The Pathogenesis and Therapeutic Approaches of Diabetic Neuropathy in the Retina
Abstract
1. Introduction
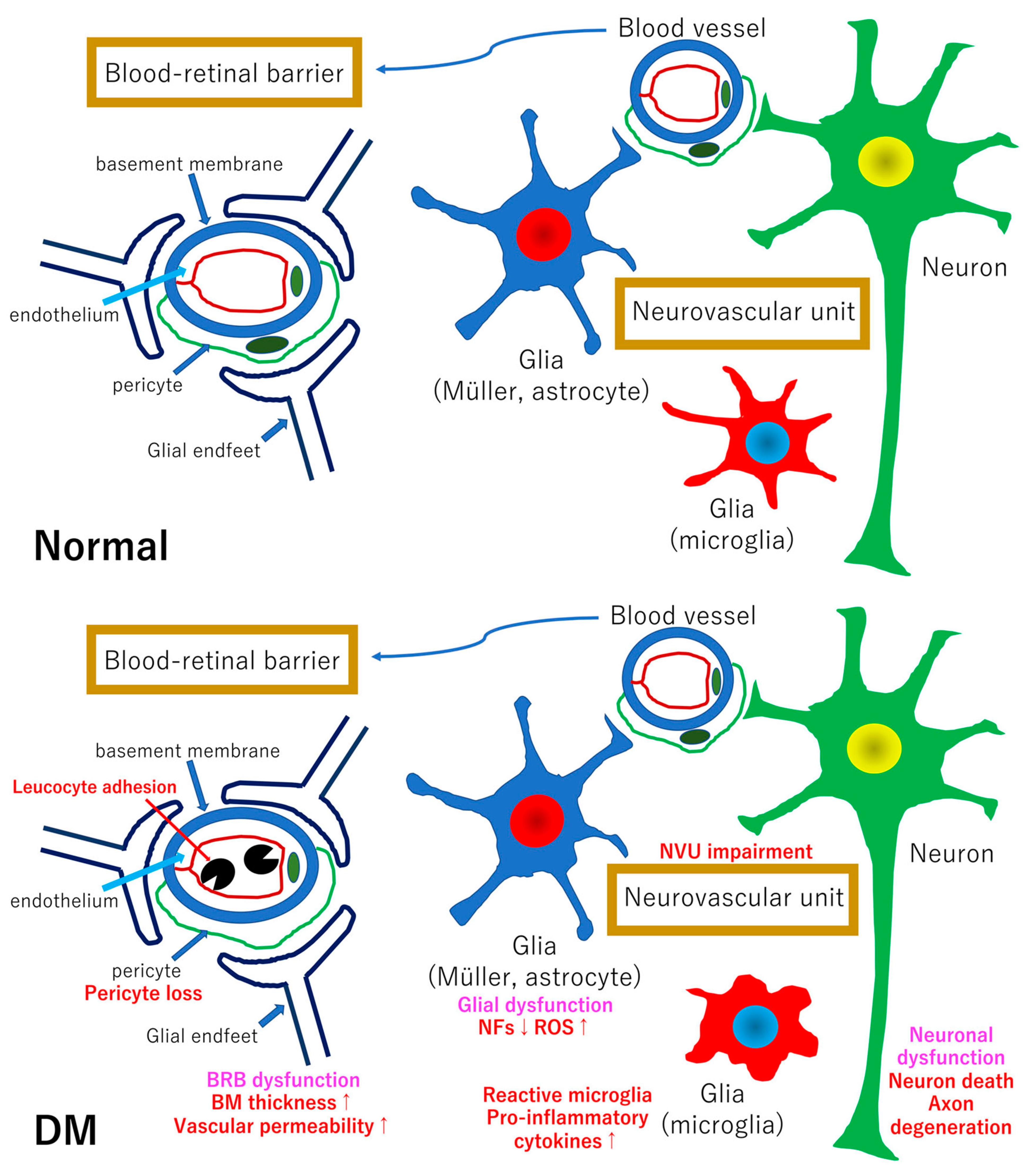
2. Pathogenesis of the Diabetic Retinopathy: The Impairment of the Neurovascular Unit
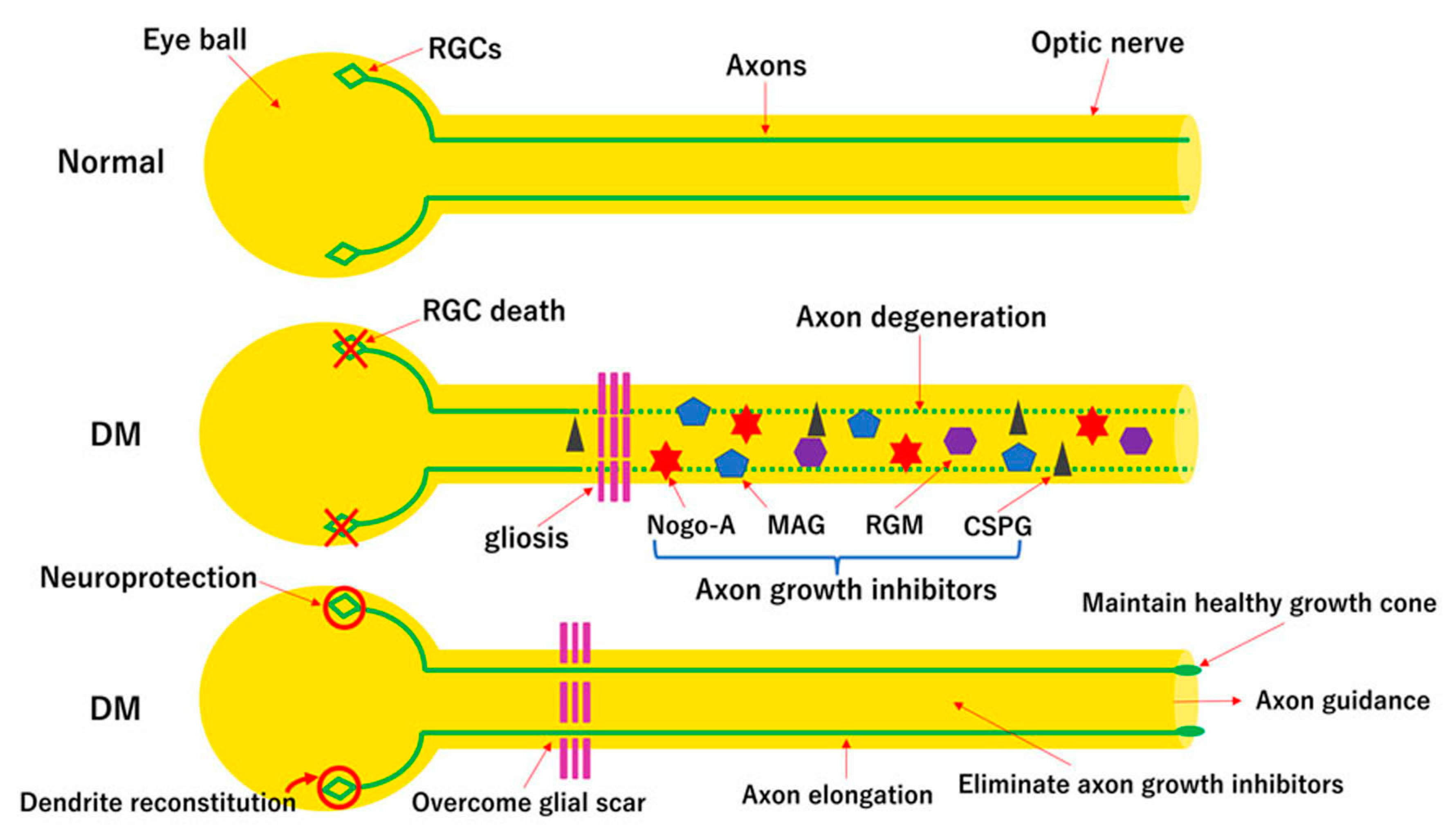
3. Multimodal Approaches for Axonal Protection and Regeneration
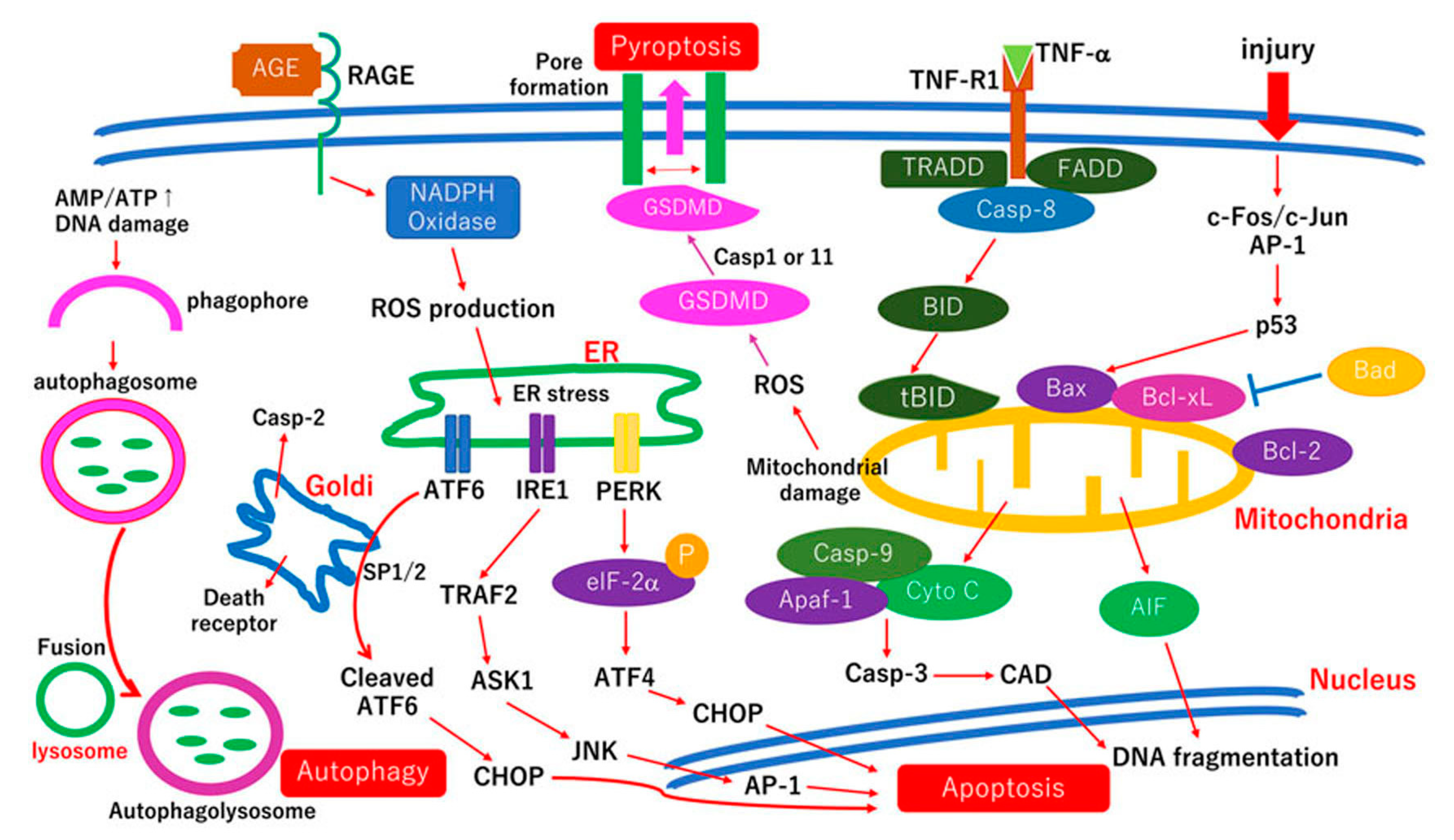
3.1. Mechanisms of Neuronal Cell Death in RGC Neuropathy in Diabetic Retina
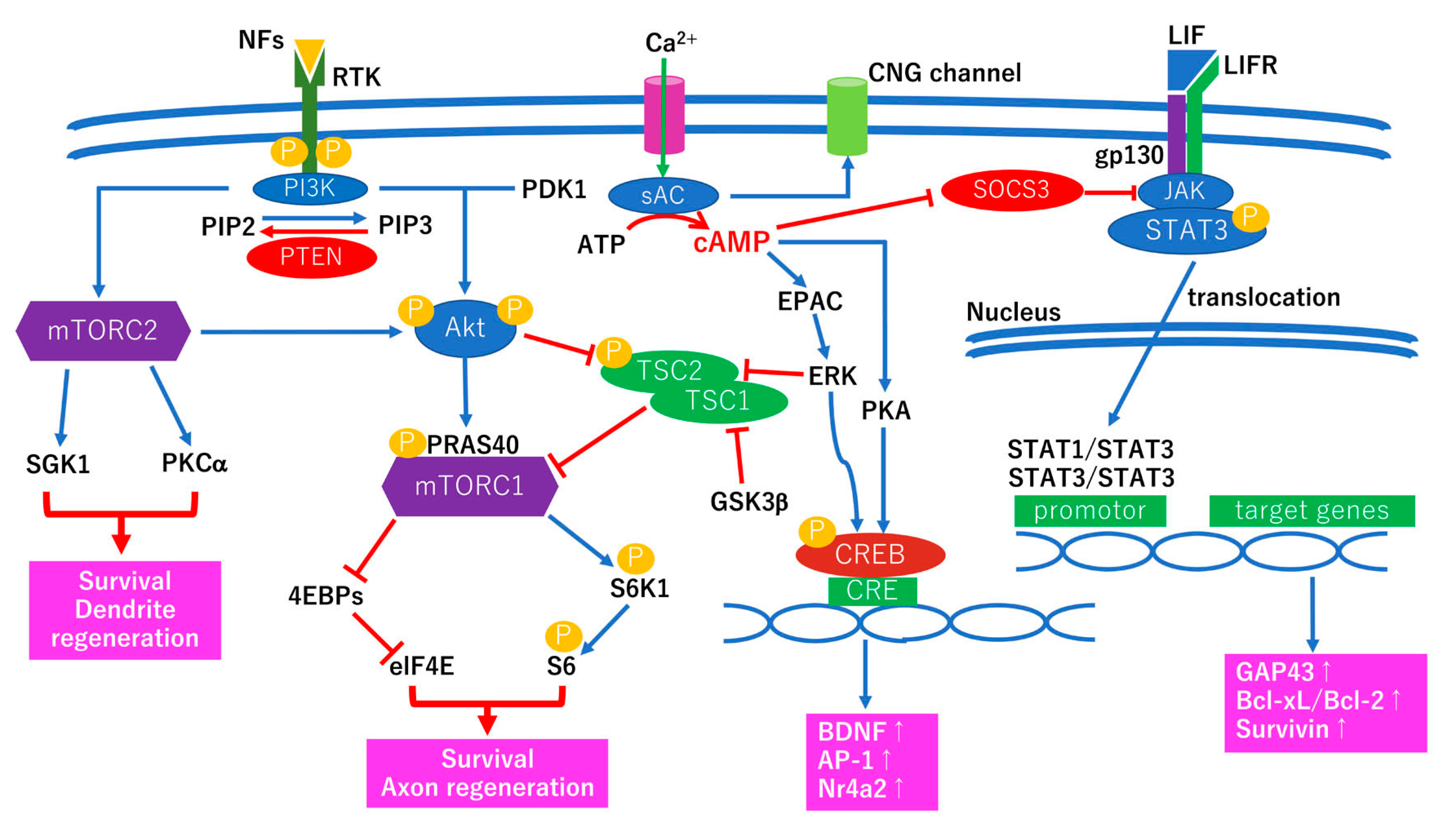
3.2. Intrinsic Survival and Regenerative Pathways for Optic Nerve Regeneration
3.3. Multimodal Strategies for Optic Nerve Regeneration
4. Neuroprotective Therapies for Diabetic Retinopathy
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Thomas, R.L.; Halim, S.; Gurudas, S.; Sivaprasad, S.; Owens, D.R. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res. Clin. Pract. 2019, 157, 107840. [Google Scholar] [CrossRef]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Araki, S.I.; Kawai, K.; Yamazaki, K.; Tomonaga, O.; Shirabe, S.I.; Maegawa, H. Declining trends of diabetic nephropathy, retinopathy and neuropathy with improving diabetes care indicators in Japanese patients with type 2 and type 1 diabetes (JDDM 46). BMJ Open Diabetes Res. Care 2018, 6, e000521. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Sango, K.; Horie, H.; Sato, M.; Iijima, Y.; Ohno, S.; Inoue, S.; Ishikawa, Y. Diabetes alters neurite regeneration from mouse retinal explants in culture. Neurosci. Lett. 1999, 275, 175–178. [Google Scholar] [CrossRef]
- Oshitari, T.; Roy, S. Diabetes: A potential enhancer of retinal injury in rat retinas. Neurosci. Lett. 2005, 390, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.R.; Cristóvão, A.J.; Santos, P.F.; Carvalho, C.M.; Ambrósio, A.F. High glucose induces caspase-independent cell death in retinal neural cells. Neurobiol. Dis. 2007, 25, 464–472. [Google Scholar] [CrossRef]
- Oshitari, T.; Yoshida-Hata, N.; Yamamoto, S. Effect of neurotrophic factors on neuronal apoptosis and neurite regeneration in cultured rat retinas exposed to high glucose. Brain Res. 2010, 1346, 43–51. [Google Scholar] [CrossRef]
- Oshitari, T.; Yoshida-Hata, N.; Yamamoto, S. Effect of neurotrophin-4 on endoplasmic reticulum stress-related neuronal apoptosis in diabetic and high glucose exposed rat retinas. Neurosci. Lett. 2011, 501, 102–106. [Google Scholar] [CrossRef]
- Bikbova, G.; Oshitari, T.; Yamamoto, S. Neurite regeneration in adult rat retinas exposed to advanced glycation end-products and regenerative effects of neurotrophin-4. Brain Res. 2013, 1534, 33–45. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, L.; Fu, X.; Zhao, Y.; Xu, X. BDNF protects retinal neurons from hyperglycemia through the TrkB/ERK/MAPK pathway. Mol. Med. Rep. 2013, 7, 1773–1778. [Google Scholar] [CrossRef]
- Oshitari, T.; Bikbova, G.; Yamamoto, S. Increased expression of phosphorylated c-Jun and phosphorylated c-Jun N-terminal kinase associated with neuronal cell death in diabetic and high glucose exposed rat retinas. Brain Res. Bull. 2014, 101, 18–25. [Google Scholar] [CrossRef]
- Bikbova, G.; Oshitari, T.; Baba, T.; Yamamoto, S. Altered expression of NF- κ B and SP1 after exposure to advanced glycation end-products and effects of neurotrophic factors in AGEs exposed rat retinas. J. Diabetes Res. 2015, 2015, 543818. [Google Scholar] [CrossRef]
- Bikbova, G.; Oshitari, T.; Baba, T.; Yamamoto, S. Mechanisms of neuronal cell death in AGE-exposed retinas-research and literature review. Curr. Diabetes Rev. 2017, 13, 280–288. [Google Scholar] [CrossRef]
- Bikbova, G.; Oshitari, T.; Baba, T.; Yamamoto, S. Combination of neuroprotective and regenerative agents for AGE-induced retinal degeneration: In vitro study. Biomed. Res. Int. 2017, 2017, 8604723. [Google Scholar] [CrossRef]
- Asnaghi, V.; Gerhardinger, C.; Hoehn, T.; Adeboje, A.; Lorenzi, M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes 2003, 52, 506–511. [Google Scholar] [CrossRef]
- Seki, M.; Tanaka, T.; Nawa, H.; Usui, T.; Fukuchi, T.; Ikeda, K.; Abe, H.; Takei, N. Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: Therapeutic potential of brain-derived neurotrophic factor for dopaminergic amacrine cells. Diabetes 2004, 53, 2412–2419. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef]
- Hernández, C.; García-Ramírez, M.; Corraliza, L.; Fernández-Carneado, J.; Farrera-Sinfreu, J.; Ponsati, B.; González-Rodríguez, A.; Valverde, A.M.; Simó, R. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes 2013, 62, 2569–2578. [Google Scholar] [CrossRef]
- Hombrebueno, J.R.; Chen, M.; Penalva, R.G.; Xu, H. Loss of synaptic connectivity, particularly in second order neurons is a key feature of diabetic retinal neuropathy in the Ins2Akita mouse. PLoS ONE 2014, 9, e97970. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef]
- Bogdanov, P.; Hernández, C.; Corraliza, L.; Carvalho, A.R.; Simó, R. Effect of fenofibrate on retinal neurodegeneration in an experimental model of type 2 diabetes. Acta Diabetol. 2015, 52, 113–122. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Litsea japonica extract inhibits neuronal apoptosis and the accumulation of advanced glycation end products in the diabetic mouse retina. Mol. Med. Rep. 2015, 12, 1075–1081. [Google Scholar] [CrossRef]
- Hernández, C.; Bogdanov, P.; Corraliza, L.; García-Ramírez, M.; Solà-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simó, R. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef]
- Hernández, C.; Bogdanov, P.; Solà-Adell, C.; Sampedro, J.; Valeri, M.; Genís, X.; Simó-Servat, O.; García-Ramírez, M.; Simó, R. Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 2017, 60, 2285–2298. [Google Scholar] [CrossRef]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid naringenin attenuates oxidative stress, apoptosis and improves neurotrophic effects in the diabetic rat retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef]
- Ma, M.; Xu, Y.; Xiong, S.; Zhang, J.; Gu, Q.; Ke, B.; Xu, X. Involvement of ciliary neurotrophic factor in early diabetic retinal neuropathy in streptozotocin-induced diabetic rats. Eye (Lond) 2018, 32, 1463–1471. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, W.; Zhao, Y.; Shu, X.; Wang, W.; Wang, D.; Yang, Y.; He, Z.; Wang, X.; Ying, Y. GSK3β-mediated tau hyperphosphorylation triggers diabetic retinal neurodegeneration by disrupting synaptic and mitochondrial functions. Mol. Neurodegener. 2018, 13, 62. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, Y.; Li, M.; Huang, X.; Yang, Y.; Zeng, J.; Zhao, Y.; Wang, X.; Zhang, W.; Ying, Y. Topical ocular administration of the GLP-1 receptor agonist liraglutide arrests hyperphosphorylated tau-triggered diabetic retinal neurodegeneration via activation of GLP-1R/Akt/GSK3β signaling. Neuropharmacology 2019, 153, 1–12. [Google Scholar] [CrossRef]
- Fan, Y.; Lai, J.; Yuan, Y.; Wang, L.; Wang, Q.; Yuan, F. Taurine protects retinal cells and improves synaptic connections in early diabetic rats. Curr. Eye Res. 2020, 45, 52–63. [Google Scholar] [CrossRef]
- Ibán-Arias, R.; Lisa, S.; Poulaki, S.; Mastrodimou, N.; Charalampopoulos, I.; Gravanis, A.; Thermos, K. Effect of topical administration of the microneurotrophin BNN27 in the diabetic rat retina. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2429–2436. [Google Scholar] [CrossRef]
- Liu, J.; Wei, L.; Wang, Z.; Song, S.; Lin, Z.; Zhu, J.; Ren, X.; Kong, L. Protective effect of Liraglutide on diabetic retinal neurodegeneration via inhibiting oxidative stress and endoplasmic reticulum stress. Neurochem. Int. 2020, 133, 104624. [Google Scholar] [CrossRef]
- Suzumura, A.; Kaneko, H.; Funahashi, Y.; Takayama, K.; Nagaya, M.; Ito, S.; Okuno, T.; Hirakata, T.; Nonobe, N.; Kataoka, K.; et al. n-3 fatty acid and its metabolite 18-HEPE ameliorate retinal neuronal cell dysfunction by enhancing Müller BDNF in diabetic retinopathy. Diabetes 2020, 69, 724–735. [Google Scholar] [CrossRef]
- Zhou, H.R.; Ma, X.F.; Lin, W.J.; Hao, M.; Yu, X.Y.; Li, H.X.; Xu, C.Y.; Kuang, H.Y. Neuroprotective role of GLP-1 analog for retinal ganglion cells via PINK1/Parkin-mediated mitophagy in diabetic retinopathy. Front. Pharmacol. 2021, 11, 589114. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T.; Yamamoto, S.; Hata, N.; Roy, S. Mitochondria- and caspase-dependent cell death pathway involved in neuronal degeneration in diabetic retinopathy. Br. J. Ophthalmol. 2008, 92, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T.; Yamamoto, S.; Roy, S. Increased expression of c-Fos, c-Jun and c-Jun N-terminal kinase associated with neuronal cell death in retinas of diabetic patients. Curr. Eye Res. 2014, 39, 527–531. [Google Scholar] [CrossRef]
- Hu, P.; Hunt, N.H.; Arfuso, F.; Shaw, L.C.; Uddin, M.N.; Zhu, M.; Devasahayam, R.; Adamson, S.J.; Benson, V.L.; Chan-Ling, T.; et al. Increased indoleamine 2,3-dioxygenase and quinolinic acid expression in microglia and Müller cells of diabetic human and rodent retina. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5043–5055. [Google Scholar] [CrossRef]
- Oshitari, T.; Hanawa, K.; Adachi-Usami, E. Changes of macular and RNFL thicknesses measured by Stratus OCT in patients with early stage diabetes. Eye (Lond) 2009, 23, 884–889. [Google Scholar] [CrossRef]
- van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Garvin, M.K.; Sonka, M.; Lee, K.; Devries, J.H.; Michels, R.P.; van Velthoven, M.E.; Schlingemann, R.O.; et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3660–3665. [Google Scholar] [CrossRef]
- van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Stehouwer, M.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abràmoff, M.D. Early neurodegeneration in the retina of type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2715–2719. [Google Scholar] [CrossRef]
- Chen, X.; Nie, C.; Gong, Y.; Zhang, Y.; Jin, X.; Wei, S.; Zhang, M. Peripapillary retinal nerve fiber layer changes in preclinical diabetic retinopathy: A meta-analysis. PLoS ONE 2015, 10, e0125919. [Google Scholar]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Cao, D.; Yu, H.; Yang, D.; Zhuang, X.; Hu, Y.; Li, J.; Yang, J.; Wu, Q.; Liu, B.; et al. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br. J. Ophthalmol. 2019, 103, 1747–1752. [Google Scholar]
- Toprak, I.; Fenkci, S.M.; Fidan Yaylali, G.; Martin, C.; Yaylali, V. Early retinal neurodegeneration in preclinical diabetic retinopathy: A multifactorial investigation. Eye (Lond) 2020, 34, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Montesano, G.; Ometto, G.; Higgins, B.E.; Das, R.; Graham, K.W.; Chakravarthy, U.; McGuiness, B.; Young, I.S.; Kee, F.; Wright, D.M.; et al. Evidence for structural and functional damage of the inner retina in diabetes with no diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 35. [Google Scholar] [CrossRef]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef]
- Metea, M.R.; Newman, E.A. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol. 2007, 92, 635–640. [Google Scholar] [CrossRef]
- Hammes, H.P. Diabetic retinopathy: Hyperglycaemia, oxidative stress and beyond. Diabetologia 2018, 61, 29–38. [Google Scholar] [CrossRef]
- Rinaldi, C.; Donato, L.; Alibrandi, S.; Scimone, C.; D’Angelo, R.; Sidoti, A. Oxidative stress and neurovascular unit. Life 2021, 11, 767. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Wang, X.; Ma, W.; Lazere, A.; Qian, H.H.; Zhang, J.; Abu-Asab, M.; Fariss, R.N.; Roger, J.E.; et al. Repopulating retinal microglia restore endogenous organization and function under CX3CL1-CX3CR1 regulation. Sci. Adv. 2018, 4, eaap8492. [Google Scholar] [CrossRef]
- Genové, G.; Mollick, T.; Johansson, K. Photoreceptor degeneration, structural remodeling and glial activation: A morphological study on a genetic mouse model for pericyte deficiency. Neuroscience 2014, 279, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Tien, T.; Barrette, K.F.; Chronopoulos, A.; Roy, S. Effects of high glucose-induced Cx43 downregulation on occludin and ZO-1 expression and tight junction barrier function in retinal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6518–6525. [Google Scholar] [CrossRef]
- Oshitari, T.; Polewski, P.; Chadda, M.; Li, A.F.; Sato, T.; Roy, S. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes 2006, 55, 86–92. [Google Scholar] [CrossRef][Green Version]
- Trost, A.; Lange, S.; Schroedl, F.; Bruckner, D.; Motloch, K.A.; Bogner, B.; Kaser-Eichberger, A.; Strohmaier, C.; Runge, C.; Aigner, L.; et al. Brain and Retinal Pericytes: Origin, Function and Role. Front. Cell Neurosci. 2016, 10, 20. [Google Scholar] [CrossRef]
- Park, D.Y.; Lee, J.; Kim, J.; Kim, K.; Hong, S.; Han, S.; Kubota, Y.; Augustin, H.G.; Ding, L.; Kim, J.W.; et al. Plastic roles of pericytes in the blood-retinal barrier. Nat. Commun. 2017, 8, 15296. [Google Scholar] [CrossRef] [PubMed]
- Pfister, F.; Feng, Y.; vom Hagen, F.; Hoffmann, S.; Molema, G.; Hillebrands, J.L.; Shani, M.; Deutsch, U.; Hammes, H.P. Pericyte migration: A novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 2008, 57, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Gerhardinger, C.; Lorenzi, M. Müller cell changes in human diabetic retinopathy. Diabetes 1998, 47, 445–449. [Google Scholar] [CrossRef]
- Pannicke, T.; Iandiev, I.; Wurm, A.; Uckermann, O.; vom Hagen, F.; Reichenbach, A.; Wiedemann, P.; Hammes, H.P.; Bringmann, A. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes 2006, 55, 633–639. [Google Scholar] [CrossRef]
- Portillo, J.C.; Lopez Corcino, Y.; Miao, Y.; Tang, J.; Sheibani, N.; Kern, T.S.; Dubyak, G.R.; Subauste, C.S. CD40 in retinal Müller cells induces P2X7-dependent cytokine expression in macrophages/microglia in diabetic mice and development of early experimental diabetic retinopathy. Diabetes 2017, 66, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Rungger-Brändle, E.; Dosso, A.A.; Leuenberger, P.M. Glial reactivity, an early feature of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1971–1980. [Google Scholar] [PubMed]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Elliott, M.H.; Zhu, M.; Le, Y.Z. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 2010, 59, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, A.; Gaddini, L.; Villa, M.; Varano, M.; Parravano, M.; Monteleone, V.; Cavallo, F.; Leo, L.; Mallozzi, C.; Malchiodi-Albedi, F.; et al. Neuroprotection by rat Müller glia against high glucose-induced neurodegeneration through a mechanism involving ERK1/2 activation. Exp. Eye Res. 2014, 125, 20–29. [Google Scholar] [CrossRef]
- Kinuthia, U.M.; Wolf, A.; Langmann, T. Microglia and inflammatory responses in diabetic retinopathy. Front. Immunol. 2020, 11, 564077. [Google Scholar] [CrossRef]
- Gaucher, D.; Chiappore, J.A.; Pâques, M.; Simonutti, M.; Boitard, C.; Sahel, J.A.; Massin, P.; Picaud, S. Microglial changes occur without neural cell death in diabetic retinopathy. Vision Res. 2007, 47, 612–623. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Gong, Y.; Wei, S.; Zhang, M. Early spatiotemporal characterization of microglial activation in the retinas of rats with streptozotocin-induced diabetes. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 519–525. [Google Scholar] [CrossRef]
- Arroba, A.I.; Alcalde-Estevez, E.; García-Ramírez, M.; Cazzoni, D.; de la Villa, P.; Sánchez-Fernández, E.M.; Mellet, C.O.; García Fernández, J.M.; Hernández, C.; Simó, R.; et al. Modulation of microglia polarization dynamics during diabetic retinopathy in db/db mice. Biochim. Biophys. Acta 2016, 1862, 1663–1674. [Google Scholar] [CrossRef]
- Dong, N.; Chang, L.; Wang, B.; Chu, L. Retinal neuronal MCP-1 induced by AGEs stimulates TNF-α expression in rat microglia via p38, ERK, and NF-κB pathways. Mol. Vis. 2014, 20, 616–628. [Google Scholar]
- Zhang, T.; Ouyang, H.; Mei, X.; Lu, B.; Yu, Z.; Chen, K.; Wang, Z.; Ji, L. Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2-NF-κB signaling pathway. FASEB J. 2019, 33, 11776–11790. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3330–3336. [Google Scholar] [CrossRef]
- Ng, J.S.; Bearse, M.A., Jr.; Schneck, M.E.; Barez, S.; Adams, A.J. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1622–1628. [Google Scholar] [CrossRef]
- Harrison, W.W.; Bearse, M.A., Jr.; Ng, J.S.; Jewell, N.P.; Barez, S.; Burger, D.; Schneck, M.E.; Adams, A.J. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration is an early event in diabetic retinopathy: Therapeutic implications. Br. J. Ophthalmol. 2012, 96, 1285–1290. [Google Scholar] [CrossRef]
- Reis, A.; Mateus, C.; Melo, P.; Figueira, J.; Cunha-Vaz, J.; Castelo-Branco, M. Neuroretinal dysfunction with intact blood-retinal barrier and absent vasculopathy in type 1 diabetes. Diabetes 2014, 63, 3926–3937. [Google Scholar] [CrossRef]
- Gaspar, J.M.; Baptista, F.I.; Galvão, J.; Castilho, A.F.; Cunha, R.A.; Ambrósio, A.F. Diabetes differentially affects the content of exocytotic proteins in hippocampal and retinal nerve terminals. Neuroscience 2010, 169, 1589–1600. [Google Scholar] [CrossRef]
- D’Cruz, T.S.; Weibley, B.N.; Kimball, S.R.; Barber, A.J. Post-translational processing of synaptophysin in the rat retina is disrupted by diabetes. PLoS ONE 2012, 7, e44711. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.I.; Pinto, M.J.; Elvas, F.; Martins, T.; Almeida, R.D.; Ambrósio, A.F. Diabetes induces changes in KIF1A, KIF5B and dynein distribution in the rat retina: Implications for axonal transport. Exp. Eye Res. 2014, 127, 91–103. [Google Scholar] [CrossRef]
- Oshitari, T. Understanding intrinsic survival and regenerative pathways through in vivo and in vitro studies: Implications for optic nerve regeneration. Expert Rev. Ophthalmol. 2021, 16, 205–215. [Google Scholar]
- Oshitari, T.; Dezawa, M.; Okada, S.; Takano, M.; Negishi, H.; Horie, H.; Sawada, H.; Tokuhisa, T.; Adachi-Usami, E. The role of c-fos in cell death and regeneration of retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2442–2449. [Google Scholar] [PubMed]
- Oshitari, T.; Adachi-Usami, E. The effect of caspase inhibitors and neurotrophic factors on damaged retinal ganglion cells. Neuroreport 2003, 14, 289–292. [Google Scholar] [CrossRef]
- Oshitari, T.; Okada, S.; Tokuhisa, T.; Adachi-Usami, E. Adenovirus-mediated gene transfer of Bcl-xL impedes neurite regeneration in vitro. Neuroreport 2003, 14, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Dralands, L.; Missotten, L.; Geboes, K. Expression of antiapoptotic and proapoptotic molecules in diabetic retinas. Eye (Lond) 2007, 21, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Abu-El-Asrar, A.M.; Dralands, L.; Missotten, L.; Al-Jadaan, I.A.; Geboes, K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T.; Fujimoto, N.; Adachi-Usami, E. Citicoline has a protective effect on damaged retinal ganglion cells in mouse culture retina. Neuroreport 2002, 13, 2109–2111. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Bradke, F. Axon Regeneration in the Central Nervous System: Facing the Challenges from the Inside. Annu. Rev. Cell Dev. Biol. 2018, 34, 495–521. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008, 322, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Miao, L.; Liang, F.; Huang, H.; Teng, X.; Li, S.; Nuriddinov, J.; Selzer, M.E.; Hu, Y. The mTORC1 effectors S6K1 and 4E-BP play different roles in CNS axon regeneration. Nat. Commun. 2014, 5, 5416. [Google Scholar] [CrossRef]
- Agostinone, J.; Alarcon-Martinez, L.; Gamlin, C.; Yu, W.Q.; Wong, R.O.L.; Di Polo, A. Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain 2018, 141, 1963–1980. [Google Scholar] [CrossRef]
- Smith, P.D.; Sun, F.; Park, K.K.; Cai, B.; Wang, C.; Kuwako, K.; Martinez-Carrasco, I.; Connolly, L.; He, Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 2009, 64, 617–623. [Google Scholar] [CrossRef]
- Cameron, E.G.; Kapiloff, M.S. Intracellular compartmentation of cAMP promotes neuroprotection and regeneration of CNS neurons. Neural Regen. Res. 2017, 12, 201–202. [Google Scholar] [CrossRef]
- Lim, J.H.; Stafford, B.K.; Nguyen, P.L.; Lien, B.V.; Wang, C.; Zukor, K.; He, Z.; Huberman, A.D. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat. Neurosci. 2016, 19, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.R.; Chen, H.Y.; Hu, Z.Z.; Xie, P.; Liu, Q.H. PTEN knockdown with the Y444F mutant AAV2 vector promotes axonal regeneration in the adult optic nerve. Neural Regen. Res. 2018, 13, 135–144. [Google Scholar] [PubMed]
- Stokoe, D.; Stephens, L.R.; Copeland, T.; Gaffney, P.R.; Reese, C.B.; Painter, G.F.; Holmes, A.B.; McCormick, F.; Hawkins, P.T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 1997, 277, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Thoreen, C.C.; Peterson, T.R.; Lindquist, R.A.; Kang, S.A.; Spooner, E.; Carr, S.A.; Sabatini, D.M. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell 2007, 25, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Losiewicz, M.K.; Elghazi, L.; Fingar, D.C.; Rajala, R.V.S.; Lentz, S.I.; Fort, P.E.; Abcouwer, S.F.; Gardner, T.W. mTORC1 and mTORC2 expression in inner retinal neurons and glial cells. Exp. Eye Res. 2020, 197, 108131. [Google Scholar] [CrossRef]
- Wang, R.; Peng, L.; Zhao, J.; Zhang, L.; Guo, C.; Zheng, W.; Chen, H. Gardenamide A Protects RGC-5 Cells from H₂O₂-Induced Oxidative Stress Insults by Activating PI3K/Akt/eNOS Signaling Pathway. Int. J. Mol. Sci. 2015, 16, 22350–22367. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, C.K. Retinal ganglion cell death is delayed by activation of retinal intrinsic cell survival program. Brain Res. 2005, 1057, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Z.H.; Chan, Y.M.; Siu, F.K.; Yip, H.K.; Wu, W.; Leung, M.C.; So, K.F. Regulation of caspase activation in axotomized retinal ganglion cells. Mol. Cell Neurosci. 2004, 25, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Leibinger, M.; Andreadaki, A.; Golla, R.; Levin, E.; Hilla, A.M.; Diekmann, H.; Fischer, D. Boosting CNS axon regeneration by harnessing antagonistic effects of GSK3 activity. Proc. Natl. Acad. Sci. USA 2017, 114, E5454–E5463. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.; Sória, M.G.; Quadrato, G.; Inak, G.; Zhou, L.; Hervera, A.; Rathore, K.I.; Elnaggar, M.; Cucchiarini, M.; Marine, J.C.; et al. The MDM4/MDM2-p53-IGF1 axis controls axonal regeneration, sprouting and functional recovery after CNS injury. Brain 2015, 138, 1843–1862. [Google Scholar] [CrossRef]
- Huang, H.; Miao, L.; Yang, L.; Liang, F.; Wang, Q.; Zhuang, P.; Sun, Y.; Hu, Y. AKT-dependent and -independent pathways mediate PTEN deletion-induced CNS axon regeneration. Cell Death Dis. 2019, 10, 203. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Peterson, W.M.; Wang, Q.; Tzekova, R.; Wiegand, S.J. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J. Neurosci. 2000, 20, 4081–4090. [Google Scholar] [CrossRef]
- Leibinger, M.; Müller, A.; Andreadaki, A.; Hauk, T.G.; Kirsch, M.; Fischer, D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J. Neurosci. 2009, 29, 14334–14341. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Li, B.; Xia, Z.; Wang, X.; Xiu, Y.; Zhang, Z.; Chen, C.; Song, H.; Li, W.; et al. The inhibition of miR-17-5p promotes cortical neuron neurite growth via STAT3/GAP-43 pathway. Mol. Biol. Rep. 2020, 47, 1795–1802. [Google Scholar] [CrossRef]
- Matsumoto, A.; Isomoto, H.; Nakayama, M.; Hisatsune, J.; Nishi, Y.; Nakashima, Y.; Matsushima, K.; Kurazono, H.; Nakao, K.; Hirayama, T.; et al. Helicobacter pylori VacA reduces the cellular expression of STAT3 and pro-survival Bcl-2 family proteins, Bcl-2 and Bcl-XL, leading to apoptosis in gastric epithelial cells. Dig. Dis. Sci. 2011, 56, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Sehara, Y.; Sawicka, K.; Hwang, J.Y.; Latuszek-Barrantes, A.; Etgen, A.M.; Zukin, R.S. Survivin Is a transcriptional target of STAT3 critical to estradiol neuroprotection in global ischemia. J. Neurosci. 2013, 33, 12364–12374. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Park, K.K.; Belin, S.; Wang, D.; Lu, T.; Chen, G.; Zhang, K.; Yeung, C.; Feng, G.; Yankner, B.A.; et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 2011, 480, 372–375. [Google Scholar] [CrossRef]
- Garcia, A.L.; Udeh, A.; Kalahasty, K.; Hackam, A.S. A growing field: The regulation of axonal regeneration by Wnt signaling. Neural Regen. Res. 2018, 13, 43–52. [Google Scholar]
- Nathan, F.M.; Ohtake, Y.; Wang, S.; Jiang, X.; Sami, A.; Guo, H.; Zhou, F.Q.; Li, S. Upregulating Lin28a Promotes Axon Regeneration in Adult Mice with Optic Nerve and Spinal Cord Injury. Mol. Ther. 2020, 28, 1902–1917. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Bian, M.; Xia, X.; Madaan, A.; Sun, C.; Wang, Q.; Li, L.; Nahmou, M.; Noro, T.; Yokota, S.; et al. Posttranslational Modification of Sox11 Regulates RGC Survival and Axon Regeneration. eNeuro 2021, 8, ENEURO.0358–20.2020. [Google Scholar] [CrossRef]
- Apara, A.; Galvao, J.; Wang, Y.; Blackmore, M.; Trillo, A.; Iwao, K.; Brown, D.P., Jr.; Fernandes, K.A.; Huang, A.; Nguyen, T.; et al. KLF9 and JNK3 Interact to Suppress Axon Regeneration in the Adult CNS. J. Neurosci. 2017, 37, 9632–9644. [Google Scholar] [CrossRef]
- Hashemian, M.; Ghasemi-Kasman, M.; Parsian, H.; Sadeghi, F. Fingolimod (FTY720) improves the functional recovery and myelin preservation of the optic pathway in focal demyelination model of rat optic chiasm. Brain Res. Bull. 2019, 153, 109–121. [Google Scholar] [CrossRef]
- Belin, S.; Nawabi, H.; Wang, C.; Tang, S.; Latremoliere, A.; Warren, P.; Schorle, H.; Uncu, C.; Woolf, C.J.; He, Z.; et al. Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron 2015, 86, 1000–1014. [Google Scholar] [CrossRef]
- Boczek, T.; Kapiloff, M.S. Compartmentalization of local cAMP signaling in neuronal growth and survival. Neural Regen. Res. 2020, 15(3), 453–454. [Google Scholar]
- de Lima, S.; Koriyama, Y.; Kurimoto, T.; Oliveira, J.T.; Yin, Y.; Li, Y.; Gilbert, H.Y.; Fagiolini, M.; Martinez, A.M.; Benowitz, L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc. Natl. Acad. Sci. USA 2012, 109, 9149–9154. [Google Scholar] [CrossRef]
- Luo, X.; Salgueiro, Y.; Beckerman, S.R.; Lemmon, V.P.; Tsoulfas, P.; Park, K.K. Three-dimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury. Exp. Neurol. 2013, 247, 653–662. [Google Scholar] [CrossRef]
- Li, S.; He, Q.; Wang, H.; Tang, X.; Ho, K.W.; Gao, X.; Zhang, Q.; Shen, Y.; Cheung, A.; Wong, F.; et al. Injured adult retinal axons with Pten and Socs3 co-deletion reform active synapses with suprachiasmatic neurons. Neurobiol. Dis. 2015, 73, 366–376. [Google Scholar] [CrossRef]
- Wang, X.W.; Yang, S.G.; Zhang, C.; Hu, M.W.; Qian, J.; Ma, J.J.; Zhang, Y.; Yang, B.B.; Weng, Y.L.; Ming, G.L.; et al. Knocking Out Non-muscle Myosin II in Retinal Ganglion Cells Promotes Long-Distance Optic Nerve Regeneration. Cell Rep. 2020, 31, 107537. [Google Scholar] [CrossRef]
- Várkuti, B.H.; Képiró, M.; Horváth, I.Á.; Végner, L.; Ráti, S.; Zsigmond, Á.; Hegyi, G.; Lenkei, Z.; Varga, M.; Málnási-Csizmadia, A. A highly soluble, non-phototoxic, non-fluorescent blebbistatin derivative. Sci. Rep. 2016, 6, 26141. [Google Scholar] [CrossRef]
- Shabanzadeh, A.P.; D’Onofrio, P.M.; Magharious, M.; Choi, K.A.B.; Monnier, P.P.; Koeberle, P.D. Modifying PTEN recruitment promotes neuron survival, regeneration, and functional recovery after CNS injury. Cell Death Dis. 2019, 10, 567. [Google Scholar] [CrossRef]
- Wang, D.; Luo, M.; Huang, B.; Gao, W.; Jiang, Y.; Li, Q.; Nan, K.; Lin, S. Localized co-delivery of CNTF and FK506 using a thermosensitive hydrogel for retina ganglion cells protection after traumatic optic nerve injury. Drug Deliv. 2020, 27, 556–564. [Google Scholar] [CrossRef]
- Laughter, M.R.; Bardill, J.R.; Ammar, D.A.; Pena, B.; Calkins, D.J.; Park, D. Injectable Neurotrophic Factor Delivery System Supporting Retinal Ganglion Cell Survival and Regeneration Following Optic Nerve Crush. ACS Biomater Sci. Eng. 2018, 4, 3374–3383. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, A.Z.; Eliasen, R.; Oswald, J.; Kempen, P.J.; Melander, F.; Andresen, T.L.; Young, M.; Baranov, P.; Urquhart, A.J. Multifarious Biologic Loaded Liposomes that Stimulate the Mammalian Target of Rapamycin Signaling Pathway Show Retina Neuroprotection after Retina Damage. ACS Nano 2018, 12, 7497–7508. [Google Scholar] [CrossRef] [PubMed]
- Leibinger, M.; Andreadaki, A.; Gobrecht, P.; Levin, E.; Diekmann, H.; Fischer, D. Boosting Central Nervous System Axon Regeneration by Circumventing Limitations of Natural Cytokine Signaling. Mol. Ther. 2016, 24, 1712–1725. [Google Scholar] [CrossRef] [PubMed]
- Mesentier-Louro, L.A.; Rosso, P.; Carito, V.; Mendez-Otero, R.; Santiago, M.F.; Rama, P.; Lambiase, A.; Tirassa, P. Nerve Growth Factor Role on Retinal Ganglion Cell Survival and Axon Regrowth: Effects of Ocular Administration in Experimental Model of Optic Nerve Injury. Mol. Neurobiol. 2019, 56, 1056–1069. [Google Scholar] [CrossRef]
- Falsini, B.; Iarossi, G.; Chiaretti, A.; Ruggiero, A.; Manni, L.; Galli-Resta, L.; Corbo, G.; Abed, E. NGF eye-drops topical administration in patients with retinitis pigmentosa, a pilot study. J. Transl. Med. 2016, 14, 8. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; et al. Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes 2019, 68, 457–463. [Google Scholar] [CrossRef]
- Kitamura, Y.; Bikbova, G.; Baba, T.; Yamamoto, S.; Oshitari, T. In vivo effects of single or combined topical neuroprotective and regenerative agents on degeneration of retinal ganglion cells in rat optic nerve crush model. Sci. Rep. 2019, 9, 101. [Google Scholar] [CrossRef]
- Fagone, P.; Jackowski, S. Phosphatidylcholine and the CDP-choline cycle. Biochim. Biophys. Acta 2013, 1831, 523–532. [Google Scholar] [CrossRef]
- Daruich, A.; Picard, E.; Boatright, J.H.; Behar-Cohen, F. Review: The bile acids urso- and tauroursodeoxycholic acid as neuroprotective therapies in retinal disease. Mol. Vis. 2019, 25, 610–624. [Google Scholar]
- Guo, W.; Li, Y.; Sun, C.; Duan, H.Q.; Liu, S.; Xu, Y.Q. Neurotrophin-4 induces myelin protein zero expression in cultured Schwann cells via the TrkB/PI3K/Akt/mTORC1 pathway. Anim. Cells Syst (Seoul) 2017, 21, 84–92. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Li, P.; Ding, Y.; Tang, J.; Chen, G.; Zhang, J.H. NT-4 attenuates neuroinflammation via TrkB/PI3K/FoxO1 pathway after germinal matrix hemorrhage in neonatal rats. J. Neuroinflamm. 2020, 17, 158. [Google Scholar] [CrossRef]
- Shen, Y.; Inoue, N.; Heese, K. Neurotrophin-4 (ntf4) mediates neurogenesis in mouse embryonic neural stem cells through the inhibition of the signal transducer and activator of transcription-3 (stat3) and the modulation of the activity of protein kinase B. Cell Mol. Neurobiol. 2010, 30, 909–916. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshitari, T. The Pathogenesis and Therapeutic Approaches of Diabetic Neuropathy in the Retina. Int. J. Mol. Sci. 2021, 22, 9050. https://doi.org/10.3390/ijms22169050
Oshitari T. The Pathogenesis and Therapeutic Approaches of Diabetic Neuropathy in the Retina. International Journal of Molecular Sciences. 2021; 22(16):9050. https://doi.org/10.3390/ijms22169050
Chicago/Turabian StyleOshitari, Toshiyuki. 2021. "The Pathogenesis and Therapeutic Approaches of Diabetic Neuropathy in the Retina" International Journal of Molecular Sciences 22, no. 16: 9050. https://doi.org/10.3390/ijms22169050
APA StyleOshitari, T. (2021). The Pathogenesis and Therapeutic Approaches of Diabetic Neuropathy in the Retina. International Journal of Molecular Sciences, 22(16), 9050. https://doi.org/10.3390/ijms22169050





