Arteriovenous Malformations—Current Understanding of the Pathogenesis with Implications for Treatment
Abstract
:1. Introduction
2. Genetics of AVMs
3. Natural History of Different AVM and Their Clinical Characteristics
4. Hypotheses of AVM Formation
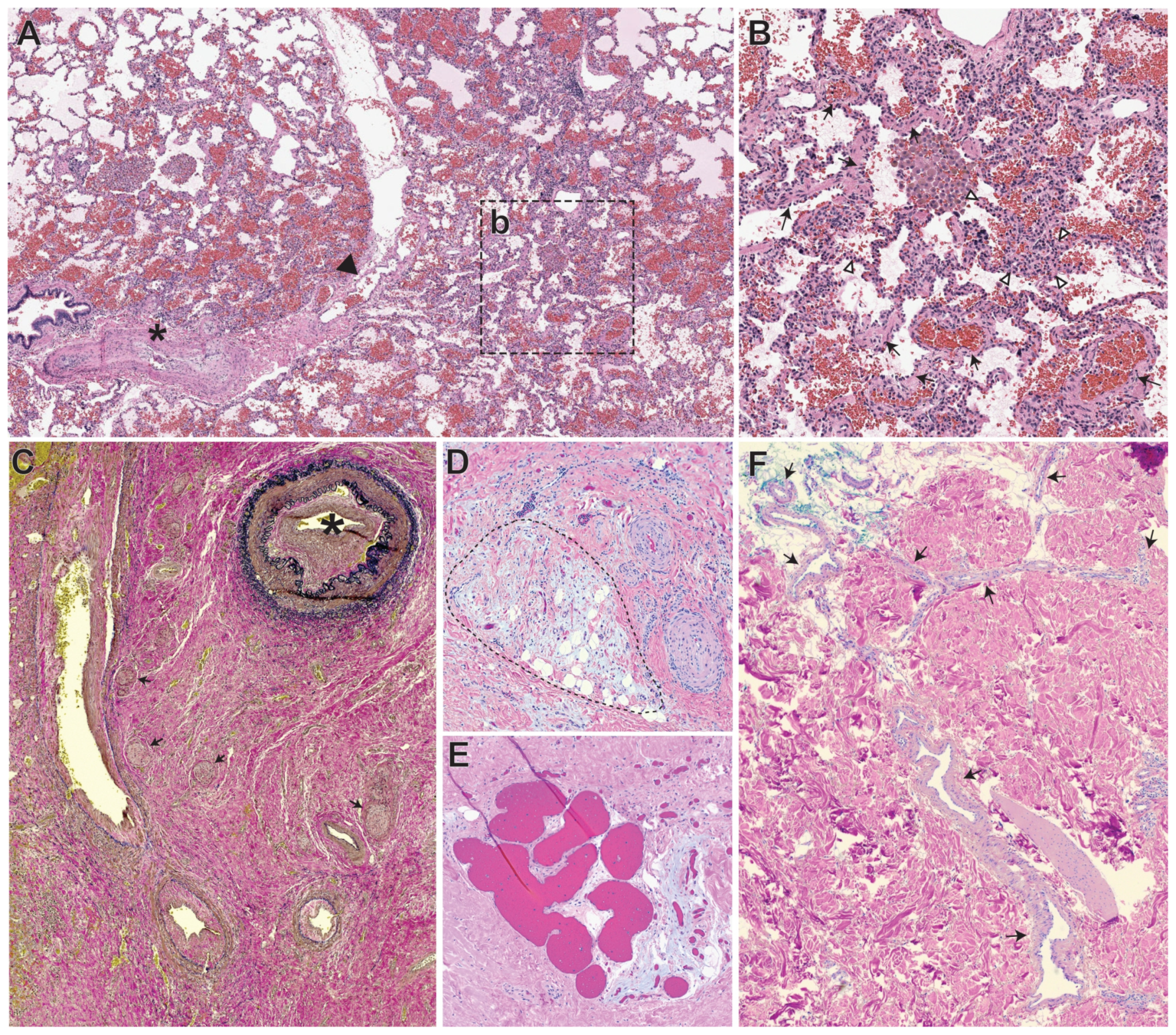
5. Overview of the Histopathology of AVMs, Vascular-Nonvascular Cells
6. Diagnosis of AVMs
6.1. Extracranial AVMs
6.2. Intracranial AVMs
7. Overview of Model Systems of AVMs In Vivo (Zebrafish, Mouse Mutants, Antibody Treatment)
7.1. Genetic Models
7.2. Antibody Based Models
7.3. Surgical Models
8. Pathways and Crosstalk between Pathways
9. Targeting Vascular Malformations with Repurposed Drugs; Benefits of Combinational Therapy (Surgical/Medical) for Complex AVM
10. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halim, A.X.; Johnston, S.C.; Singh, V.; McCulloch, C.E.; Bennett, J.P.; Achrol, A.S.; Sidney, S.; Young, W.L. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke 2004, 35, 1697–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shovlin, C.L.; Jackson, J.E.; Bamford, K.B.; Jenkins, I.H.; Benjamin, A.R.; Ramadan, H.; Kulinskaya, E. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 2008, 63, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleetwood, I.G.; Steinberg, G.K. Arteriovenous malformations. Lancet 2002, 359, 863–873. [Google Scholar] [CrossRef]
- Faughnan, M.E.; Mager, J.J.; Hetts, S.W.; Palda, V.A.; Lang-Robertson, K.; Buscarini, E.; Deslandres, E.; Kasthuri, R.S.; Lausman, A.; Poetker, D.; et al. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann. Intern. Med. 2020, 173, 989–1001. [Google Scholar] [CrossRef]
- Rosen, R.J.; Nassiri, N.; Drury, J.E. Interventional management of high-flow vascular malformations. Tech. Vasc. Interv. Radiol. 2013, 16, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Tau, N.; Atar, E.; Mei-Zahav, M.; Bachar, G.N.; Dagan, T.; Birk, E.; Bruckheimer, E. Amplatzer Vascular Plugs Versus Coils for Embolization of Pulmonary Arteriovenous Malformations in Patients with Hereditary Hemorrhagic Telangiectasia. Cardiovasc. Interv. Radiol. 2016, 39, 1110–1114. [Google Scholar] [CrossRef]
- Milic, A.; Chan, R.P.; Cohen, J.H.; Faughnan, M.E. Reperfusion of pulmonary arteriovenous malformations after embolotherapy. J. Vasc. Interv. Radiol. 2005, 16, 1675–1683. [Google Scholar] [CrossRef]
- Liu, A.S.; Mulliken, J.B.; Zurakowski, D.; Fishman, S.J.; Greene, A.K. Extracranial arteriovenous malformations: Natural progression and recurrence after treatment. Plast Reconstr. Surg. 2010, 125, 1185–1194. [Google Scholar] [CrossRef]
- Limaye, N.; Boon, L.M.; Vikkula, M. From germline towards somatic mutations in the pathophysiology of vascular anomalies. Hum. Mol. Genet. 2009, 18, R65–R74. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Choe, S.W.; Kim, Y.H.; Acharya, A.P.; Keselowsky, B.G.; Sorg, B.S.; Lee, Y.J.; Oh, S.P. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis 2014, 17, 823–830. [Google Scholar] [CrossRef]
- Lacout, A.; Marcy, P.Y.; Thariat, J.; El Hajjam, M.; Lacombe, P. VEGF target in HHT lung patients: The role of bevacizumab as a possible alternative to embolization. Med. Hypotheses 2012, 78, 689–690. [Google Scholar] [CrossRef] [PubMed]
- Ardelean, D.S.; Letarte, M. Anti-angiogenic therapeutic strategies in hereditary hemorrhagic telangiectasia. Front. Genet. 2015, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Snellings, D.A.; Gallione, C.J.; Clark, D.S.; Vozoris, N.T.; Faughnan, M.E.; Marchuk, D.A. Somatic Mutations in Vascular Malformations of Hereditary Hemorrhagic Telangiectasia Result in Bi-allelic Loss of ENG or ACVRL1. Am. J. Hum. Genet. 2019, 105, 894–906. [Google Scholar] [CrossRef]
- Choi, E.J.; Chen, W.; Jun, K.; Arthur, H.M.; Young, W.L.; Su, H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS ONE 2014, 9, e88511. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Martin, E.M.; Nguyen, H.L.; Cunningham, T.A.; Choe, S.W.; Jiang, Z.; Arthur, H.M.; Lee, Y.J.; Oh, S.P. Common and distinctive pathogenetic features of arteriovenous malformations in hereditary hemorrhagic telangiectasia 1 and hereditary hemorrhagic telangiectasia 2 animal models--brief report. Arter. Thromb. Vasc. Biol. 2014, 34, 2232–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, M.; Allinson, K.R.; Zhai, Z.; Oakenfull, R.; Ghandi, P.; Adams, R.H.; Fruttiger, M.; Arthur, H.M. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ. Res. 2010, 106, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Park, S.O.; Wankhede, M.; Lee, Y.J.; Choi, E.J.; Fliess, N.; Choe, S.W.; Oh, S.H.; Walter, G.; Raizada, M.K.; Sorg, B.S.; et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J. Clin. Investig. 2009, 119, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Tual-Chalot, S.; Mahmoud, M.; Allinson, K.R.; Redgrave, R.E.; Zhai, Z.; Oh, S.P.; Fruttiger, M.; Arthur, H.M. Endothelial depletion of Acvrl1 in mice leads to arteriovenous malformations associated with reduced endoglin expression. PLoS ONE 2014, 9, e98646. [Google Scholar] [CrossRef] [Green Version]
- Ola, R.; Dubrac, A.; Han, J.; Zhang, F.; Fang, J.S.; Larrivée, B.; Lee, M.; Urarte, A.A.; Kraehling, J.R.; Genet, G.; et al. PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nat. Commun. 2016, 7, 13650. [Google Scholar] [CrossRef]
- Jin, Y.; Muhl, L.; Burmakin, M.; Wang, Y.; Duchez, A.C.; Betsholtz, C.; Arthur, H.M.; Jakobsson, L. Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling. Nat. Cell Biol. 2017, 19, 639–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsina-Sanchís, E.; García-Ibáñez, Y.; Figueiredo, A.M.; Riera-Domingo, C.; Figueras, A.; Matias-Guiu, X.; Casanovas, O.; Botella, L.M.; Pujana, M.A.; Riera-Mestre, A.; et al. ALK1 Loss Results in Vascular Hyperplasia in Mice and Humans through PI3K Activation. Arter. Thromb. Vasc. Biol. 2018, 38, 1216–1229. [Google Scholar] [CrossRef] [Green Version]
- Shovlin, C.L.; Guttmacher, A.E.; Buscarini, E.; Faughnan, M.E.; Hyland, R.H.; Westermann, C.J.; Kjeldsen, A.D.; Plauchu, H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am. J. Med. Genet. 2000, 91, 66–67. [Google Scholar] [CrossRef]
- Marchuk, D.A.; Guttmacher, A.E.; Penner, J.A.; Ganguly, P. Report on the workshop on Hereditary Hemorrhagic Telangiectasia, 10–11 July 1997. Am. J. Med. Genet. 1998, 76, 269–273. [Google Scholar] [CrossRef]
- McDonald, J.; Wooderchak-Donahue, W.; VanSant Webb, C.; Whitehead, K.; Stevenson, D.A.; Bayrak-Toydemir, P. Hereditary hemorrhagic telangiectasia: Genetics and molecular diagnostics in a new era. Front. Genet. 2015, 6, 1. [Google Scholar] [CrossRef]
- Wooderchak-Donahue, W.L.; Akay, G.; Whitehead, K.; Briggs, E.; Stevenson, D.A.; O’Fallon, B.; Velinder, M.; Farrell, A.; Shen, W.; Bedoukian, E.; et al. Phenotype of CM-AVM2 caused by variants in EPHB4: How much overlap with hereditary hemorrhagic telangiectasia (HHT)? Genet. Med. 2019, 21, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Wooderchak-Donahue, W.L.; Johnson, P.; McDonald, J.; Blei, F.; Berenstein, A.; Sorscher, M.; Mayer, J.; Scheuerle, A.E.; Lewis, T.; Grimmer, J.F.; et al. Expanding the clinical and molecular findings in RASA1 capillary malformation-arteriovenous malformation. Eur. J. Hum. Genet. 2018, 26, 1521–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macmurdo, C.F.; Wooderchak-Donahue, W.; Bayrak-Toydemir, P.; Le, J.; Wallenstein, M.B.; Milla, C.; Teng, J.M.; Bernstein, J.A.; Stevenson, D.A. RASA1 somatic mutation and variable expressivity in capillary malformation/arteriovenous malformation (CM/AVM) syndrome. Am. J. Med. Genet. A 2016, 170, 1450–1454. [Google Scholar] [CrossRef]
- Tan, M.H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer Res. 2012, 18, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.H.; Baris, H.N.; Burrows, P.E.; Robson, C.D.; Alomari, A.I.; Mulliken, J.B.; Fishman, S.J.; Irons, M.B. The spectrum of vascular anomalies in patients with PTEN mutations: Implications for diagnosis and management. J. Med. Genet. 2007, 44, 594–602. [Google Scholar] [CrossRef] [Green Version]
- Couto, J.A.; Huang, A.Y.; Konczyk, D.J.; Goss, J.A.; Fishman, S.J.; Mulliken, J.B.; Warman, M.L.; Greene, A.K. Somatic MAP2K1 Mutations Are Associated with Extracranial Arteriovenous Malformation. Am. J. Hum. Genet. 2017, 100, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, P.J.; Konczyk, D.J.; Sudduth, C.L.; Goss, J.A.; Greene, A.K. Endothelial MAP2K1 mutations in arteriovenous malformation activate the RAS/MAPK pathway. Biochem. Biophys. Res. Commun. 2020, 529, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Kohout, M.P.; Hansen, M.; Pribaz, J.J.; Mulliken, J.B. Arteriovenous malformations of the head and neck: Natural history and management. Plast Reconstr. Surg. 1998, 102, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Lee, J.W.; Choi, K.Y.; Yang, J.D.; Cho, B.C.; Lee, S.J.; Kim, Y.S.; Lee, J.M.; Huh, S.; Chung, H.Y. Clinical Characteristics of Arteriovenous Malformations of the Head and Neck. Dermatol. Surg. 2017, 43, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Sadick, M.; Müller-Wille, R.; Wildgruber, M.; Wohlgemuth, W.A. Vascular Anomalies (Part I): Classification and Diagnostics of Vascular Anomalies. Rofo 2018, 190, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, P.; Dubois, J.; Giroux, M.F.; Soulez, G. New Treatment Approaches to Arteriovenous Malformations. Semin. Interv. Radiol. 2017, 34, 258–271. [Google Scholar] [CrossRef]
- Weitz, N.A.; Lauren, C.T.; Behr, G.G.; Wu, J.K.; Kandel, J.J.; Meyers, P.M.; Sultan, S.; Anyane-Yeboa, K.; Morel, K.D.; Garzon, M.C. Clinical spectrum of capillary malformation-arteriovenous malformation syndrome presenting to a pediatric dermatology practice: A retrospective study. Pediatr. Dermatol. 2015, 32, 76–84. [Google Scholar] [CrossRef]
- Braverman, I.M.; Keh, A.; Jacobson, B.S. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J. Investig. Dermatol. 1990, 95, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Sugden, W.W.; Meissner, R.; Aegerter-Wilmsen, T.; Tsaryk, R.; Leonard, E.V.; Bussmann, J.; Hamm, M.J.; Herzog, W.; Jin, Y.; Jakobsson, L.; et al. Endoglin controls blood vessel diameter through endothelial cell shape changes in response to haemodynamic cues. Nat. Cell Biol. 2017, 19, 653–665. [Google Scholar] [CrossRef] [Green Version]
- Clark, E.R. The transparent chamber technique for the microscopic study of living blood vessels. Anat. Rec. 1954, 120, 241–251. [Google Scholar] [CrossRef]
- Bourdeau, A.; Dumont, D.J.; Letarte, M. A murine model of hereditary hemorrhagic telangiectasia. J. Clin. Investig. 1999, 104, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, S.; Hanes, M.A.; Dickens, T.; Porteous, M.E.; Oh, S.P.; Hale, L.P.; Marchuk, D.A. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum. Mol. Genet. 2003, 12, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Torsney, E.; Charlton, R.; Diamond, A.G.; Burn, J.; Soames, J.V.; Arthur, H.M. Mouse model for hereditary hemorrhagic telangiectasia has a generalized vascular abnormality. Circulation 2003, 107, 1653–1657. [Google Scholar] [CrossRef] [Green Version]
- Hawinkels, L.J.; Kuiper, P.; Wiercinska, E.; Verspaget, H.W.; Liu, Z.; Pardali, E.; Sier, C.F.; ten Dijke, P. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010, 70, 4141–4150. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Hao, Q.; Kim, H.; Su, H.; Letarte, M.; Karumanchi, S.A.; Lawton, M.T.; Barbaro, N.M.; Yang, G.Y.; Young, W.L. Soluble endoglin modulates aberrant cerebral vascular remodeling. Ann. Neurol. 2009, 66, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Monroe, E.J. Brief Description of ISSVA Classification for Radiologists. Tech. Vasc. Interv. Radiol. 2019, 22, 100628. [Google Scholar] [CrossRef]
- Eerola, I.; Boon, L.M.; Mulliken, J.B.; Burrows, P.E.; Dompmartin, A.; Watanabe, S.; Vanwijck, R.; Vikkula, M. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am. J. Hum. Genet. 2003, 73, 1240–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amyere, M.; Revencu, N.; Helaers, R.; Pairet, E.; Baselga, E.; Cordisco, M.; Chung, W.; Dubois, J.; Lacour, J.P.; Martorell, L.; et al. Germline Loss-of-Function Mutations in EPHB4 Cause a Second Form of Capillary Malformation-Arteriovenous Malformation (CM-AVM2) Deregulating RAS-MAPK Signaling. Circulation 2017, 136, 1037–1048. [Google Scholar] [CrossRef]
- Nickel, N.P.; Spiekerkoetter, E.; Gu, M.; Li, C.G.; Li, H.; Kaschwich, M.; Diebold, I.; Hennigs, J.K.; Kim, K.Y.; Miyagawa, K.; et al. Elafin Reverses Pulmonary Hypertension via Caveolin-1-Dependent Bone Morphogenetic Protein Signaling. Am. J. Respir. Crit. Care Med. 2015, 191, 1273–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdivielso-Ramos, M.; Torrelo, A.; Martin-Santiago, A.; Hernández-Nuñez, A.; Azaña, J.M.; Campos, M.; Berenguer, B.; Garnacho, G.; Moreno, R.; Colmenero, I. Histopathological hallmarks of cutaneous lesions of capillary malformation-arteriovenous malformation syndrome. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Kurek, K.C.; Howard, E.; Tennant, L.B.; Upton, J.; Alomari, A.I.; Burrows, P.E.; Chalache, K.; Harris, D.J.; Trenor, C.C., 3rd; Eng, C.; et al. PTEN hamartoma of soft tissue: A distinctive lesion in PTEN syndromes. Am. J. Surg. Pathol. 2012, 36, 671–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccara, O.; Galmiche-Rolland, L.; Dadone-Montaudié, B.; Ariche-Maman, S.; Coulet, F.; Eyries, M.; Pannier, S.; Soupre, V.; Molina, T.; Pedeutour, F.; et al. Soft tissue angiomatosis: Another PIK3CA-related disorder. Histopathology 2020, 76, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Al-Olabi, L.; Polubothu, S.; Dowsett, K.; Andrews, K.A.; Stadnik, P.; Joseph, A.P.; Knox, R.; Pittman, A.; Clark, G.; Baird, W.; et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J. Clin. Investig. 2018, 128, 1496–1508. [Google Scholar] [CrossRef]
- Goss, J.A.; Konczyk, D.J.; Smits, P.J.; Kozakewich, H.P.W.; Alomari, A.I.; Al-Ibraheemi, A.; Taghinia, A.H.; Dickie, B.H.; Adams, D.M.; Fishman, S.J.; et al. Intramuscular fast-flow vascular anomaly contains somatic MAP2K1 and KRAS mutations. Angiogenesis 2019, 22, 547–552. [Google Scholar] [CrossRef]
- Steiner, J.E.; Drolet, B.A. Classification of Vascular Anomalies: An Update. Semin. Intervent. Radiol. 2017, 34, 225–232. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.E.; Dolati, P.; Mitha, A.P.; Wong, J.H.; Frayne, R. Flow and pressure measurements in aneurysms and arteriovenous malformations with phase contrast MR imaging. Magn. Reson. Imaging 2016, 34, 1322–1328. [Google Scholar] [CrossRef]
- Bashir, U.; Shah, S.; Jeph, S.; O’Keeffe, M.; Khosa, F. Magnetic Resonance (MR) Imaging of Vascular Malformations. Pol. J. Radiol. 2017, 82, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Hou, K.; Li, C.; Su, H.; Yu, J. Imaging Characteristics and Endovascular Treatment of Brain Arteriovenous Malformations Mainly Fed by the Posterior Cerebral Artery. Front. Neurol. 2020, 11, 609461. [Google Scholar] [CrossRef] [PubMed]
- De Gussem, E.M.; Kroon, S.; Hosman, A.E.; Kelder, J.C.; Post, M.C.; Snijder, R.J.; Mager, J.J. Hereditary Hemorrhagic Telangiectasia (HHT) and Survival: The Importance of Systematic Screening and Treatment in HHT Centers of Excellence. J. Clin. Med. 2020, 9, 3581. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, P.; Buscarini, E.; Leandro, G.; Reduzzi, L.; Grosso, M.; Pongiglione, G.; Pedrinazzi, C.; Lanzarini, L.; Portugalli, V.; Blotta, P.; et al. Contrast echocardiography for pulmonary arteriovenous malformations screening: Does any bubble matter? Eur. J. Echocardiogr. 2009, 10, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinjikji, W.; Iyer, V.N.; Wood, C.P.; Lanzino, G. Prevalence and characteristics of brain arteriovenous malformations in hereditary hemorrhagic telangiectasia: A systematic review and meta-analysis. J. Neurosurg. 2017, 127, 302–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, R.; Hashimoto, T.; Tihan, T.; Young, W.L.; Perry, V.; Lawton, M.T. Growth and regression of arteriovenous malformations in a patient with hereditary hemorrhagic telangiectasia. Case report. J. Neurosurg. 2007, 106, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latino, G.A.; Al-Saleh, S.; Carpenter, S.; Ratjen, F. The diagnostic yield of rescreening for arteriovenous malformations in children with hereditary hemorrhagic telangiectasia. J. Pediatr. 2014, 165, 197–199. [Google Scholar] [CrossRef]
- Giordano, P.; Lenato, G.M.; Suppressa, P.; Lastella, P.; Dicuonzo, F.; Chiumarulo, L.; Sangerardi, M.; Piccarreta, P.; Valerio, R.; Scardapane, A.; et al. Hereditary hemorrhagic telangiectasia: Arteriovenous malformations in children. J. Pediatr. 2013, 163, 179–186. [Google Scholar] [CrossRef]
- Togao, O.; Obara, M.; Helle, M.; Yamashita, K.; Kikuchi, K.; Momosaka, D.; Kikuchi, Y.; Nishimura, A.; Arimura, K.; Wada, T.; et al. Vessel-selective 4D-MR angiography using super-selective pseudo-continuous arterial spin labeling may be a useful tool for assessing brain AVM hemodynamics. Eur. Radiol. 2020, 30, 6452–6463. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.J.; Thakur, N.H.; Iv, M.; Fischbein, N.J.; Wintermark, M.; Dodd, R.L.; Steinberg, G.K.; Chang, S.D.; Kapadia, K.B.; Zaharchuk, G. Arterial-spin labeling MRI identifies residual cerebral arteriovenous malformation following stereotactic radiosurgery treatment. J. Neuroradiol. 2020, 47, 13–19. [Google Scholar] [CrossRef]
- Madhugiri, V.S.; Teo, M.K.C.; Westbroek, E.M.; Chang, S.D.; Marks, M.P.; Do, H.M.; Levy, R.P.; Steinberg, G.K. Multimodal management of arteriovenous malformations of the basal ganglia and thalamus: Factors affecting obliteration and outcome. J. Neurosurg. 2018, 131, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Krings, T.; Chng, S.M.; Ozanne, A.; Alvarez, H.; Rodesch, G.; Lasjaunias, P.L. Hereditary haemorrhagic telangiectasia in children. Endovascular treatment of neurovascular malformations. Results in 31 patients. Interv. Neuroradiol. 2005, 11, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.P.; Marcellus, M.L.; Santarelli, J.; Dodd, R.L.; Do, H.M.; Chang, S.D.; Adler, J.R.; Mlynash, M.; Steinberg, G.K. Embolization Followed by Radiosurgery for the Treatment of Brain Arteriovenous Malformations (AVMs). World Neurosurg. 2017, 99, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Gavin, C.G.; Ian Sabin, H. Stereotactic diffusion tensor imaging tractography for Gamma Knife radiosurgery. J. Neurosurg. 2016, 125, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Zenner, K.; Jensen, D.M.; Cook, T.T.; Dmyterko, V.; Bly, R.A.; Ganti, S.; Mirzaa, G.M.; Dobyns, W.B.; Perkins, J.A.; Bennett, J.T. Cell-free DNA as a diagnostic analyte for molecular diagnosis of vascular malformations. Genet. Med. 2021, 23, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Raj, J.A.; Stoodley, M. Experimental Animal Models of Arteriovenous Malformation: A Review. Vet. Sci. 2015, 2, 97–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tual-Chalot, S.; Oh, S.P.; Arthur, H.M. Mouse models of hereditary hemorrhagic telangiectasia: Recent advances and future challenges. Front. Genet. 2015, 6, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.Y.; Sorensen, L.K.; Brooke, B.S.; Urness, L.D.; Davis, E.C.; Taylor, D.G.; Boak, B.B.; Wendel, D.P. Defective angiogenesis in mice lacking endoglin. Science 1999, 284, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Arthur, H.M.; Ure, J.; Smith, A.J.; Renforth, G.; Wilson, D.I.; Torsney, E.; Charlton, R.; Parums, D.V.; Jowett, T.; Marchuk, D.A.; et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev. Biol. 2000, 217, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.P.; Seki, T.; Goss, K.A.; Imamura, T.; Yi, Y.; Donahoe, P.K.; Li, L.; Miyazono, K.; ten Dijke, P.; Kim, S.; et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 2626–2631. [Google Scholar] [CrossRef] [Green Version]
- Urness, L.D.; Sorensen, L.K.; Li, D.Y. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat. Genet. 2000, 26, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Milton, I.; Ouyang, D.; Allen, C.J.; Yanasak, N.E.; Gossage, J.R.; Alleyne, C.H., Jr.; Seki, T. Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke 2012, 43, 1432–1435. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Sun, Z.; Han, Z.; Jun, K.; Camus, M.; Wankhede, M.; Mao, L.; Arnold, T.; Young, W.L.; Su, H. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 2014, 45, 900–902. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Young, W.L.; Su, H. Induction of brain arteriovenous malformation in the adult mouse. Methods Mol. Biol. 2014, 1135, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Corti, P.; Young, S.; Chen, C.Y.; Patrick, M.J.; Rochon, E.R.; Pekkan, K.; Roman, B.L. Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development 2011, 138, 1573–1582. [Google Scholar] [CrossRef] [Green Version]
- Roman, B.L.; Pham, V.N.; Lawson, N.D.; Kulik, M.; Childs, S.; Lekven, A.C.; Garrity, D.M.; Moon, R.T.; Fishman, M.C.; Lechleider, R.J.; et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 2002, 129, 3009–3019. [Google Scholar] [CrossRef] [PubMed]
- Walcott, B.P.; Peterson, R.T. Zebrafish models of cerebrovascular disease. J. Cereb. Blood Flow Metab. 2014, 34, 571–577. [Google Scholar] [CrossRef]
- Ruiz, S.; Zhao, H.; Chandakkar, P.; Chatterjee, P.K.; Papoin, J.; Blanc, L.; Metz, C.N.; Campagne, F.; Marambaud, P. A mouse model of hereditary hemorrhagic telangiectasia generated by transmammary-delivered immunoblocking of BMP9 and BMP10. Sci. Rep. 2016, 5, 37366. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Chandakkar, P.; Zhao, H.; Papoin, J.; Chatterjee, P.K.; Christen, E.; Metz, C.N.; Blanc, L.; Campagne, F.; Marambaud, P. Tacrolimus rescues the signaling and gene expression signature of endothelial ALK1 loss-of-function and improves HHT vascular pathology. Hum. Mol. Genet. 2017, 26, 4786–4798. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Zhao, H.; Chandakkar, P.; Papoin, J.; Choi, H.; Nomura-Kitabayashi, A.; Patel, R.; Gillen, M.; Diao, L.; Chatterjee, P.K.; et al. Correcting Smad1/5/8, mTOR, and VEGFR2 treats pathology in hereditary hemorrhagic telangiectasia models. J. Clin. Investig. 2020, 130, 942–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, D.; Starke, R.M.; Sheehan, J.P. Radiosurgery for the management of cerebral arteriovenous malformations. Handb. Clin. Neurol. 2017, 143, 69–83. [Google Scholar] [CrossRef]
- Jahan, R.; Solberg, T.D.; Lee, D.; Medin, P.; Tateshima, S.; De Salles, A.; Sayre, J.; Vinters, H.V.; Viñuela, F. An arteriovenous malformation model for stereotactic radiosurgery research. Neurosurgery 2007, 61, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg-Schwager, M. Induction, repair and biological relevance of radiation-induced DNA lesions in eukaryotic cells. Radiat. Environ. Biophys. 1990, 29, 273–292. [Google Scholar] [CrossRef]
- Mut, M.; Oge, K.; Zorlu, F.; Undeğer, U.; Erdem, S.; Ozcan, O.E. Effects of ionizing radiation on brain tissue surrounding arteriovenous malformations: An experimental study in a rat caroticojugular fistula model. Neurosurg. Rev. 2004, 27, 121–127. [Google Scholar] [CrossRef]
- Lunec, J. Free radicals: Their involvement in disease processes. Ann. Clin. Biochem 1990, 27, 173–182. [Google Scholar] [CrossRef]
- Kiliç, K.; Konya, D.; Kurtkaya, O.; Sav, A.; Pamir, M.N.; Kiliç, T. Inhibition of angiogenesis induced by cerebral arteriovenous malformations using gamma knife irradiation. J. Neurosurg. 2007, 106, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Storer, K.; Tu, J.; Karunanayaka, A.; Smee, R.; Short, R.; Thorpe, P.; Stoodley, M. Coadministration of low-dose lipopolysaccharide and soluble tissue factor induces thrombosis after radiosurgery in an animal arteriovenous malformation model. Neurosurgery 2007, 61, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Storer, K.P.; Tu, J.; Stoodley, M.A.; Smee, R.I. Expression of endothelial adhesion molecules after radiosurgery in an animal model of arteriovenous malformation. Neurosurgery 2010, 67, 976–983. [Google Scholar] [CrossRef]
- Cai, J.; Pardali, E.; Sanchez-Duffhues, G.; ten Dijke, P. BMP signaling in vascular diseases. FEBS Lett. 2012, 586, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.H.; Trembath, R.C.; Morse, J.A.; Grunig, E.; Loyd, J.E.; Adnot, S.; Coccolo, F.; Ventura, C.; Phillips, J.A., 3rd; Knowles, J.A.; et al. Genetic basis of pulmonary arterial hypertension: Current understanding and future directions. J. Am. Coll Cardiol. 2004, 43, 33S–39S. [Google Scholar] [CrossRef] [Green Version]
- Garcia de Vinuesa, A.; Abdelilah-Seyfried, S.; Knaus, P.; Zwijsen, A.; Bailly, S. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev. 2016, 27, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Tillet, E.; Bailly, S. Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia. Front. Genet. 2014, 5, 456. [Google Scholar] [CrossRef] [Green Version]
- McAllister, K.A.; Grogg, K.M.; Johnson, D.W.; Gallione, C.J.; Baldwin, M.A.; Jackson, C.E.; Helmbold, E.A.; Markel, D.S.; McKinnon, W.C.; Murrell, J.; et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994, 8, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Ricard, N.; Bidart, M.; Mallet, C.; Lesca, G.; Giraud, S.; Prudent, R.; Feige, J.J.; Bailly, S. Functional analysis of the BMP9 response of ALK1 mutants from HHT2 patients: A diagnostic tool for novel ACVRL1 mutations. Blood 2010, 116, 1604–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaa El Din, F.; Patri, S.; Thoreau, V.; Rodriguez-Ballesteros, M.; Hamade, E.; Bailly, S.; Gilbert-Dussardier, B.; Abou Merhi, R.; Kitzis, A. Functional and splicing defect analysis of 23 ACVRL1 mutations in a cohort of patients affected by Hereditary Hemorrhagic Telangiectasia. PLoS ONE 2015, 10, e0132111. [Google Scholar] [CrossRef] [Green Version]
- Mallet, C.; Lamribet, K.; Giraud, S.; Dupuis-Girod, S.; Feige, J.J.; Bailly, S.; Tillet, E. Functional analysis of endoglin mutations from hereditary hemorrhagic telangiectasia type 1 patients reveals different mechanisms for endoglin loss of function. Hum. Mol. Genet. 2015, 24, 1142–1154. [Google Scholar] [CrossRef]
- Crist, A.M.; Zhou, X.; Garai, J.; Lee, A.R.; Thoele, J.; Ullmer, C.; Klein, C.; Zabaleta, J.; Meadows, S.M. Angiopoietin-2 Inhibition Rescues Arteriovenous Malformation in a Smad4 Hereditary Hemorrhagic Telangiectasia Mouse Model. Circulation 2019, 139, 2049–2063. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, Y.H.; Choe, S.W.; Tak, Y.G.; Garrido-Martin, E.M.; Chang, M.; Lee, Y.J.; Oh, S.P. Enhanced responses to angiogenic cues underlie the pathogenesis of hereditary hemorrhagic telangiectasia 2. PLoS ONE 2013, 8, e63138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, T.; Emala, C.W.; Joshi, S.; Mesa-Tejada, R.; Quick, C.M.; Feng, L.; Libow, A.; Marchuk, D.A.; Young, W.L. Abnormal pattern of Tie-2 and vascular endothelial growth factor receptor expression in human cerebral arteriovenous malformations. Neurosurgery 2000, 47, 910–918. [Google Scholar] [CrossRef]
- Hashimoto, T.; Wu, Y.; Lawton, M.T.; Yang, G.Y.; Barbaro, N.M.; Young, W.L. Coexpression of angiogenic factors in brain arteriovenous malformations. Neurosurgery 2005, 56, 1058–1065. [Google Scholar] [PubMed]
- Kashiwazaki, D.; Kobayashi, R.; Houkin, K.; Kuroda, S. Increased expression of vascular endothelial growth factor and its receptor in enlarging brain arteriovenous malformations—A case report. Br. J. Neurosurg. 2014, 28, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Kilic, T.; Pamir, M.N.; Kullu, S.; Eren, F.; Ozek, M.M.; Black, P.M. Expression of structural proteins and angiogenic factors in cerebrovascular anomalies. Neurosurgery 2000, 46, 1179–1191. [Google Scholar] [CrossRef]
- Koizumi, T.; Shiraishi, T.; Hagihara, N.; Tabuchi, K.; Hayashi, T.; Kawano, T. Expression of vascular endothelial growth factors and their receptors in and around intracranial arteriovenous malformations. Neurosurgery 2002, 50, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sadick, H.; Naim, R.; Sadick, M.; Hormann, K.; Riedel, F. Plasma level and tissue expression of angiogenic factors in patients with hereditary hemorrhagic telangiectasia. Int. J. Mol. Med. 2005, 15, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Sadick, H.; Naim, R.; Gossler, U.; Hormann, K.; Riedel, F. Angiogenesis in hereditary hemorrhagic telangiectasia: VEGF165 plasma concentration in correlation to the VEGF expression and microvessel density. Int. J. Mol. Med. 2005, 15, 15–19. [Google Scholar] [CrossRef]
- Botella, L.M.; Albinana, V.; Ojeda-Fernandez, L.; Recio-Poveda, L.; Bernabeu, C. Research on potential biomarkers in hereditary hemorrhagic telangiectasia. Front. Genet. 2015, 6, 115. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Wu, Y.Q.; Huey, M.; Arthur, H.M.; Marchuk, D.A.; Hashimoto, T.; Young, W.L.; Yang, G.Y. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J. Cereb. Blood Flow Metab. 2004, 24, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.; Ma, L.; Shaligram, S.; Walker, E.J.; Yang, S.T.; Tang, C.; Zhu, W.; Zhan, L.; Li, Q.; Zhu, X.; et al. Effect of elevation of vascular endothelial growth factor level on exacerbation of hemorrhage in mouse brain arteriovenous malformation. J. Neurosurg. 2019, 132, 1566–1573. [Google Scholar] [CrossRef] [Green Version]
- Shao, E.S.; Lin, L.; Yao, Y.; Bostrom, K.I. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood 2009, 114, 2197–2206. [Google Scholar] [CrossRef] [Green Version]
- Al-Samkari, H.; Kasthuri, R.S.; Parambil, J.G.; Albitar, H.A.; Almodallal, Y.A.; Vazquez, C.; Serra, M.M.; Dupuis-Girod, S.; Wilsen, C.B.; McWilliams, J.P.; et al. An international, multicenter study of intravenous bevacizumab for bleeding in hereditary hemorrhagic telangiectasia: The InHIBIT-Bleed study. Haematologica 2020, 106, 2161. [Google Scholar] [CrossRef] [PubMed]
- Ola, R.; Kunzel, S.H.; Zhang, F.; Genet, G.; Chakraborty, R.; Pibouin-Fragner, L.; Martin, K.; Sessa, W.; Dubrac, A.; Eichmann, A. SMAD4 Prevents Flow Induced Arteriovenous Malformations by Inhibiting Casein Kinase 2. Circulation 2018, 138, 2379–2394. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [Green Version]
- Nikolaev, S.I.; Vetiska, S.; Bonilla, X.; Boudreau, E.; Jauhiainen, S.; Rezai Jahromi, B.; Khyzha, N.; DiStefano, P.V.; Suutarinen, S.; Kiehl, T.R.; et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N. Engl. J. Med. 2018, 378, 250–261. [Google Scholar] [CrossRef]
- Zúñiga-Castillo, M.; Teng, C.L.; Teng, J.M.C. Genetics of vascular malformation and therapeutic implications. Curr. Opin. Pediatr. 2019, 31, 498–508. [Google Scholar] [CrossRef]
- Guba, M.; von Breitenbuch, P.; Steinbauer, M.; Koehl, G.; Flegel, S.; Hornung, M.; Bruns, C.J.; Zuelke, C.; Farkas, S.; Anthuber, M.; et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat. Med. 2002, 8, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Phung, T.L.; Ziv, K.; Dabydeen, D.; Eyiah-Mensah, G.; Riveros, M.; Perruzzi, C.; Sun, J.; Monahan-Earley, R.A.; Shiojima, I.; Nagy, J.A.; et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 2006, 10, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.M.; Trenor, C.C., 3rd; Hammill, A.M.; Vinks, A.A.; Patel, M.N.; Chaudry, G.; Wentzel, M.S.; Mobberley-Schuman, P.S.; Campbell, L.M.; Brookbank, C.; et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics 2016, 137, e20153257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelliah, M.P.; Do, H.M.; Zinn, Z.; Patel, V.; Jeng, M.; Khosla, R.K.; Truong, M.T.; Marqueling, A.; Teng, J.M.C. Management of Complex Arteriovenous Malformations Using a Novel Combination Therapeutic Algorithm. JAMA Dermatol. 2018, 154, 1316–1319. [Google Scholar] [CrossRef]
- Colletti, G.; Dalmonte, P.; Moneghini, L.; Ferrari, D.; Allevi, F. Adjuvant role of anti-angiogenic drugs in the management of head and neck arteriovenous malformations. Med. Hypotheses 2015, 85, 298–302. [Google Scholar] [CrossRef]
- Lekwuttikarn, R.; Lim, Y.H.; Admani, S.; Choate, K.A.; Teng, J.M.C. Genotype-Guided Medical Treatment of an Arteriovenous Malformation in a Child. JAMA Dermatol. 2019, 155, 256–257. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schimmel, K.; Ali, M.K.; Tan, S.Y.; Teng, J.; Do, H.M.; Steinberg, G.K.; Stevenson, D.A.; Spiekerkoetter, E. Arteriovenous Malformations—Current Understanding of the Pathogenesis with Implications for Treatment. Int. J. Mol. Sci. 2021, 22, 9037. https://doi.org/10.3390/ijms22169037
Schimmel K, Ali MK, Tan SY, Teng J, Do HM, Steinberg GK, Stevenson DA, Spiekerkoetter E. Arteriovenous Malformations—Current Understanding of the Pathogenesis with Implications for Treatment. International Journal of Molecular Sciences. 2021; 22(16):9037. https://doi.org/10.3390/ijms22169037
Chicago/Turabian StyleSchimmel, Katharina, Md Khadem Ali, Serena Y. Tan, Joyce Teng, Huy M. Do, Gary K. Steinberg, David A. Stevenson, and Edda Spiekerkoetter. 2021. "Arteriovenous Malformations—Current Understanding of the Pathogenesis with Implications for Treatment" International Journal of Molecular Sciences 22, no. 16: 9037. https://doi.org/10.3390/ijms22169037
APA StyleSchimmel, K., Ali, M. K., Tan, S. Y., Teng, J., Do, H. M., Steinberg, G. K., Stevenson, D. A., & Spiekerkoetter, E. (2021). Arteriovenous Malformations—Current Understanding of the Pathogenesis with Implications for Treatment. International Journal of Molecular Sciences, 22(16), 9037. https://doi.org/10.3390/ijms22169037






