Role of the Myokine Irisin on Bone Homeostasis: Review of the Current Evidence
Abstract
:1. Introduction
1.1. Bone Homeostasis
1.2. Irisin
2. Role of Irisin on Bone Homeostasis
2.1. Role of Irisin on Bone Homeostasis: In Vitro Evidence
2.1.1. Osteoblasts
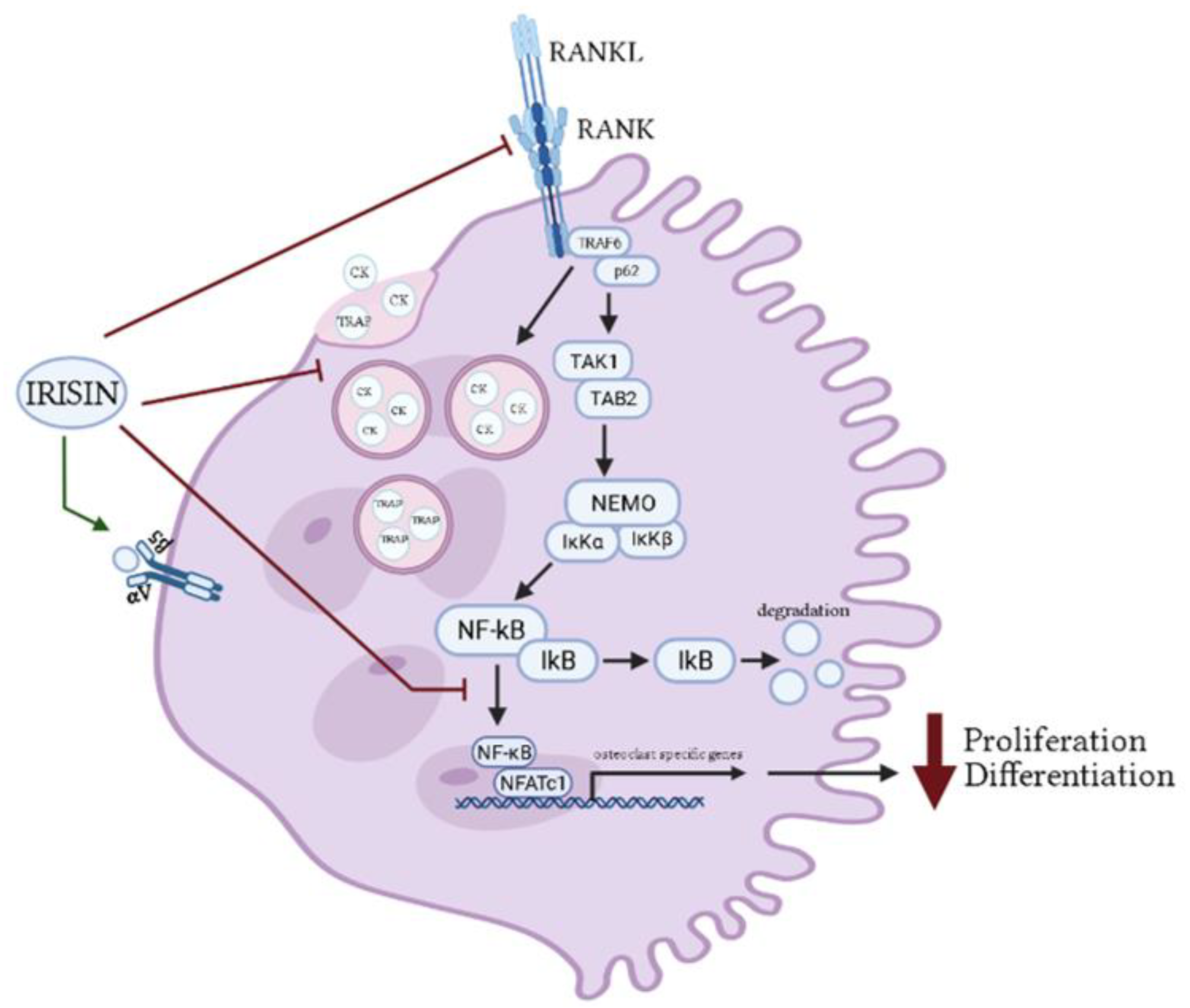
2.1.2. Osteoclasts
2.1.3. Osteocytes
2.1.4. Chondrocytes
2.2. Role of Irisin on Bone Homeostasis: In Vivo Evidence
2.3. Role of Irisin on Bone Homeostasis: Clinical Evidence
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seeman, E.; Delmas, P.D. Bone Quality—The Material and Structural Basis of Bone Strength and Fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [Green Version]
- Caetano-Lopes, J.; Canhão, H.; Fonseca, J.E. Osteoblasts and Bone Formation. Acta Reumatol. Port. 2007, 32, 103–110. [Google Scholar] [PubMed]
- Vander, A.J.; Widmaier, E.P.; Raff, H.; Strang, K.T.; Shoepe, T.C. Vander’s Human Physiology: The Mechanisms of Body Function; McGraw-Hill Education: New York, NY, USA, 2019; ISBN 978-1-260-08522-8. [Google Scholar]
- Grigoriadis, A.E.; Heersche, J.N.; Aubin, J.E. Differentiation of Muscle, Fat, Cartilage, and Bone from Progenitor Cells Present in a Bone-Derived Clonal Cell Population: Effect of Dexamethasone. J. Cell Biol. 1988, 106, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Available online: https://www.hindawi.com/journals/bmri/2015/421746/ (accessed on 17 December 2020).
- Li, J.; Sarosi, I.; Yan, X.-Q.; Morony, S.; Capparelli, C.; Tan, H.-L.; McCabe, S.; Elliott, R.; Scully, S.; Van, G.; et al. RANK Is the Intrinsic Hematopoietic Cell Surface Receptor That Controls Osteoclastogenesis and Regulation of Bone Mass and Calcium Metabolism. Proc. Natl. Acad. Sci. USA 2000, 97, 1566–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povolny, B.T.; Lee, M.Y. The Role of Recombinant Human M-CSF, IL-3, GM-CSF and Calcitriol in Clonal Development of Osteoclast Precursors in Primate Bone Marrow. Exp. Hematol. 1993, 21, 532–537. [Google Scholar] [PubMed]
- Orcel, P.; Bielakoff, J.; Vernejoul, M.C.D. Formation of Multinucleated Cells with Osteoclast Precursor Features in Human Cord Monocytes Cultures. Anat. Rec. 1990, 226, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.M.W.; Neale, S.; Fujikawa, Y.; McGee, J.O.D.; Athanasou, N.A. Human Osteoclast Formation from Blood Monocytes, Peritoneal Macrophages, and Bone Marrow Cells. Calcif. Tissue Int. 1998, 62, 527–531. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zhang, X.; Moreno, J.; Celluzzi, C.; Tondravi, M.; Shi, Y. Regulation of Activation-Induced Receptor Activator of NF-ΚB Ligand (RANKL) Expression in T Cells. Eur. J. Immunol. 2002, 32, 1090–1098. [Google Scholar] [CrossRef]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried Alive: How Osteoblasts Become Osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M.; Sims, N.A.; Hunzelman, J.L.; Knight, M.C.; Giovannetti, A.; Saxton, J.M.; Kronenberg, H.M.; Baron, R.; Schipani, E. Activated Parathyroid Hormone/Parathyroid Hormone–Related Protein Receptor in Osteoblastic Cells Differentially Affects Cortical and Trabecular Bone. J. Clin. Investig. 2001, 107, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, M.; Inzerillo, A.M.; Moonga, B.S.; Bevis, P.J.R.; Huang, C.L.-H. Forty Years of Calcitonin—Where Are We Now? A Tribute to the Work of Iain Macintyre, FRS. Bone 2002, 30, 655–663. [Google Scholar] [CrossRef]
- Miao, D.; Scutt, A. Recruitment, Augmentation and Apoptosis of Rat Osteoclasts in 1,25-(OH)2D3 Response to Short-Term Treatment with 1,25-Dihydroxyvitamin D3 in Vivo. BMC Musculoskelet. Disord. 2002, 3, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderschueren, D.; Vandenput, L.; Boonen, S.; Lindberg, M.K.; Bouillon, R.; Ohlsson, C. Androgens and Bone. Endocr. Rev. 2004, 25, 389–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the Skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Bezooijen, R.L.; Roelen, B.A.J.; Visser, A.; van der Wee-Pals, L.; de Wilt, E.; Karperien, M.; Hamersma, H.; Papapoulos, S.E.; ten Dijke, P.; Löwik, C.W.G.M. Sclerostin Is an Osteocyte-Expressed Negative Regulator of Bone Formation, but Not a Classical BMP Antagonist. J. Exp. Med. 2004, 199, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin Binds to LRP5/6 and Antagonizes Canonical Wnt Signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [Green Version]
- Roudier, M.; Li, X.; Niu, Q.-T.; Pacheco, E.; Pretorius, J.K.; Graham, K.; Yoon, B.-R.P.; Gong, J.; Warmington, K.; Ke, H.Z.; et al. Sclerostin Is Expressed in Articular Cartilage but Loss or Inhibition Does Not Affect Cartilage Remodeling during Aging or Following Mechanical Injury. Arthritis Rheum. 2013, 65, 721–731. [Google Scholar] [CrossRef]
- Baron, R.; Rawadi, G. Targeting the Wnt/Beta-Catenin Pathway to Regulate Bone Formation in the Adult Skeleton. Endocrinology 2007, 148, 2635–2643. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, N.; Garnero, P.; Ferrari, S. Periostin Action in Bone. Mol. Cell Endocrinol. 2016, 432, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Morra, L.; Moch, H. Periostin Expression and Epithelial-Mesenchymal Transition in Cancer: A Review and an Update. Virchows. Arch. 2011, 459, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.R. The Underappreciated Role of Muscle in Health and Disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone Biomaterials and Interactions with Stem Cells. Bone Res. 2017, 5, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal Muscle Performance and Ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.R.; Kim, S.W. Effects of Resistance Exercise on Bone Health. Endocrinol. Metab. 2018, 33, 435–444. [Google Scholar] [CrossRef] [PubMed]
- He, C.; He, W.; Hou, J.; Chen, K.; Huang, M.; Yang, M.; Luo, X.; Li, C. Bone and Muscle Crosstalk in Aging. Front. Cell Dev. Biol. 2020, 8, 585644. [Google Scholar] [CrossRef]
- Yakabe, M.; Hosoi, T.; Akishita, M.; Ogawa, S. Updated Concept of Sarcopenia Based on Muscle–Bone Relationship. J. Bone Miner. Metab. 2020, 38, 7–13. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Wang, D.; AIQudsy, L.; Jiang, J.X.; Xu, H.; Shang, P. Muscle-bone Crosstalk and Potential Therapies for Sarco-osteoporosis. J. Cell Biochem. 2019, 120, 14262–14273. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Akerström, T.C.A.; Nielsen, A.R.; Fischer, C.P. Role of Myokines in Exercise and Metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [Green Version]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Lara-Castillo, N.; Johnson, M.L. Bone-Muscle Mutual Interactions. Curr. Osteoporos. Rep. 2020, 18, 408–421. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Teufel, A.; Malik, N.; Mukhopadhyay, M.; Westphal, H. Frcp1 and Frcp2, Two Novel Fibronectin Type III Repeat Containing Genes. Gene 2002, 297, 79–83. [Google Scholar] [CrossRef]
- Erickson, H.P. Irisin and FNDC5 in Retrospect. Adipocyte 2013, 2, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin Exerts Dual Effects on Browning and Adipogenesis of Human White Adipocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef]
- Dinas, P.C.; Lahart, I.M.; Timmons, J.A.; Svensson, P.-A.; Koutedakis, Y.; Flouris, A.D.; Metsios, G.S. Effects of Physical Activity on the Link between PGC-1a and FNDC5 in Muscle, Circulating Ιrisin and UCP1 of White Adipocytes in Humans: A Systematic Review. F1000Research 2017, 6, 286. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Cakmak, T.; Albayrak, S.; Gungor, S.; Colakoglu, N.; Ozercan, I.H. A Comprehensive Immunohistochemical Examination of the Distribution of the Fat-Burning Protein Irisin in Biological Tissues. Peptides 2014, 61, 130–136. [Google Scholar] [CrossRef]
- Lv, J.; Pan, Y.; Li, X.; Cheng, D.; Ju, H.; Tian, J.; Shi, H.; Zhang, Y. Study on the Distribution and Elimination of the New Hormone Irisin in Vivo: New Discoveries Regarding Irisin. Horm. Metab. Res. 2015, 47, 591–595. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via AV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmons, J.A.; Baar, K.; Davidsen, P.K.; Atherton, P.J. Is Irisin a Human Exercise Gene? Nature 2012, 488, E9–E10. [Google Scholar] [CrossRef]
- Arad, A.D.; DiMenna, F.J.; Thomas, N.; Tamis-Holland, J.; Weil, R.; Geliebter, A.; Albu, J.B. High-Intensity Interval Training without Weight Loss Improves Exercise but Not Basal or Insulin-Induced Metabolism in Overweight/Obese African American Women. J. Appl. Physiol. 2015, 119, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, M.C.; Vella, J.P.; Cafeo, F.R.; Affonso Fonseca, F.L.; Bacci, M.R. Association between Irisin and Major Chronic Diseases: A Review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4072–4077. [Google Scholar] [PubMed]
- Martinez Munoz, I.Y.; Camarillo Romero, E.D.S.; de Jesus Garduno Garcia, J. Irisin a Novel Metabolic Biomarker: Present Knowledge and Future Directions. Int. J. Endocrinol. 2018, 2018, 7816806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Buccoliero, C.; Oranger, A.; Colaianni, G.; Pignataro, P.; Zerlotin, R.; Lovero, R.; Errede, M.; Grano, M. The effect of Irisin on bone cells in vivo and in vitro. Biochem. Soc. Trans. 2021, 49, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Tsiani, E.; Tsakiridis, N.; Kouvelioti, R.; Jaglanian, A.; Klentrou, P. Current Evidence of the Role of the Myokine Irisin in Cancer. Cancers 2021, 13, 2628. [Google Scholar] [CrossRef]
- Hee Park, K.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating Irisin in Relation to Insulin Resistance and the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Benedini, S.; Dozio, E.; Invernizzi, P.L.; Vianello, E.; Banfi, G.; Terruzzi, I.; Luzi, L.; Corsi Romanelli, M.M. Irisin: A Potential Link between Physical Exercise and Metabolism—An Observational Study in Differently Trained Subjects, from Elite Athletes to Sedentary People. J. Diabetes Res. 2017, 2017, e1039161. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Mayer, A.; Maderova, D.; Belan, V.; Wolfrum, C.; Ukropec, J.; Ukropcova, B. Exercise-Mimicking Treatment Fails to Increase Fndc5 MRNA & Irisin Secretion in Primary Human Myotubes. Peptides 2014, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.; Becerril, S.; Méndez-Giménez, L.; Ramírez, B.; Sáinz, N.; Catalán, V.; Gómez-Ambrosi, J.; Frühbeck, G. Leptin Administration Activates Irisin-Induced Myogenesis via Nitric Oxide-Dependent Mechanisms, but Reduces Its Effect on Subcutaneous Fat Browning in Mice. Int. J. Obes. 2015, 39, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin enhances osteoblast differentiation in vitro. Int. J. Endocrinol. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Yong Qiao, X.; Nie, Y.; Ma, Y.; Xian Ma, Y.; Chen, Y.; Cheng, R.; Yin, W.; Yao Yinrg, W.; Hu, Y.; et al. Irisin Promotes Osteoblast Proliferation and Differentiation via Activating the MAP Kinase Signaling Pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Valverde, P.; Zhu, X.; Murray, D.; Wu, Y.; Yu, L.; Jiang, H.; Dard, M.M.; Huang, J.; Xu, Z.; et al. Exercise-Induced Irisin in Bone and Systemic Irisin Administration Reveal New Regulatory Mechanisms of Bone Metabolism. Bone Res. 2017, 5, 16056. [Google Scholar] [CrossRef] [Green Version]
- Kawao, N.; Moritake, A.; Tatsumi, K.; Kaji, H. Roles of Irisin in the Linkage from Muscle to Bone During Mechanical Unloading in Mice. Calcif. Tissue Int. 2018, 103, 24–34. [Google Scholar] [CrossRef]
- Zhang, D.; Bae, C.; Lee, J.; Lee, J.; Jin, Z.; Kang, M.; Cho, Y.S.; Kim, J.-H.; Lee, W.; Lim, S.-K. The Bone Anabolic Effects of Irisin Are through Preferential Stimulation of Aerobic Glycolysis. Bone 2018, 114, 150–160. [Google Scholar] [CrossRef]
- Zeng, R.; Ma, Y.; Qiao, X.; Zhang, J.; Luo, Y.; Li, S.; Liu, L.; Xu, L. The Effect of His-Tag and Point Mutation on the Activity of Irisin on MC3T3-E1 Cells. Biosci. Trends 2018, 12, 580–586. [Google Scholar] [CrossRef]
- Palermo, A.; Sanesi, L.; Colaianni, G.; Tabacco, G.; Naciu, A.M.; Cesareo, R.; Pedone, C.; Lelli, D.; Brunetti, G.; Mori, G.; et al. A Novel Interplay Between Irisin and PTH: From Basic Studies to Clinical Evidence in Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2019, 104, 3088–3096. [Google Scholar] [CrossRef]
- Chen, X.; Sun, K.; Zhao, S.; Geng, T.; Fan, X.; Sun, S.; Zheng, M.; Jin, Q. Irisin Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Activating Autophagy via the Wnt//β-Catenin Signal Pathway. Cytokine 2020, 136, 155292. [Google Scholar] [CrossRef] [PubMed]
- Colucci, S.; Colaianni, G.; Brunetti, G.; Ferranti, F.; Mascetti, G.; Mori, G.; Grano, M. Irisin Prevents Microgravity-Induced Impairment of Osteoblast Differentiation in Vitro during the Space Flight CRS-14 Mission. FASEB J. 2020, 34, 10096–10106. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wang, J.; Lin, D.; Ding, Z. The Immunomodulatory Role of Irisin on Osteogenesis via AMPK-Mediated Macrophage Polarization. Int. J. Biol. Macromol. 2020, 146, 25–35. [Google Scholar] [CrossRef]
- Pullisaar, H.; Colaianni, G.; Lian, A.-M.; Vandevska-Radunovic, V.; Grano, M.; Reseland, J.E. Irisin Promotes Growth, Migration and Matrix Formation in Human Periodontal Ligament Cells. Arch. Oral Biol. 2020, 111, 104635. [Google Scholar] [CrossRef]
- Estell, E.G.; Le, P.T.; Vegting, Y.; Kim, H.; Wrann, C.; Bouxsein, M.L.; Nagano, K.; Baron, R.; Spiegelman, B.M.; Rosen, C.J. Irisin Directly Stimulates Osteoclastogenesis and Bone Resorption in Vitro and in Vivo. eLife 2020, 9, e58172. [Google Scholar] [CrossRef]
- Ma, Y.; Qiao, X.; Zeng, R.; Cheng, R.; Zhang, J.; Luo, Y.; Nie, Y.; Hu, Y.; Yang, Z.; Zhang, J.; et al. Irisin Promotes Proliferation but Inhibits Differentiation in Osteoclast Precursor Cells. FASEB J. 2018, fj201700983RR. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Li, X.; Wang, X.; Chen, T.; Tao, F.; Liu, C.; Tu, Q.; Shen, G.; Chen, J.J. Irisin Deficiency Disturbs Bone Metabolism. J. Cell Physiol. 2021, 236, 664–676. [Google Scholar] [CrossRef]
- He, Z.; Li, H.; Han, X.; Zhou, F.; Du, J.; Yang, Y.; Xu, Q.; Zhang, S.; Zhang, S.; Zhao, N.; et al. Irisin Inhibits Osteocyte Apoptosis by Activating the Erk Signaling Pathway in Vitro and Attenuates ALCT-Induced Osteoarthritis in Mice. Bone 2020, 141, 115573. [Google Scholar] [CrossRef]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef]
- Vadalà, G.; Di Giacomo, G.; Ambrosio, L.; Cannata, F.; Cicione, C.; Papalia, R. Irisin Recovers Osteoarthritic Chondrocytes In Vitro. Cells 2020, 9, 1478. [Google Scholar] [CrossRef]
- Wang, F.-S.; Kuo, C.-W.; Ko, J.-Y.; Chen, Y.-S.; Wang, S.-Y.; Ke, H.-J.; Kuo, P.-C.; Lee, C.-H.; Wu, J.-C.; Lu, W.-B.; et al. Irisin Mitigates Oxidative Stress, Chondrocyte Dysfunction and Osteoarthritis Development through Regulating Mitochondrial Integrity and Autophagy. Antioxidants 2020, 9, 810. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The Myokine Irisin Increases Cortical Bone Mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Qiao, X.; Ma, Y.; Deng, H.; Xu, C.C.; Xu, L. Disordered Metabolism in Mice Lacking Irisin. Sci. Rep. 2020, 10, 17368. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Zhao, F.; Yin, C.; Yang, C.; Wang, X.; Wu, Z.; Liang, S.; Li, D.; Lin, X.; et al. Recombinant Irisin Prevents the Reduction of Osteoblast Differentiation Induced by Stimulated Microgravity through Increasing β-Catenin Expression. Int. J. Mol. Sci. 2020, 21, 1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, C.E.; Anand Narayanan, S.; Phan, P.H.; Bloomfield, S.A. Hindlimb Unloading Causes Regional Loading-Dependent Changes in Osteocyte Inflammatory Cytokines That Are Modulated by Exogenous Irisin Treatment. NPJ Microgravity 2020, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, Y.; Qiao, X.; Zeng, R.; Cheng, R.; Nie, Y.; Li, S.; Shen, X.; Yang, M.; Xu, L.; et al. Irisin Ameliorates Bone Loss in Ovariectomized Mice. Climacteric 2020, 23, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shen, L.; Yu, X.; Li, P.; Wang, Q.; Li, C. Effects of Irisin on Osteoblast Apoptosis and Osteoporosis in Postmenopausal Osteoporosis Rats through Upregulating Nrf2 and Inhibiting NLRP3 Inflammasome. Exp. Ther. Med. 2020, 19, 1084–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawao, N.; Iemura, S.; Kawaguchi, M.; Mizukami, Y.; Takafuji, Y.; Kaji, H. Role of Irisin in Effects of Chronic Exercise on Muscle and Bone in Ovariectomized Mice. J. Bone Miner. Metab. 2021, 39, 547–557. [Google Scholar] [CrossRef]
- Iemura, S.; Kawao, N.; Okumoto, K.; Akagi, M.; Kaji, H. Role of Irisin in Androgen-Deficient Muscle Wasting and Osteopenia in Mice. J. Bone Miner. Metab. 2020, 38, 161–171. [Google Scholar] [CrossRef]
- Narayanan, S.A.; Metzger, C.E.; Bloomfield, S.A.; Zawieja, D.C. Inflammation-Induced Lymphatic Architecture and Bone Turnover Changes Are Ameliorated by Irisin Treatment in Chronic Inflammatory Bowel Disease. FASEB J. 2018, 32, 4848–4861. [Google Scholar] [CrossRef]
- Metzger, C.E.; Narayanan, S.A.; Elizondo, J.P.; Carter, A.M.; Zawieja, D.C.; Hogan, H.A.; Bloomfield, S.A. DSS-Induced Colitis Produces Inflammation-Induced Bone Loss While Irisin Treatment Mitigates the Inflammatory State in Both Gut and Bone. Sci. Rep. 2019, 9, 15144. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.-S.; Kim, J.-C.; Kim, J.-S.; Kim, S.H. Effects of Swimming Exercise on Serum Irisin and Bone FNDC5 in Rat Models of High-Fat Diet-Induced Osteoporosis. J. Sports Sci. Med. 2019, 18, 596–603. [Google Scholar] [PubMed]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdés, S.; Botas, P.; Ricart, W.; Fernández-Real, J.M. Circulating Irisin Levels Are Positively Associated with Metabolic Risk Factors in Sedentary Subjects. PLoS ONE 2015, 10, e0124100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colaianni, G.; Faienza, M.F.; Sanesi, L.; Brunetti, G.; Pignataro, P.; Lippo, L.; Bortolotti, S.; Storlino, G.; Piacente, L.; D’Amato, G.; et al. Irisin Serum Levels Are Positively Correlated with Bone Mineral Status in a Population of Healthy Children. Pediatr. Res. 2019, 85, 484–488. [Google Scholar] [CrossRef]
- Sanderson, M.; McKinlay, B.J.; Theocharidis, A.; Kouvelioti, R.; Falk, B.; Klentrou, P. Changes in Inflammatory Cytokines and Irisin in Response to High Intensity Swimming in Adolescent versus Adult Male Swimmers. Sports 2020, 8, 157. [Google Scholar] [CrossRef]
- Colaianni, G.; Notarnicola, A.; Sanesi, L.; Brunetti, G.; Lippo, L.; Celi, M.; Moretti, L.; Pesce, V.; Vicenti, G.; Moretti, B.; et al. Irisin Levels Correlate with Bone Mineral Density in Soccer Players. J. Biol. Regul. Homeost. Agents 2017, 31, 21–28. [Google Scholar]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Gkiomisi, A.; Bisbinas, I.; Katsarou, A.; Filippaios, A.; Mantzoros, C.S. Circulating Irisin Is Associated with Osteoporotic Fractures in Postmenopausal Women with Low Bone Mass but Is Not Affected by Either Teriparatide or Denosumab Treatment for 3 Months. Osteoporos. Int. 2014, 25, 1633–1642. [Google Scholar] [CrossRef]
- Engin-Üstün, Y.; Çağlayan, E.K.; Göçmen, A.Y.; Polat, M.F. Postmenopausal Osteoporosis Is Associated with Serum Chemerin and Irisin but Not with Apolipoprotein M Levels. J. Menopausal. Med. 2016, 22, 76–79. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.-F.; Zhu, D.-C.; Tang, C.-H.; Ge, B.; Shi, J.; Wang, B.-H.; Lu, Y.-H.; He, P.; Wang, W.-Y.; Lu, S.-Q.; et al. Association of Plasma Irisin with Bone Mineral Density in a Large Chinese Population Using an Extreme Sampling Design. Calcif. Tissue Int. 2018, 103, 246–251. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Yu, R.; Wang, Y.; Gao, C. Circulating Irisin Is Linked to Bone Mineral Density in Geriatric Chinese Men. Open Med. 2020, 15, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Lavrova, D.P.; Zavodovsky, B.V.; Akhverdyan, Y.R.; Polyakova, Y.V.; Sivordova, L.E.; Zborovskaya, I.A.; Yakovlev, A.T. [Irisin as a new marker for the early diagnosis of low-traumatic fractures in rheumatoid arthritis]. Klin. Lab. Diagn. 2018, 63, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, H.-J.; Guo, W.-C.; Yang, J. Low Serum Concentrations of Irisin Are Associated with Increased Risk of Hip Fracture in Chinese Older Women. Jt. Bone Spine 2018, 85, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Errede, M.; Sanesi, L.; Notarnicola, A.; Celi, M.; Zerlotin, R.; Storlino, G.; Pignataro, P.; Oranger, A.; Pesce, V.; et al. Irisin Correlates Positively with BMD in a Cohort of Older Adult Patients and Downregulates the Senescent Marker P21 in Osteoblasts. J. Bone Miner. Res. 2021, 36, 305–314. [Google Scholar] [CrossRef]
- Lu, C.-W.; Wang, C.-H.; Lin, Y.-L.; Kuo, C.-H.; Lai, Y.-H.; Hsu, B.-G.; Tsai, J.-P. Serum Irisin Level Is Positively Associated with Bone Mineral Density in Patients on Maintenance Hemodialysis. Int. J. Endocrinol. 2021, 2021, 8890042. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Strollo, R.; Maddaloni, E.; Tuccinardi, D.; D’Onofrio, L.; Briganti, S.I.; Defeudis, G.; De Pascalis, M.; Lazzaro, M.C.; Colleluori, G.; et al. Irisin Is Associated with Osteoporotic Fractures Independently of Bone Mineral Density, Body Composition or Daily Physical Activity. Clin. Endocrinol. 2015, 82, 615–619. [Google Scholar] [CrossRef]
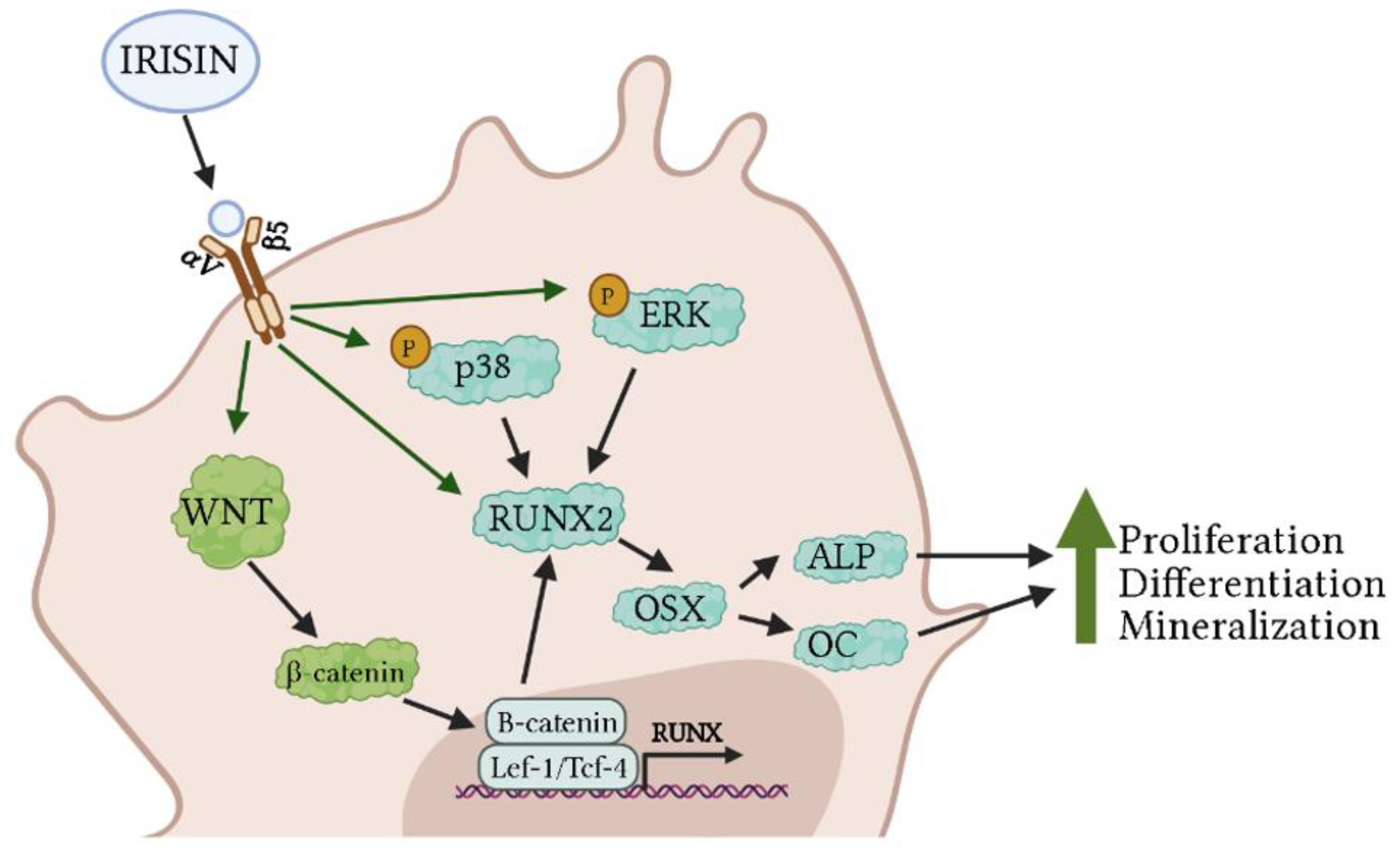



| Cell Type | Irisin Concentration/Duration | Effect | Reference |
|---|---|---|---|
| Bone marrow stromal cells cultured in exercised conditioned muscle cell-derived media | N/A | ↑ Osteoblast differentiation ↑ FNDC5 mRNA and protein ↑ ALP mRNA and protein ↑ Col1a1 mRNA and protein | [56] |
| Primary rat osteoblasts and MC3T3-E1 cells | Irisin 100 ng/mL; 24 h | ↑ Proliferation ↑ Osteoblast differentiation ↑ Runx2 mRNA ↑ Osx mRNA ↑ ALP mRNA ↑ Col1a1 mRNA ↑ OC mRNA ↑ OPG mRNA ↑ p-p38 protein ↑ p-ERK protein | [57] |
| MC3T3-E1 cells | r-irisin 20 μmol/L; 6 h, 10 days, and 6 weeks | 6 weeks: ↑ Osteoblast differentiation ↑ Mineralization 10 days: ↑ Osx mRNA ↑ Runx2 mRNA ↑ SATB2 mRNA ↑ BSP mRNA ↑ Col1a1 mRNA 6 h: ↑ Nuclear β-catenin ↓ Cytoplasmic β-catenin | [58] |
| Mouse bone marrow cells primary osteoblasts | Irisin 10, 20, 50, and 100 nM; 24 h | ↓ osteoclast formation ↓ RANKL mRNA in primary osteoblasts | [59] |
| MC3T3E1 cells and bone marrow stromal cells | r-irisin 10 nM; 48 h | ↑ Proliferation ↑ Osteoblast differentiation ↑ Osx mRNA ↑ Runx2 mRNA ↑ ALP mRNA ↑ Col1a1 mRNA ↑ ALP-positive colonies ↑ LDHA protein ↑ PDK1 protein ↑ Lactate | [60] |
| MC3T3E1 cells | r-irisin 20 nM; 14 days | irisin point mutation: ↓ R-irisin activity ↓ Proliferation ↓ Osteogenesis | [61] |
| MC3T3-E1 cells | r-irisin 100 ng/mL; 8 h | ↓ Parathyroid hormone receptor mRNA | [62] |
| Bone marrow mesenchymal stem cells | Irisin 40 μM; 48 h | ↑ Osteogenic differentiation ↑ Calcified nodules ↑ Autophagy ↑ Lc3I/II mRNA ↑ Atg5 mRNA ↑ Runx2 mRNA ↑ ALP mRNA ↑ OCN mRNA ↑ β-catenin mRNA ↑ Lef-1 mRNA ↑ Tcf-4 mRNA | [63] |
| Osteoblasts, osteoclasts, and endothelial cells seeded on Skelite discs | r-irisin 100 ng/mL; 14 days | ↑ Osteoblast differentiation ↓ Osteoclastogenesis ↑ Atf4 mRNA ↑ Runx2 mRNA ↑ Osx mRNA ↑ Collagen I mRNA ↑ Osteoprotegerin mRNA | [64] |
| Pre-osteoblast MC3T3-L1 cells and M0/1 macrophages | Irisin 200 ng/mL; 48 h | ↑ M2 macrophage phenotype ↑ Osteogenesis ↑ Mineralized particle formation ↑ CD206 cell surface expression ↑ ARG-1 ↑ TGF-B1 ↓ CD86 cell surface expression ↑ AMPK activity ↑ p-AMPK protein | [65] |
| Cell Type | Irisin Concentration/Duration | Effect | Reference |
|---|---|---|---|
| Pre-osteoclast RAW264.7 cells | r-irisin 20 nmol/L; 3 days | ↓ RANKL-induced differentiation ↓ TRAP mRNA ↓ CK mRNA ↓ TRAP-positive multinucleated cells ↓ NFATc1 mRNA and protein ↓ Calcineurin protein ↓ p-Akt protein ↓ p-JNK protein | [58] |
| Pre-osteoclast RAW264.7 cells | Irisin 2, 5, 10, and 20 ng/mL; 4 h, 24 h, 7 days | ↑ Proliferation ↑ Cell size ↑ αV/β5 integrin receptor mRNA ↑ Resorption pit area ↑ Adamts5 mRNA ↑ Lox13 mRNA ↑ Postn mRNA ↑ Igfbp5 mRNA ↑ Tgfb2 mRNA ↑ Sparc mRNA ↑ Atp6vod2 mRNA ↑ c-fps mRNA ↑ Fam102a mRNA ↑ Itgb3 mRNA ↑ Nrf2 mRNA ↑ Dcstamp mRNA ↑ Rank mRNA ↑ Rela mRNA ↑ Rgs12 mRNA ↑ Mst1r mRNA ↑ Itgax mRNA ↓ Slamf8 mRNA ↓ H2-aa mRNA | [67] |
| Pre-osteoclast Raw264.7 cells and mouse bone marrow monocytes | Irisin 20 nM; 60 min, 4 days | ↑ Osteoclast precursor cell proliferation ↓ Osteoclast differentiation ↓ Receptor activators of NF-κB mRNA ↓ NFATc1 mRNA ↓ Cytoplasmic 1 mRNA ↓ CK mRNA ↓ TRAP mRNA ↓ TRAP-positive multinucleated cell number ↓ IkBα protein ↓ p-p65 protein ↓ p-JNK protein ↑ p-p38 protein | [68] |
| Primary BMSCs isolated from Osx-Cre: F/I KO mice | Irisin 4 and 20 μmol/L; 5 days | ↑ Osteoblastogenesis ↑ Mineralization ↓ Osteoclastogenesis ↑ Runx2 mRNA ↑ Satb2 mRNA ↑ Bsp mRNA ↑ Col1a1 mRNA ↑ ALP mRNA ↓ Trap mRNA ↓ MMP9 mRNA ↓ NFATc1 mRNA and protein ↓ p-AKT protein ↓ p-p38 protein ↓ Calcineurin protein ↓ TRAP staining ↓ TRAP-positive multinucleated cell number ↓ Cytosolic β-catenin ↑ Nuclear β-catenin | [69] |
| Cell Type | Irisin Concentration/Duration | Effect | Reference |
|---|---|---|---|
| MLO-Y4 cells | Irisin 1–500 ng/mL; 16 h | ↓ H2O2–induced apoptosis ↑ Sclerostin mRNA ↑ p-FAK protein ↑ p-AKT protein ↑ p-CREB protein ↑ p-Zyxin protein | [42] |
| MLO-Y4 cells | Irisin 100 ng/mL; 24 h | ↓ Cyclic stretching-induced apoptosis ↑ Osteocyte proliferation ↑ p-Erk protein ↑ p-p38 protein ↓ Sclerostin mRNA ↑ OPG/RANKL ratio | [70] |
| MLO-Y4 cells | r-irisin 100 ng/mL; 8 and 24 h | ↓ H2O2–induced apoptosis ↓ Caspase-9 protein ↓ Cleaved caspase-3 protein ↑ Pdpn mRNA ↓ Dkk1 mRNA ↑ Atf4 mRNA ↓ Sclerostin mRNA ↑ Tfam mRNA ↑ Bcl2/Bax ratio ↑ p-ERK protein | [71] |
| Cell Type | Irisin Concentration/Duration | Effect | Reference |
|---|---|---|---|
| hOAC cells | Human r-irisin 25 ng/mL; 7 days | ↑ Cell proliferation ↑ GAG production ↑ TIMP-1 mRNA ↑ TIMP-3 mRNA ↓ IL-1 mRNA ↓ IL-6 mRNA ↓ iNOS mRNA ↓ MMP-1 mRNA ↓ MMP-13 mRNA ↑ Type II collagen mRNA and protein ↓ p-p38 protein ↓ p-Akt protein ↓ p-JNK protein ↓ p-NF-κB protein | [72] |
| Primary mouse chondrocytes isolated from mice with DMM-induced osteoarthritis | Irisin 5 and 10 ng/mL; 6 and 72 h | ↑ Proliferation ↑ Mitophagy ↑ Autophagosome formation ↓ ROS production ↑ FNDC5 expression ↑ LC3 expression ↑ PCNA immunoreactivity ↑ GAG production ↑ ECM accumulation ↑ Collagen II mRNA ↑ Aggrecan mRNA ↑ Sox9 mRNA ↓ MMP9 mRNA ↓ CEGF mRNA ↑ PGC-1a mRNA ↑ Tfam mRNA ↑ ATP production ↑ Mito membrane potential ↑ Sirt3 protein ↑ UCP-1 protein | [73] |
| Animal Model | Irisin Concentration/Duration | Effect | Reference |
|---|---|---|---|
| C57BL6 male mice | Hind-limb suspension + irisin injections (100 µg/kg b.w.); once a week for 4 weeks | ↓ Disuse-induced bone mass loss ↑ BV/TV ratio ↑ Femoral and tibia radiodensity ↑ Polar moment of inertia ↑ Bending strength | [74] |
| C57BL/6J mice and FNDC5-KO mice | r-irisin injection (1 mg/kg b.w.); 6 days | ↑ Bone and plasma sclerostin mRNA ↑ Ucp1 mRNA and protein FNDC5-KO: ↓ RANKL mRNA ↑ Femoral bone mass ↑ Femoral connectivity | [42] |
| C57BL/6J mice with forced expression of FNDC5 | N/A | ↓ Cortical bone area ↓ Trabecular bone volume ↓ Cortical thickness ↑ Osteoclast number and size | [67] |
| Mice lacking functional irisin | N/A | ↓ Bone strength ↓ Bone mass ↑ Osteoclast number ↑ RANKL surface expression ↓ Browning response ↑ Intraperitoneal white adipose cell size ↑ Hyperlipidemia ↑ Insulin resistance ↑ LDL-cholesterol level ↓ HDL-cholesterol level | [75] |
| Osx-Cre:FNDC5/irisin KO mice | r-irisin (undisclosed dosage); 14 days | ↓ Irisin mRNA and protein ↓ BMD ↓ Bone development ↓ Bone mineralization ↓ Trabecular bone mass ↓ Trabecular bone area ↓ Osteoblast number ↓ Cortical BMD ↓ Trabecular BV/TV ↑ Cortical BS/BV ↓ Runx2 mRNA ↓ Bsp mRNA ↓ Osx mRNA ↓ Alp mRNA ↑ Cathepsin K mRNA ↑ Mmp9 mRNA ↑ Trap mRNA r-irisin: ↑ Bone strength ↑ Osteoblastogenesis ↓ Osteoclastogenesis | [69] |
| Wild-type C57BL/6J | 2 weeks voluntary Wheel-running exercise Irisin injection 3.24 ng daily | ↑ FNDC5 mRNA in bone tissue ↑ Irisin | [58] |
| Hindlimb unloaded and sciatic neurectomic mice | N/A | ↓ Trabecular BMD ↓ Muscle volume ↓ Soleus FNDC5 mRNA Soleus FNDC5 = ↑ Tibia trabecular BMD Soleus FNDC5 = ↓ RANKL mRNA | [59] |
| Hindlimb unloaded mice and primary osteoblasts with stimulated microgravity | Primary osteoblasts: r-irisin (1 nM); 14 days | Hindlimb unloaded mice: ↑ Femur bone loss ↓ Mineral apposition rates ↓ Alp mRNA ↓ ColIa1 mRNA ↓ Fndc5 mRNA Primary osteoblasts: ↑ Osteoblast differentiation ↑ Osteoblast proliferation ↑ Alp mRNA ↑ ColIa1 mRNA ↑ ALP activity ↑ Mineralization ↑ CyclinA2, D1, and E1 mRNA ↑ CDK2 and 12 mRNA ↑ β-catenin mRNA | [76] |
| Hindlimb unloaded Sprague–Dawley rats | r-irisin (18 ng/mL); three times per week for 8 weeks | ↑ Bone homeostasis ↑ Bone formation rate ↓ Osteoclast surface ↓ TNF-α level ↓ IL-17 level ↓ RANKL level ↓ Sclerostin level | [77] |
| Hindlimb unloaded C57BL6 male mice | hindlimb suspension irisin injections (100 µg/kg b.w.); 4 weeks | ↓ Osteocyte apoptosis ↑ Bcl2/Bax ratio ↓ Caspase 3 protein ↓ Caspase 9 protein | [71] |
| C57BL/6J mice and FNDC5-KO mice | r-irisin injection (1 mg/kg b.w.); 6 days cRGDyK (1 mg/kg) | r-irisin: ↑ Bone and plasma sclerostin mRNA Co-injection r-irisin and cRGDyK: ↓ Ucp1 mRNA and protein ↓ Dio2 mRNA | [42] |
| Ovariectomized (OVX) mice | r-irisin (100 ug/kg b.w.); 5 weeks | ↓ Trabecular bone loss ↑ Greater bone microarchitecture ↑ BMD ↑ BV/VR ↑ Connection density ↑ Bone stiffness ↑ Osteoblast number ↓ Osteoclast number ↑ Osteocalcin serum level ↓ TRAP serum level | [78] |
| Postmenopausal Sprague–Dawley rats with osteoporosis | Irisin (1 mmol/L) | ↑ Trabecular thickness ↑ Trabecular number ↑ Trabecular BMD ↓ Osteoblast apoptosis ↓ ALP serum level ↓ Caspase-2 mRNA and protein ↓ NLRP3 mRNA and protein ↑ RUNX2 mRNA ↑ OC mRNA ↑ Bcl-2 mRNA and protein ↑ Nrf2 mRNA and protein | [79] |
| OVX mice that underwent moderate intensity treadmill exercise | N/A | ↑ Irisin protein ↑ Fndc5 mRNA Fndc5 mRNA = ↑ Trabecular BMD | [80] |
| Androgen deficient and osteopenic mice | r-irisin (100 ug/kg b.w.); once a week for 8 weeks | ↑ Trabecular BMD Soleus Fndc5 mRNA = ↑ Trabecular BMD Soleus Fndc5 mRNA ≠ ↑ Cortical BMD | [81] |
| Sprague–Dawley rats with IBD | r-irisin (18 ng/mL b.w.); 2 times per week for 3 weeks | ↓ Gut inflammation ↓ Bone inflammation ↑ Bone structure ↑ Bone formation rate ↓ Lymphatic hyperproliferation ↓ Osteoblast surface expression ↓ TNF-α protein ↓ RANKL protein | [82] |
| Sprague–Dawley rats with IBD | r-irisin (18 ng/mL b.w.); 2 times per week for 3 weeks | ↓ DSS-stimulated colon inflammation ↑ Bone formation ↓ Osteoclast surface expression ↓ TNF-α positive expression ↓ RANKL positive expression ↓ OPG positive expression ↓ IL-6 positive expression | [83] |
| C57BL6 mice | r-irisin (100 µg/kg b.w.); 4 weeks | ↓ Cartilage degradation ↑ Tibial hyaline cartilage ↓ Calcified cartilage ↑ BV/TV ↑ Trabecular bone number ↑ Connective density ↓ Osteocyte apoptosis ↓ TRAP positive expression ↓ MMP-13 ↓ Caspase 3 | [70] |
| C57BL6 mice with destabilized medial meniscus | r-irisin (10 µL) injected into injured knee joint | ↓ Cartilage injury ↓ Synovitis ↑ Walking ability ↑ LC3 expression ↓ Proliferating cell nuclear antigen ↓ TUNEL | [73] |
| Sprague–Dawley rats fed an HFD and underwent 8 weeks exercise regimen | N/A | ↑ Body weight ↓ BMD ↓ Femur and tibia microstructure Exercise: ↑ BMD ↑ Femur and tibia microstructure ↑ β-catenin ↑ PGC-1α ↑ FNDC5 ↑ Osteocalcin serum level ↑ Irisin serum level | [84] |
| Clinical Model | Effect | Reference |
|---|---|---|
| Healthy children | Irisin = ↑ bone mineral content Irisin = ↑ osteocalcin serum level Irisin = ↑ CTX serum level Irisin = ↓ DKK-1 serum level | [87] |
| Adolescent and adult swimmers | Both groups: ↑ IL-1β ↑ IL-10 Adults: ↑ IL-6 ↑ TNF-α ↓ Irisin | [88] |
| Athletic Caucasian football players | Irisin = ↑ BMD Irisin = ↑ bone mineral content | [89] |
| Women that are postmenopausal and with low bone mass | ↓ Irisin serum level Age = ↓ irisin Parathyroid hormone = ↓ irisin Creatinine = ↓ irisin Osteoporotic fracture occurrence = ↓ irisin | [90] |
| Women with postmenopausal osteoporosis | ↓ Irisin serum level ↓ Chemerin serum level ↑ C-reactive protein level | [91] |
| Chinese elderly population with extremely high hip bone marrow density | ↑ Irisin serum levels (males) Irisin = ↑ BMD Irisin = ↑ triglyceride level | [92] |
| Geriatric Chinese men with osteoporosis or osteopenia | ↓ Irisin serum level Irisin = ↑ BMD | [93] |
| Patients with RA | ↓ Irisin serum level ↑ Low-fracture bone fractures ↑ Extra-articular manifestations ↑ Function joint failure ↓ Vitamin D serum level | [94] |
| Patients with end-stage osteoarthritis | ↑ Cartilage damage ↑ 8-OHdG expression ↑ TUNEL expression ↓ LC3-II expression ↓ FNDC5 expression | [73] |
| Elderly Chinese women that had suffered minimal trauma hip fractures | ↓ Irisin serum level Irisin = ↑ BMD Low irisin level = ↑ risk of hip fracture | [95] |
| Individuals with total hip/knee replacement and osteopenia/osteoporosis | Age = ↓ irisin serum level Irisin = ↑ osteocalcin mRNA FNDC5 = ↑ total femur BMD FNDC5 = ↑ femoral neck BMD | [96] |
| Individuals with osteoporosis and osteopenia | Lumbar bone marrow density = ↑ serum irisin level Lumbar T-score = ↓ serum irisin level | [97] |
| Overweight individuals with a previous vertebral osteoporotic fracture | ↓ Irisin serum level Irisin ≠ BMD Irisin ≠ daily physical activity | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornel, A.; Den Hartogh, D.J.; Klentrou, P.; Tsiani, E. Role of the Myokine Irisin on Bone Homeostasis: Review of the Current Evidence. Int. J. Mol. Sci. 2021, 22, 9136. https://doi.org/10.3390/ijms22179136
Kornel A, Den Hartogh DJ, Klentrou P, Tsiani E. Role of the Myokine Irisin on Bone Homeostasis: Review of the Current Evidence. International Journal of Molecular Sciences. 2021; 22(17):9136. https://doi.org/10.3390/ijms22179136
Chicago/Turabian StyleKornel, Amanda, Danja J. Den Hartogh, Panagiota Klentrou, and Evangelia Tsiani. 2021. "Role of the Myokine Irisin on Bone Homeostasis: Review of the Current Evidence" International Journal of Molecular Sciences 22, no. 17: 9136. https://doi.org/10.3390/ijms22179136







