Local Preirradiation of Infarcted Cardiac Tissue Substantially Enhances Cell Engraftment
Abstract
:1. Introduction
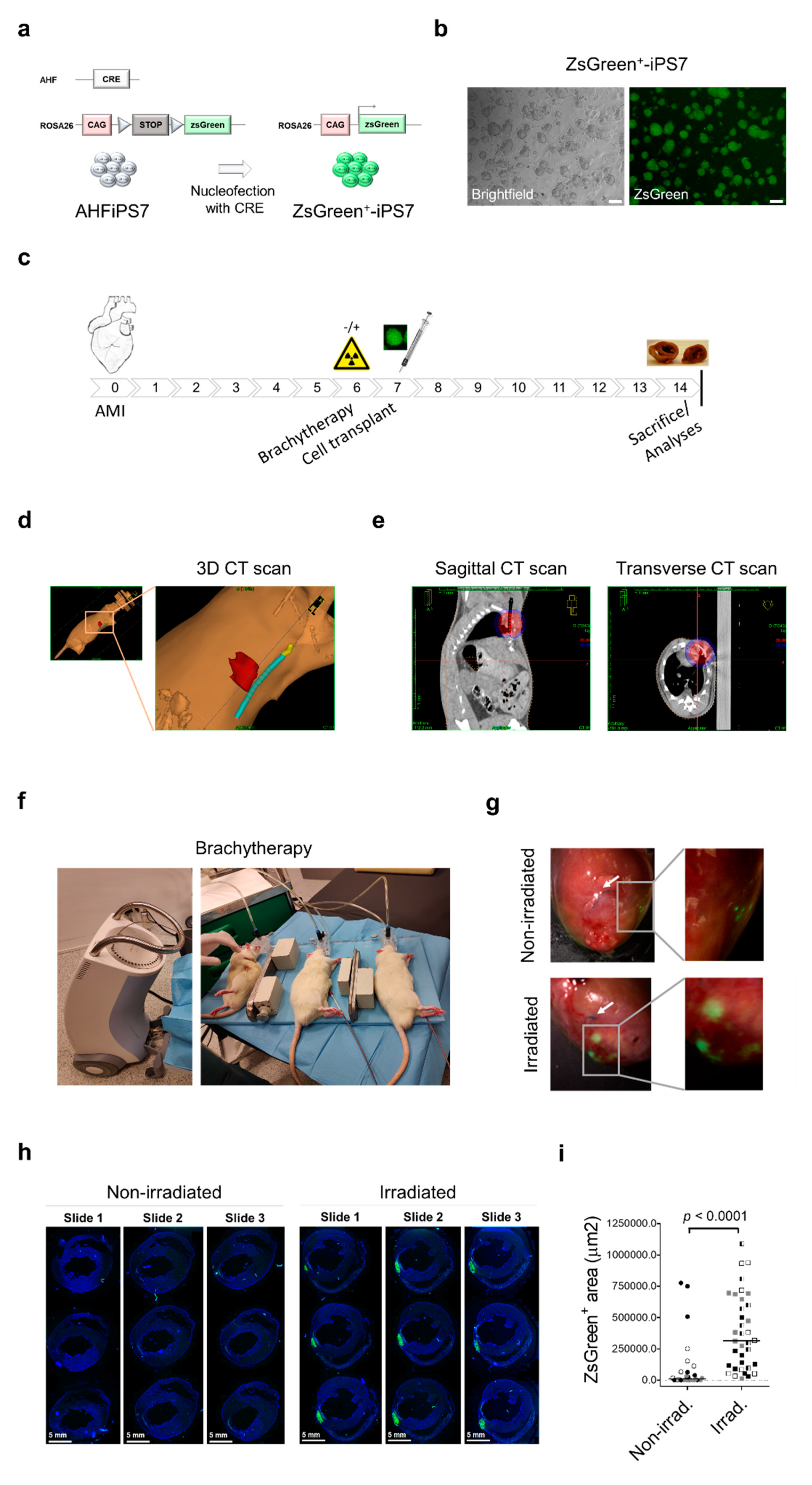
2. Results
3. Discussion
4. Materials and Methods
4.1. ZsGreen-iPS7 Cells Derivation and Culture Conditions
4.2. Experimental Animals
4.3. Tissue Processing and Staining
4.4. Brachytherapy in Rats
4.5. Fluorescence Microscopy and ZsGreen Quantification
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European society of Cardiology: Cardiovascular disease statistics 2019 (executive summary). Eur. Hear. J.-Qual. Care Clin. Outcomes 2020, 6, 7–9. [Google Scholar] [CrossRef]
- Steinhauser, M.L.; Lee, R.T. Regeneration of the heart. EMBO Mol. Med. 2011, 3, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.G.; Sharpe, N. Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy. Circulation 2000, 101, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Cambria, E.; Pasqualini, F.S.; Wolint, P.; Günter, J.; Steiger, J.; Bopp, A.; Hoerstrup, S.P.; Emmert, M.Y. Translational cardiac stem cell therapy: Advancing from first-generation to next-generation cell types. NPJ Regen. Med. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Hu, X.; Wang, J. Concise Review: Optimized Strategies for Stem Cell-Based Therapy in Myocardial Repair: Clinical Translatability and Potential Limitation. Stem Cells 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madonna, R.; Van Laake, L.W.; Botker, H.E.; Davidson, S.M.; De Caterina, R.; Engel, F.B.; Eschenhagen, T.; Fernandez-Aviles, F.; Hausenloy, D.J.; Hulot, J.S.; et al. ESC working group on cellular biology of the heart: Position paper for Cardiovascular Research: Tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc. Res. 2019, 115, 488–500. [Google Scholar] [CrossRef] [Green Version]
- Tu, C.; Mezynski, R.; Wu, J.C. Improving the engraftment and integration of cell transplantation for cardiac regeneration. Cardiovasc. Res. 2020, 116, 473–475. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.U.; Guo, Y.; Li, Q.-H.; Cao, P.; Al-Maqtari, T.; Vajravelu, B.N.; Du, J.; Book, M.J.; Zhu, X.; Nong, Y.; et al. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS ONE 2014, 9, e96725. [Google Scholar] [CrossRef] [Green Version]
- Lemcke, H.; Voronina, N.; Steinhoff, G.; David, R. Recent Progress in Stem Cell Modification for Cardiac Regeneration. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- López-Muneta, L.; Miranda-Arrubla, J.; Carvajal-Vergara, X. The future of direct cardiac reprogramming: Any gmt cocktail variety? Int. J. Mol. Sci. 2020, 21, 7950. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, L.; Neal, A.; Zammit, P.S.; Muntoni, F.; Morgan, J.E. Donor Satellite Cell Engraftment Is SignificantlyAugmented When the Host Niche Is Preserved and Endogenous Satellite Cells Are Incapacitated. Stem Cells 2012, 30, 1971–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosrotehrani, K. Mesenchymal stem cell therapy in skin: Why and what for? Exp. Dermatol. 2013, 22, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Down, J.D.; Tarbell, N.J.; Thames, H.D.; Mauch, P.M. Syngeneic and allogeneic bone marrow engraftment after total body irradiation: Dependence on dose, dose rate, and fractionation. Blood 1991, 77, 661–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltas, D.; Zamboglou, N.; Sakelliou, L. The Physics of Modern Brachytherapy for Oncology; CRC Press: Boca Raton, FL, USA, 2007; 647p. [Google Scholar]
- Linares, J.; Arellano-Viera, E.; Iglesias-García, O.; Ferreira, C.; Iglesias, E.; Abizanda, G.; Prósper, F.; Carvajal-Vergara, X. Generation of iPSC from cardiac and tail-tip fibroblasts derived from a second heart field reporter mouse. Stem Cell Res. 2016, 16, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Agbulut, O.; Mazo, M.; Bressolle, C.; Gutierrez, M.; Azarnoush, K.; Sabbah, L.; Niederlander, N.; Abizanda, G.; Andreu, E.J.; Pelacho, B.; et al. Can bone marrow-derived multipotent adult progenitor cells regenerate infarcted myocardium? Cardiovasc. Res. 2006, 72, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Khalil, H.; Kanisicak, O.; Boyer, J.G.; Vagnozzi, R.J.; Maliken, B.D.; Sargent, M.A.; Prasad, V.; Valiente-Alandi, I.; Blaxall, B.C.; et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Investig. 2018, 128, 2127–2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.E.; Gross, J.G.; Pagel, C.N.; Beauchamp, J.R.; Fassati, A.; Thrasher, A.J.; Di Santo, J.P.; Fisher, I.B.; Shiwen, X.; Abraham, D.J.; et al. Myogenic cell proliferation and generation of a reversible tumorigenic phenotype are triggered by preirradiation of the recipient site. J. Cell Biol. 2002, 157, 693–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameters of the Scanning Protocol | |
|---|---|
| Mode | Axial |
| Pitch | 0 |
| KVp | 80 |
| mA | 320 |
| Rotation time (s) | 1 |
| mAs | 320 |
| FOV (cm) | 20 |
| Reconstruction diameter (cm) | 20 |
| Slice thickness (mm) | 1 |
| Convolution kernel | H31s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abizanda, G.; López-Muneta, L.; Linares, J.; Ramos, L.I.; Baraibar-Churio, A.; Bobadilla, M.; Iglesias, E.; Coppiello, G.; Ripalda-Cemboráin, P.; Aranguren, X.L.; et al. Local Preirradiation of Infarcted Cardiac Tissue Substantially Enhances Cell Engraftment. Int. J. Mol. Sci. 2021, 22, 9126. https://doi.org/10.3390/ijms22179126
Abizanda G, López-Muneta L, Linares J, Ramos LI, Baraibar-Churio A, Bobadilla M, Iglesias E, Coppiello G, Ripalda-Cemboráin P, Aranguren XL, et al. Local Preirradiation of Infarcted Cardiac Tissue Substantially Enhances Cell Engraftment. International Journal of Molecular Sciences. 2021; 22(17):9126. https://doi.org/10.3390/ijms22179126
Chicago/Turabian StyleAbizanda, Gloria, Leyre López-Muneta, Javier Linares, Luis I. Ramos, Arantxa Baraibar-Churio, Miriam Bobadilla, Elena Iglesias, Giulia Coppiello, Purificación Ripalda-Cemboráin, Xabier L. Aranguren, and et al. 2021. "Local Preirradiation of Infarcted Cardiac Tissue Substantially Enhances Cell Engraftment" International Journal of Molecular Sciences 22, no. 17: 9126. https://doi.org/10.3390/ijms22179126







