scholar] [CrossRef] [PubMed]
- Brea, D.; Sobrino, T.; Blanco, M.; Fraga, M.; Agulla, J.; Rodríguez-Yáñez, M.; Rodríguez-González, R.; Pérez de la Ossa, N.; Leira, R.; Forteza, J.; et al. Usefulness of Haptoglobin and Serum Amyloid A Proteins as Biomarkers for Atherothrombotic Ischemic Stroke Diagnosis Confirmation. Atherosclerosis 2009, 205, 561–567. [Google Scholar] [CrossRef]
- Ijäs, P.; Melkas, S.; Saksi, J.; Jula, A.; Jauhiainen, M.; Oksala, N.; Pohjasvaara, T.; Kaste, M.; Karhunen, P.J.; Lindsberg, P.; et al. Haptoglobin Hp2 Variant Promotes Premature Cardiovascular Death in Stroke Survivors. Stroke 2017, 48, 1463–1469. [Google Scholar] [CrossRef]
- Ijäs, P.; Saksi, J.; Soinne, L.; Tuimala, J.; Jauhiainen, M.; Jula, A.; Kähönen, M.; Kesäniemi, Y.A.; Kovanen, P.T.; Kaste, M.; et al. Haptoglobin 2 Allele Associates with Unstable Carotid Plaque and Major Cardiovascular Events. Atherosclerosis 2013, 230, 228–234. [Google Scholar] [CrossRef]
- Merkler, A.; Sertić, J.; Bazina Martinović, A.; Križ, T.; Miličić, I.; Šimić, M.; Caban, D.; Ljubić, H.; Markeljević, J.; Šimičević, L.; et al. Haptoglobin Genotype 2-2 Associated with Atherosclerosis in Patients with Ischemic Stroke. Gene 2020, 752, 144786. [Google Scholar] [CrossRef]
- Blum, S.; Vardi, M.; Brown, J.B.; Russell, A.; Milman, U.; Shapira, C.; Levy, N.S.; Miller-Lotan, R.; Asleh, R.; Levy, A.P. Vitamin E Reduces Cardiovascular Disease in Individuals with Diabetes Mellitus and the Haptoglobin 2-2 Genotype. Pharmacogenomics 2010, 11, 675–684. [Google Scholar] [CrossRef]
- Zhao, Y.; He, X.; Shi, X.; Huang, C.; Liu, J.; Zhou, S.; Heng, C.-K. Association between Serum Amyloid A and Obesity: A Meta-Analysis and Systematic Review. Inflamm. Res. 2010, 59, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, H.J.; de Veber, G.A.; Hills, N.K.; Dowling, M.M.; Fox, C.K.; Mackay, M.T.; Kirton, A.; Yager, J.Y.; Bernard, T.J.; Hod, E.A.; et al. Inflammatory Biomarkers in Childhood Arterial Ischemic Stroke: Correlates of Stroke Cause and Recurrence. Stroke 2016, 47, 2221–2228. [Google Scholar] [CrossRef]
- Azurmendi, L.; Lapierre-Fetaud, V.; Schneider, J.; Montaner, J.; Katan, M.; Sanchez, J.-C. Proteomic Discovery and Verification of Serum Amyloid A as a Predictor Marker of Patients at Risk of Post-Stroke Infection: A Pilot Study. Clin. Proteom 2017, 14, 27. [Google Scholar] [CrossRef]
- Schweizer, J.; Bustamante, A.; Lapierre-Fétaud, V.; Faura, J.; Scherrer, N.; Azurmendi Gil, L.; Fluri, F.; Schütz, V.; Luft, A.; Boned, S.; et al. SAA (Serum Amyloid A): A Novel Predictor of Stroke-Associated Infections. Stroke 2020, 51, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, Q.-X.; Peng, B.; Chen, Y.; Yao, T.; Wang, G. Microalbuminuria in Patients with Acute Ischemic Stroke. Neurol. Res. 2019, 41, 498–503. [Google Scholar] [CrossRef]
- Elyas, S.; Shore, A.C.; Kingwell, H.; Keenan, S.; Boxall, L.; Stewart, J.; James, M.A.; Strain, W.D. Microalbuminuria Could Improve Risk Stratification in Patients with TIA and Minor Stroke. Ann. Clin. Transl. Neurol. 2016, 3, 678–683. [Google Scholar] [CrossRef]
- Mykkänen, L.; Zaccaro, D.J.; O’Leary, D.H.; Howard, G.; Robbins, D.C.; Haffner, S.M. Microalbuminuria and Carotid Artery Intima-Media Thickness in Nondiabetic and NIDDM Subjects: The Insulin Resistance Atherosclerosis Study (IRAS). Stroke 1997, 28, 1710–1716. [Google Scholar] [CrossRef]
- Kong, X.; Jia, X.; Wei, Y.; Cui, M.; Wang, Z.; Tang, L.; Li, W.; Zhu, Z.; Chen, P.; Xu, D. Association between Microalbuminuria and Subclinical Atherosclerosis Evaluated by Carotid Artery Intima-Media in Elderly Patients with Normal Renal Function. BMC Nephrol. 2012, 13, 37. [Google Scholar] [CrossRef]
- Thampy, A. Early Clinical Implications of Microalbuminuria in Patients with Acute Ischaemic Stroke. JCDR 2016. [Google Scholar] [CrossRef]
- Sander, D.; Weimar, C.; Bramlage, P.; Brandt, T.; Rosin, L.; Siebler, M. Microalbuminuria Indicates Long-Term Vascular Risk in Patients after Acute Stroke Undergoing in-Patient Rehabilitation. BMC Neurol. 2012, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Ruilope, L.; Locatelli, F.; Ptaszynska, A.; Pieske, B.; de Champlain, J.; Weber, M.A.; Raz, I. Treatment of Microalbuminuria in Hypertensive Subjects with Elevated Cardiovascular Risk: Results of the IMPROVE Trial. Kidney Int. 2007, 72, 879–885. [Google Scholar] [CrossRef]
- Basi, S.; Fesler, P.; Mimran, A.; Lewis, J.B. Microalbuminuria in Type 2 Diabetes and Hypertension: A Marker, Treatment Target, or Innocent Bystander? Diabetes Care 2008, 31 (Suppl. 2), S194–S201. [Google Scholar] [CrossRef]
- Piñón, P.; Kaski, J.C. Inflammation, Atherosclerosis, and Cardiovascular Disease Risk: PAPP-A, Lp-PLA2, and Cystatin C. New Insights or Redundant Information? Rev. Española Cardiol. (Engl. Ed.) 2006, 59, 247–258. [Google Scholar] [CrossRef]
- Fialová, L.; Pilecková, N.; Bauer, J.; Soukupová, J.; Kalousová, M.; Malbohan, I.; Pernický, A.; Kraml, P.; Zima, T. Pregnancy-Associated Plasma Protein-A in Patients with Cerebrovascular Diseases--a Pilot Study. Prague Med. Rep. 2006, 107, 37–45. [Google Scholar] [PubMed]
- Consuegra-Sanchez, L.; Fredericks, S.; Kaski, J.C. Pregnancy-Associated Plasma Protein-A (PAPP-A) and Cardiovascular Risk. Atherosclerosis 2009, 203, 346–352. [Google Scholar] [CrossRef]
- Kalousová, M.; Zima, T.; Krane, V.; März, W.; Wanner, C.; Tesař, V.; Drechsler, C. Pregnancy-Associated Plasma Protein A Associates with Cardiovascular Events in Diabetic Hemodialysis Patients. Atherosclerosis 2014, 236, 263–269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aso, Y.; Okumura, K.; Wakabayashi, S.; Takebayashi, K.; Taki, S.; Inukai, T. Elevated Pregnancy-Associated Plasma Protein-A in Sera from Type 2 Diabetic Patients with Hypercholesterolemia: Associations with Carotid Atherosclerosis and Toe-Brachial Index. J. Clin. Endocrinol. Metab. 2004, 89, 5713–5717. [Google Scholar] [CrossRef] [PubMed]
- Heider, P.; Pfäffle, N.; Pelisek, J.; Wildgruber, M.; Poppert, H.; Rudelius, M.; Eckstein, H.-H. Is Serum Pregnancy-Associated Plasma Protein A Really a Potential Marker of Atherosclerotic Carotid Plaque Stability? Eur. J. Vasc. Endovasc. Surg. 2010, 39, 668–675. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.J.; Gong, Z.Q.; Bi, X.M.; Li, Y.L. Correlation of Plasma Soluble Cluster of Differentiation 40 Ligand, Alpha Fetoprotein A, and Pregnancy-Associated Plasma Protein A with Carotid Plaque in Patients with Ischemic Stroke. Genet. Mol. Res. 2015, 14, 8091–8099. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Du, L.; Zhang, X.; Wang, C. The Prognostic Value of Serum Pregnancy-Associated Plasma Protein A, S100 and High Sensitivity C-Reactive Protein in Acute Ischemic Stroke Patients without Heparin Administration. Clin. Biochem. 2014, 47, 187–191. [Google Scholar] [CrossRef]
- Manso, H.; Krug, T.; Sobral, J.; Albergaria, I.; Gaspar, G.; Ferro, J.M.; Oliveira, S.A.; Vicente, A.M. Variants of the Matrix Metalloproteinase-2 but Not the Matrix Metalloproteinase-9 Genes Significantly Influence Functional Outcome after Stroke. BMC Med. Genet. 2010, 11, 40. [Google Scholar] [CrossRef]
- Buraczynska, K.; Kurzepa, J.; Ksiazek, A.; Buraczynska, M.; Rejdak, K. Matrix Metalloproteinase-9 (MMP-9) Gene Polymorphism in Stroke Patients. Neuromol. Med. 2015, 17, 385–390. [Google Scholar] [CrossRef]
- Loftus, I.M.; Naylor, A.R.; Bell, P.R.F.; Thompson, M.M. Matrix Metalloproteinases and Atherosclerotic Plaque Instability. Br. J. Surg. 2002, 89, 680–694. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Q.; Ding, R.; Liu, D.; Chen, Z. Carotid Thickness and Atherosclerotic Plaque Stability, Serum Inflammation, Serum MMP-2 and MMP-9 Were Associated with Acute Cerebral Infarction. Exp. Med. 2018, 16, 5253–5257. [Google Scholar] [CrossRef]
- Heo, S.H.; Cho, C.-H.; Kim, H.O.; Jo, Y.H.; Yoon, K.-S.; Lee, J.H.; Park, J.-C.; Park, K.C.; Ahn, T.-B.; Chung, K.C.; et al. Plaque Rupture Is a Determinant of Vascular Events in Carotid Artery Atherosclerotic Disease: Involvement of Matrix Metalloproteinases 2 and 9. J. Clin. Neurol. 2011, 7, 69. [Google Scholar] [CrossRef]
- Nie, S.-W.; Wang, X.-F.; Tang, Z.-C. Correlations between MMP-2/MMP-9 Promoter Polymorphisms and Ischemic Stroke. Int. J. Clin. Exp. Med. 2014, 7, 400–404. [Google Scholar]
- Hao, Y.; Tian, S.; Sun, M.; Zhu, Y.; Nie, Z.; Yang, S. Association between Matrix Metalloproteinase Gene Polymorphisms and Development of Ischemic Stroke. Int. J. Clin. Exp. Pathol. 2015, 8, 11647–11652. [Google Scholar]
- Lin, H.-F.; Hsi, E.; Huang, L.-C.; Liao, Y.-C.; Juo, S.-H.H.; Lin, R.-T. Methylation in the Matrix Metalloproteinase-2 Gene Is Associated with Cerebral Ischemic Stroke. J. Investig. Med. 2017, 65, 794–799. [Google Scholar] [CrossRef]
- Zhong, C.; Yang, J.; Xu, T.; Xu, T.; Peng, Y.; Wang, A.; Wang, J.; Peng, H.; Li, Q.; Ju, Z.; et al. For the CATIS Investigators. Serum Matrix Metalloproteinase-9 Levels and Prognosis of Acute Ischemic Stroke. Neurology 2017, 89, 805–812. [Google Scholar] [CrossRef]
- Murata, Y.; Rosell, A.; Scannevin, R.H.; Rhodes, K.J.; Wang, X.; Lo, E.H. Extension of the Thrombolytic Time Window with Minocycline in Experimental Stroke. Stroke 2008, 39, 3372–3377. [Google Scholar] [CrossRef]
- Montaner, J.; Ramiro, L.; Simats, A.; Hernández-Guillamon, M.; Delgado, P.; Bustamante, A.; Rosell, A. Matrix Metalloproteinases and ADAMs in Stroke. Cell. Mol. Life Sci. 2019, 76, 3117–3140. [Google Scholar] [CrossRef]
- Kurzepa, J.; Kurzepa, J.; Golab, P.; Czerska, S.; Bielewicz, J. The Significance of Matrix Metalloproteinase (MMP)-2 and MMP-9 in the Ischemic Stroke. Int. J. Neurosci. 2014, 124, 707–716. [Google Scholar] [CrossRef]
- Kumar, G.; Patnaik, R. Inhibition of Gelatinases (MMP-2 and MMP-9) by Withania Somnifera Phytochemicals Confers Neuroprotection in Stroke: An In Silico Analysis. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 722–733. [Google Scholar] [CrossRef]
- Kato, Y.; Furusyo, N.; Tanaka, Y.; Yamasaki, S.; Ueyama, T.; Takayama, K.; Mitsumoto-Kaseida, F.; Murata, M.; Ikezaki, H.; Hayashi, J. Association of the Serum Endostatin Level, Renal Function, and Carotid Atherosclerosis of Healthy Residents of Japan: Results from the Kyushu and Okinawa Population Study (KOPS). J. Atheroscler. Thromb. 2018, 25, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Furusyo, N.; Tanaka, Y.; Ueyama, T.; Yamasaki, S.; Murata, M.; Hayashi, J. The Relation between Serum Endostatin Level and Carotid Atherosclerosis in Healthy Residents of Japan: Results from the Kyushu and Okinawa Population Study (KOPS). J. Atheroscler. Thromb. 2017, 24, 1023–1030. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, C.; Qian, S.; Zhang, R.; Guo, D.; Wang, A.; Peng, Y.; Peng, H.; Li, Q.; Ju, Z.; Geng, D.; et al. Endostatin as a Novel Prognostic Biomarker in Acute Ischemic Stroke. Atherosclerosis 2020, 293, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Li, R.; Zhang, C.; Zhang, R.; Guo, D.; Bu, X.; Wang, A.; Peng, H.; Chen, J.; Zhang, Y.; et al. Plasma Endostatin Levels at Acute Phase of Ischemic Stroke Are Associated with Post-Stroke Cognitive Impairment. Neurotox. Res. 2020, 37, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Chen, H.; Zhang, T.; Chen, J.; Geng, Z.; Zhao, Y. Changes in Serum Vascular Endothelial Growth Factor and Endostatin Concentrations Associated with Circulating Endothelial Progenitor Cells after Acute Ischemic Stroke. Metab. Brain Dis. 2017, 32, 641–648. [Google Scholar] [CrossRef]
- Fang, Y.; Jinfeng, L.; Zhibin, L.; Xiaoqing, Z.; Sian, Z.; Qiong, Z.; Mei, Y.; Qidong, Y.; Jian, X. Correlation of Plasma Vascular Endothelial Growth Factor and Endostatin Levels with Symptomatic Intra- and Extracranial Atherosclerotic Stenosis in a Chinese Han Population. J. Stroke Cerebrovasc. Dis. 2017, 26, 1061–1070. [Google Scholar] [CrossRef]
- Lee, S.-C.; Lee, K.-Y.; Kim, Y.-J.; Kim, S.H.; Koh, S.-H.; Lee, Y.J. Serum VEGF Levels in Acute Ischaemic Strokes Are Correlated with Long-Term Prognosis: Serum VEGF Levels and Long-Term Prognosis. Eur. J. Neurol. 2010, 17, 45–51. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, J. Role of Alternative Splicing of VEGF-A in the Development of Atherosclerosis. Aging 2018, 10, 2695–2708. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Lemnitzer, P.; Gall, J.; Schwager, S.; Toska, A.; Yvan-Charvet, L.; Detmar, M.; Soehnlein, O. Arterial Delivery of VEGF-C Stabilizes Atherosclerotic Lesions. Circ. Res. 2021, 128, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Tozzi, M.; Schembri, L.; Ferraro, S.; Tarallo, A.; Scanzano, A.; Legnaro, M.; Castelli, P.; Cosentino, M. Production of IL-8, VEGF and Elastase by Circulating and Intraplaque Neutrophils in Patients with Carotid Atherosclerosis. PLoS ONE 2015, 10, e0124565. [Google Scholar] [CrossRef]
- Cui, S.; Li, W.; Lv, X.; Wang, P.; Gao, Y.; Huang, G. Folic Acid Supplementation Delays Atherosclerotic Lesion Development by Modulating MCP1 and VEGF DNA Methylation Levels In Vivo and In Vitro. IJMS 2017, 18, 990. [Google Scholar] [CrossRef]
- Yueniwati, Y.; Darmiastini, N.K.; Arisetijono, E. Thicker Carotid Intima-Media Thickness and Increased Plasma VEGF Levels Suffered by Post-Acute Thrombotic Stroke Patients. IJGM 2016, 9, 447–452. [Google Scholar] [CrossRef]


| Universal Characteristics of Biomarkers |
|---|
| Non-invasive |
| Easily measured |
| Inexpensive |
| Produce rapid results |
| Measured from easily available sources (blood/urine) |
| Allow early detection |
| High sensitivity and specificity |
| Biomarker levels should vary according to therapy |
| Biomarker levels should aid in risk stratification |
| Biomarker levels should possess prognostic value |
| Evidence of the underlying disease mechanism and the role of the biomarker should be available |
| Biomarker | Sensitivity | Specificity | Cut-off Value | Source |
|---|---|---|---|---|
| CRP—predictive of severe ischemic stroke | 80% | 75% | 10.25 mg/L | Shoaeb et al. [25] |
| CRP—predictive of poor outcome | 75% | 82% | 10.25 mg/L | Shoaeb et al. [25] |
| TAT—stroke diagnosis | 58.1% | 87.8% | 1.75 ng/mL | Naifang et al. [29] |
| TAT—complete recanalisation | 53% | 89% | 24 ug/L | Fernandez-Cadenas et al. [30] |
| a2-PI | NA | NA | NA | NA |
| PAI-1 | NA | NA | NA | NA |
| sP-selectin | NA | NA | NA | NA |
| E-selectin—predictive of poor outcome | 88% | 76% | 29 ng/mL | Richard et al. [51] |
| Adiponectin—moderate to high stroke diagnosis | 63.6% | 62.4% | 7.0 ug/mL | Wang et al. [56] |
| Adiponectin—predictive of poor outcomes | 78.6% | 72.9% | 9.0 ug/mL | Wang et al. [56] |
| Leptin—predictive of depression development at 1 month after stroke | 86% | 84% | 20.7 ng/mL | Jiménez et al. [60] |
| Leptin—predictive of major depression within the first month after stroke | 100% | 99% | 85 ng/mL | Jiménez et al. [60] |
| Leptin—day 1 concentration predictive of poor 90 days outcome | 43% | 92% | 3.14 ng/mL | Carbone et al. [61] |
| Leptin/Adiponectin— day 1 concentration predictive of poor 90 days outcome | 88% | 61% | 1.16 | Carbone et al. [61] |
| Resistin | NA | NA | NA | NA |
| D-Dimer—diagnosis of acute aortic disection in stroke patients | 100% | 86% | 4.1 ug/mL | Ohara et al. [74] |
| D-Dimer—stroke diagnosis | 60.2% | 88.9% | 0.38 mg/L | Naifang et al. [29] |
| Hcy—predictive for post-stroke depression | 82.5% | 63.3% | 16.5 mmol/L | Yan et al. [85] |
| ADMA | NA | NA | NA | NA |
| Lp(a) | NA | NA | NA | NA |
| Lp-PLA2 activity—predictive of 30-day recurrent stroke/TIA | 78% | 66% | 207 nmol/mL/min | Delgado et al. [110] |
| Lp-PLA2 activity—predictive of recurrent vascular event | 72% | 59% | 153.36 nmol/mL/min | Massot et al. [111] |
| Hp—diagnosis of atherothrombotic stroke | 95% | 88% | 1040 ug/mL | Brea et al. [118] |
| SAA—diagnosis of atherothrombotic stroke | 91% | 83% | 160 ug/mL | Brea et al. [118] |
| Microalbuminuria | NA | NA | NA | NA |
| PAPP-A—marker for plaque stability | 100% | 62.5% | 0.395 ug/mL | Heider et al. [140] |
| PAPP-A—marker for pressence of neurological symptoms | 82.8% | 35.3% | 0.555 ug/mL | Heider et al. [140] |
| MMP-9—predictor for MCA infarction development | 64% | 88% | 140 ng/mL | Montaner et al. [153] |
| MMP-9—predictor for hemorhagic transformation after tPA | 85% | 79% | 140-191 ng/mL | Montaner et al. [153] |
| Endostatin—predictor for death or severe disability | 49.5% | 60.31% | 83.78 ng/mL | Zhang et al. [158] |
| VEGF—marker for intracranial atherosclerotic stenosis | 93.1% | 36.7% | 64.05 pg/mL | Fang et al. [161] |
| Biomarker | Has Role in Risk Stratification? | Has Role in Diagnosis/Detection? | Has Role in Outcome? | Has Therapeutic Role? Or Is It Affected by Stroke Treatment? |
|---|---|---|---|---|
| C-reactive protein (CRP) | YES, high values are associated with increased risk of stroke and TIA, as well as increased risk of recurrence. | UNCLEAR, although high values appear in acute phases of stroke. | YES, high values are associated with poor outcomes, both in the short and long term. In addition, high values are associated with increased mortality. | YES, statins have been observed to reduce hs-CRP levels. |
| Thrombin-antithrombin complex (TAT) | Not enough data is available. | UNCLEAR, although higher values have been found in acute ischemic stroke patients. | YES, higher values are associated with poor outcomes. | YES, lower values are associated with better rt-PA success rates. |
| Alpha2-plasmin inhibitor (a2-PI) | YES, high plasma values are associated with increased stroke recurrence risk. | YES, higher values have been detected in patients with atherothrombotic stroke. | UNCLEAR, although in experimental studies, there was a slight reduction of brain lesion volume by administering human plasmin in order to inhibit a2-PI effects. | YES, higher values are associated with lower rt-PA success rates. |
| Plasminogen activator inhibitor-1 (PAI-1) | YES, high values are linked to stroke risk and stroke recurrence risk. | UNCLEAR, although higher PAI-1 levels can be found in stroke patients. | UNCLEAR, even if no statistically significant difference was found regarding PAI-1 polymorphism, there is an increased risk of hemorrhagic transformation. | YES, PAI-1 5G/5G is associated with an increased risk of hemorrhage after rt-PA treatment. |
| Soluble P-selectin (sP-selectin) | UNCLEAR, although increased values are found in patients with aortic atherosclerosis. | YES, high values have been found in stroke patients, with a decrease in value after three months. | YES, high values were found in progressive ischemic stroke. | YES, patients that received statin treatment showed a decrease in blood values of sP-selectin. |
| E-selectin | YES, genetic polymorphism is associated with increased stroke risk. Alleles association increases the risk even more. | Not enough data is available. | YES, independent predictor of poor three-month outcome | NEED FURTHER EVIDENCE. Experimental studies on mice showed improved outcomes after administering anti-E-selectin monoclonal antibodies. |
| Adiponectin (APN) | YES, higher values are associated with a lower prevalence of carotid atherosclerotic plaques. | YES, low values can be found at admission in stroke patients compared to control patients. | UNCLEAR, patients with higher levels at admission were more likely to have a poor outcome and increased mortality risk. However, low levels were associated with increased five-year mortality rates. | UNCLEAR. Further studies regarding a viable agonist of APN could hold the answer. |
| Leptin | YES, higher leptin values were associated with increased stroke incidence. Leptin/Adiponectin ratio could offer additional predictive value for subclinical atherosclerotic changes. | YES, both ischemic and hemorrhagic stroke patients had higher leptin values compared to healthy ones. | YES, patients with stroke history that had higher leptin values had an increased risk of recurrence. A high Leptin/Adiponectin ratio is associated with good outcomes. | YES, leptin values can be decreased by healthy lifestyle changes, statins, and antidiabetic drugs. |
| Resistin | YES, higher values of resistin are a risk factor for atherothrombotic stroke. The risk is further increased when diabetes or hypertension are present. Specific genotypes such as -420(C>G) are an additional risk factor. | YES, higher values have been found in the early phase of stroke. Experimental studies on mice showed resistin peak values around 12 h from the onset. | YES, high values of resistin were associated with increased stroke severity, increased disability after stroke, and a higher five-year mortality rate. | UNCLEAR. Additional studies need to be performed to confirm the evidence from experimental studies, where resistin was able to decrease brain edema. |
| D-Dimer (DDs) | YES, increased DDs value is associated with increased risk of ischemic stroke. | UNCLEAR, although in specific etiological subtypes of stroke, like occult cancer or undetected atrial fibrillation, it was shown that higher values of DD could be found. Because of the poor specificity and sensitivity of DD in stroke, the role is still unclear. | YES, higher DD values have been associated with progressive stroke. Also, combined with other biomarkers, it can be used as a predictive factor for the poor outcome of stroke patients. | UNCLEAR, although patients that had poor outcomes after rt-PA treatment had higher values compared to those who had good outcomes post rt-PA. This is suggestive of a decrease in the success rate of rt-PA in high D-dimers level patients. |
| Homocysteine (Hcy) | YES, high levels are associated with increased stroke risk. Also, patients that already had a stroke and high plasma values have an increased risk of recurrence. | YES, higher values were detected in stroke patients. | YES, higher blood values were correlated with higher severity of stroke using NIHSS score. | YES, a decrease in stroke risk was shown in groups of patients treated with folic acid in order to lower the total Hcy levels. Further studies are required to show the benefits in the general population. |
| Asymmetric dimethylarginine (ADMA) | YES, several studies showed that ADMA could be linked with the atherosclerosis process and with increased stroke risk. | YES, higher blood values of ADMA can be found in the acute phase of the stroke. | YES, higher values of ADMA have been associated with poor outcomes at 90 days after stroke. | UNCLEAR, rt-PA seems to decrease ADMA levels. A decrease in ADMA value was also seen in patients who received folic acid and B12 vitamin supplements. |
| Lipoprotein(a) (Lp (a)) | YES, Lp (a) is a risk factor for both atherosclerosis and stroke, especially in young patients. High values are also associated with an increased risk of recurrence after a first-time stroke. | YES, higher values were found in stroke patients compared to healthy subjects. | YES, high values of Lp (a) are associated with poor short-term outcomes. | UNCLEAR, but increased values of Lp (a) were documented in patients that received statin treatment. |
| Lipoprotein lipase (LPL) | YES, polymorphism of LPL is a direct influence on the risk of developing ischemic stroke. Genetic variants have different effects in different ethnic populations, ranging from increasing stroke risk to having a protective effect. There is suggestive data showing LPL proatherogenic effect. | Not enough data. | Not enough data. | Not enough data. |
| Lipoprotein-associated phospholipase A2 (Lp-PLA2) | YES, high values are strongly associated with large-vessel disease and risk of transient ischemic attack, and also with a high risk of stroke reoccurrence. | YES, high values were found in patients with TIA and in stroke patients with arterial stenosis, especially intracranial stenosis. | YES, high values are associated with increased one-year mortality rates. | Not enough data. |
| Haptoglobin (Hp) | YES, Hp2 allele and its Hp2-2 genotype are associated with carotid atherosclerosis leading to potential strokes and TIA. | YES, high levels of Hp in the first 12 h of onset are strongly associated with atherothrombotic stroke. | YES, Hp2 allele is associated with premature cardiovascular deaths in patients with first-time ischemic stroke. | UNCLEAR, however, in the Hp2-2 genotype, there was an observed decrease of cardiovascular events after administering Vitamin E. |
| Serum amyloid A (SAA) | YES, in arteriopathic children with atherothrombotic stroke, SAA correlated with both occurrence and recurrence of stroke. | YES, high values of SAA in the first 12 h were associated with atherothrombotic stroke. | YES, patients with increased SAA at admission are more likely to develop infections, thus increasing mortality. | Not enough data. |
| Microalbuminuria (MAU) | YES, patients with MAU have an increased incidence of large vessel disease and recurrence of stroke. | UNCLEAR; however, MAU was detected in one-third of stroke patients. | YES, patients with MAU have a worse short-term and long-term prognosis. | UNCLEAR for stroke patients, but angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have been shown to reduce MAU in patients with cardiovascular comorbidities. |
| Pregnancy-associated plasma protein-A (PAPP-A) | YES, PAPP-A has been associated with atherosclerosis, especially unstable atherosclerotic plaques. | YES, high PAPP-A levels are found in stroke patients that have coronary artery disease. | YES, patients with increased levels of PAPP-A have increased stroke severity and are more likely to have a poor outcome. | UNCLEAR, although statin treatment can decrease PAPP-A levels. |
| Matrix metalloproteinases (MMPs) | YES, high MMPs values are correlated with the presence of unstable atherosclerotic plaques. Also, specific genotypes have proven to be independent risk factors for ischemic stroke. | YES, high values of MMPs can be found in the acute phase of stroke. | YES, increased values of MMPs at admission are associated with poor outcomes and increased mortality risk. | YES, inhibition of MMPs activity in the acute phase of the stroke could be a viable treatment strategy. Statins are a drug class capable of decreasing MMPs activity. Other specific inhibitors could be used in future studies, such as minocycline alongside rt-PA, as a possibility to increase the therapeutic window and also reduce its secondary effects, such as brain hemorrhage. Another specific inhibitor could be phytochemicals derived from Withaniasomnifera, which have the ability to inhibit specifically MMP-2 and MMP-9 and have a potential neuroprotective effect against stroke. |
| Endostatin | YES, high endostatin values are associated with subclinical atherosclerosis changes. | YES, increased endostatin values can be found in stroke patients. | YES, increased endostatin values are associated with severe disability, increased mortality risk, and three-month cognitive impairment. | Not enough data. |
| VEGF | YES, low values of VEGF are correlated with intracranial arterial stenosis. | YES, High values of VEGF have been found in the acute phase of atherothrombotic stroke but with no correlation with atherosclerotic changes, such as IMT. | YES, high values of VEGF have been correlated with good clinical outcomes. The endostatin/VEGF ratio is another predictor for good outcomes. | UNCLEAR, administering VEGF-C to mice showed increased plaque stability. Acid folic affects VEGF DNA methylation and slows down the atherosclerotic process. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andone, S.; Bajko, Z.; Motataianu, A.; Mosora, O.; Balasa, R. The Role of Biomarkers in Atherothrombotic Stroke—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9032. https://doi.org/10.3390/ijms22169032
Andone S, Bajko Z, Motataianu A, Mosora O, Balasa R. The Role of Biomarkers in Atherothrombotic Stroke—A Systematic Review. International Journal of Molecular Sciences. 2021; 22(16):9032. https://doi.org/10.3390/ijms22169032
Chicago/Turabian StyleAndone, Sebastian, Zoltan Bajko, Anca Motataianu, Oana Mosora, and Rodica Balasa. 2021. "The Role of Biomarkers in Atherothrombotic Stroke—A Systematic Review" International Journal of Molecular Sciences 22, no. 16: 9032. https://doi.org/10.3390/ijms22169032
APA StyleAndone, S., Bajko, Z., Motataianu, A., Mosora, O., & Balasa, R. (2021). The Role of Biomarkers in Atherothrombotic Stroke—A Systematic Review. International Journal of Molecular Sciences, 22(16), 9032. https://doi.org/10.3390/ijms22169032






