The Oxidation of Equol by Tyrosinase Produces a Unique Di-ortho-Quinone: Possible Implications for Melanocyte Toxicity
Abstract
:1. Introduction
2. Results
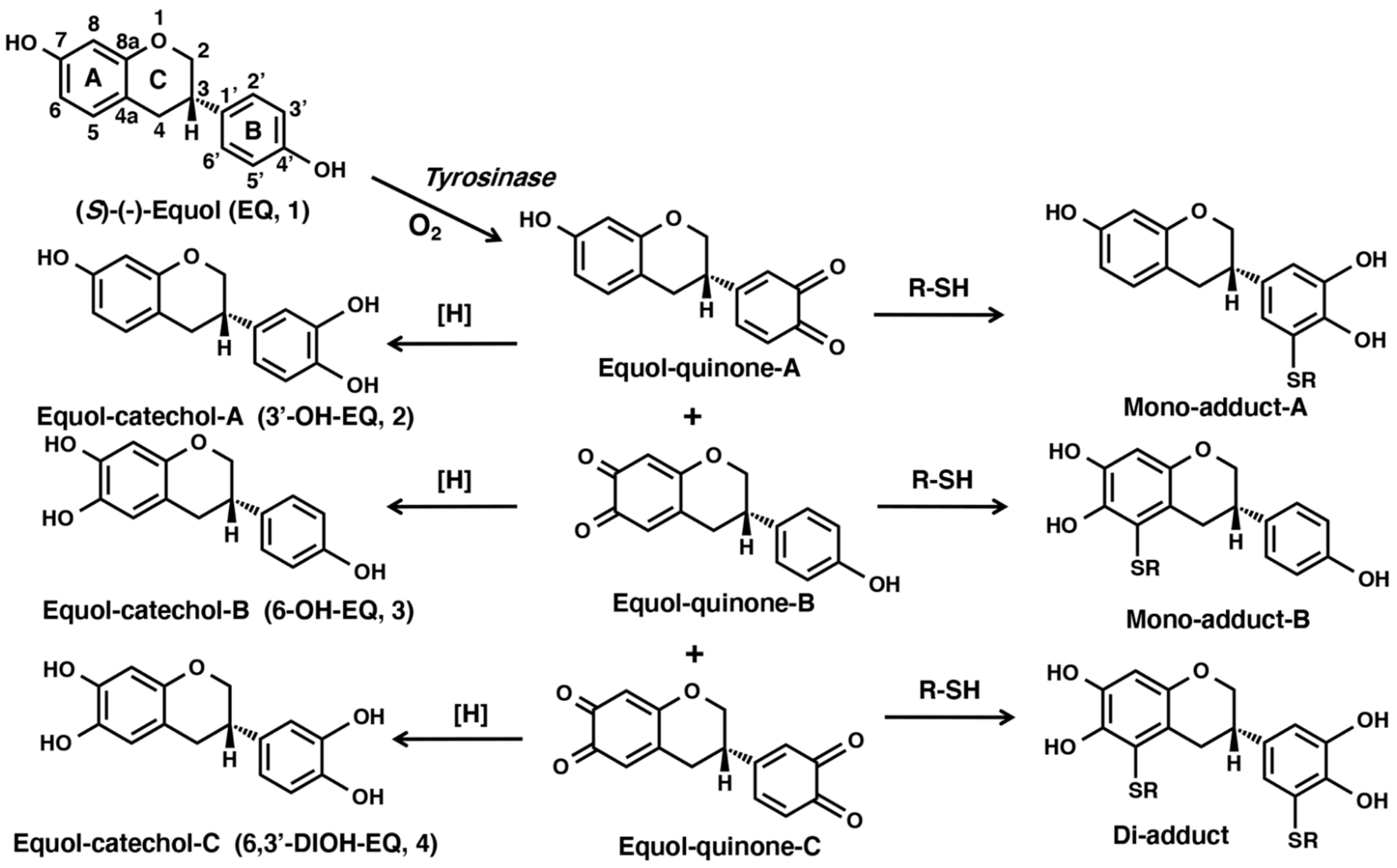
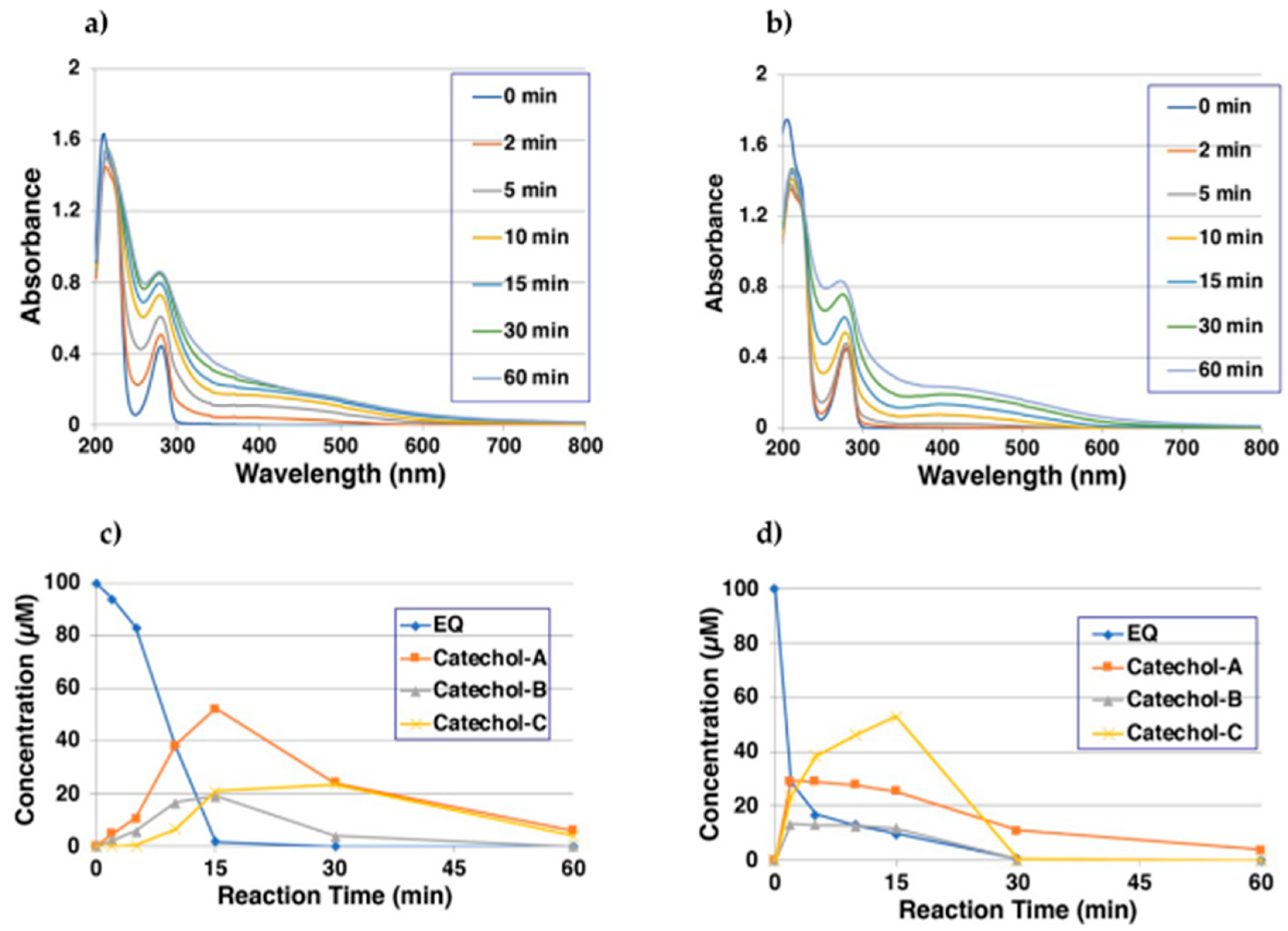
2.1. Tyrosinase-Catalyzed Oxidation of EQ 1 Produces EQ-Quinones
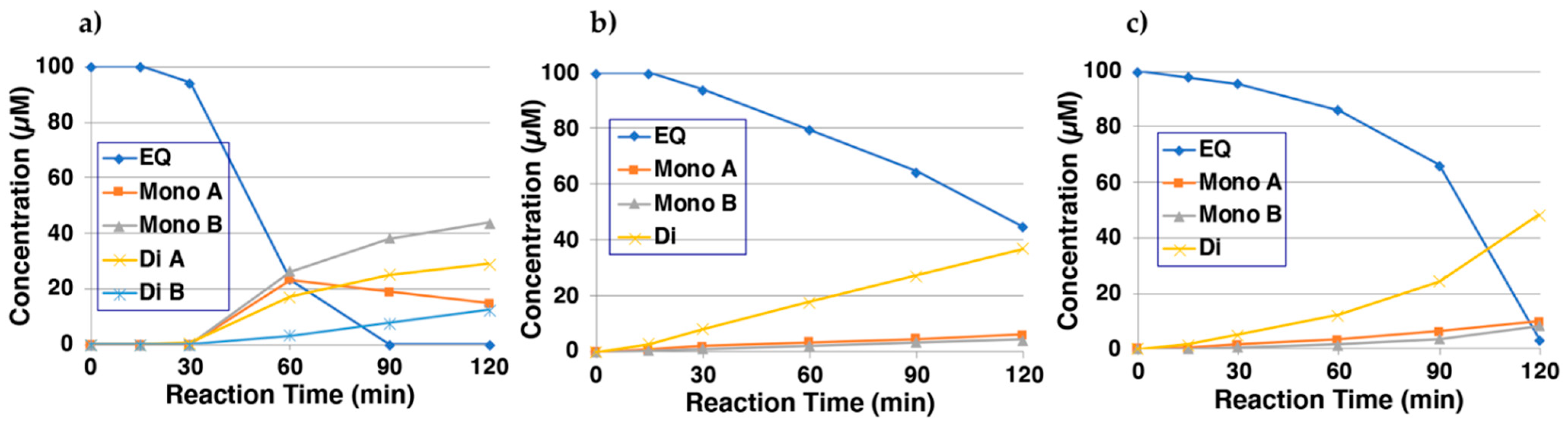
2.2. Reaction of EQ-Quinones with Non-Protein Thiol Compounds Produces Mono- and Di-Adducts
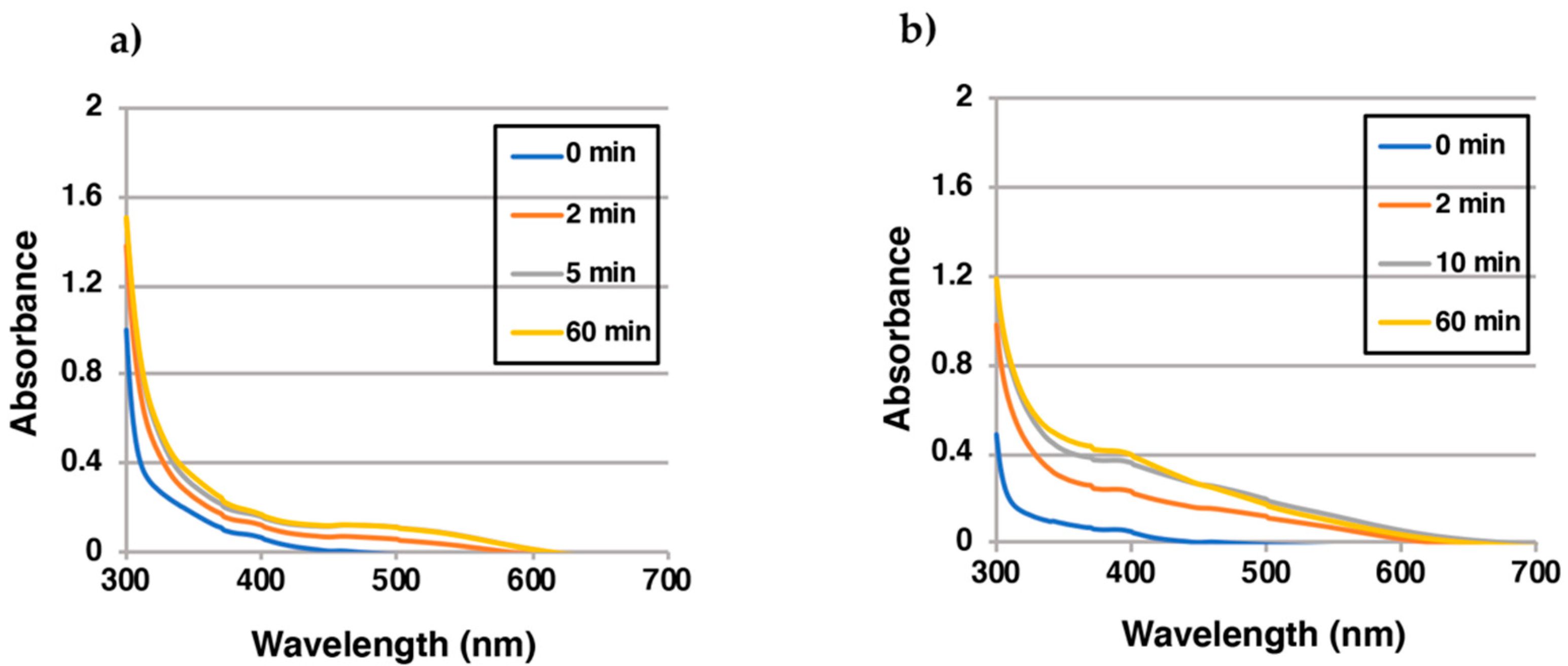
2.3. Reaction of EQ-Quinones with BSA
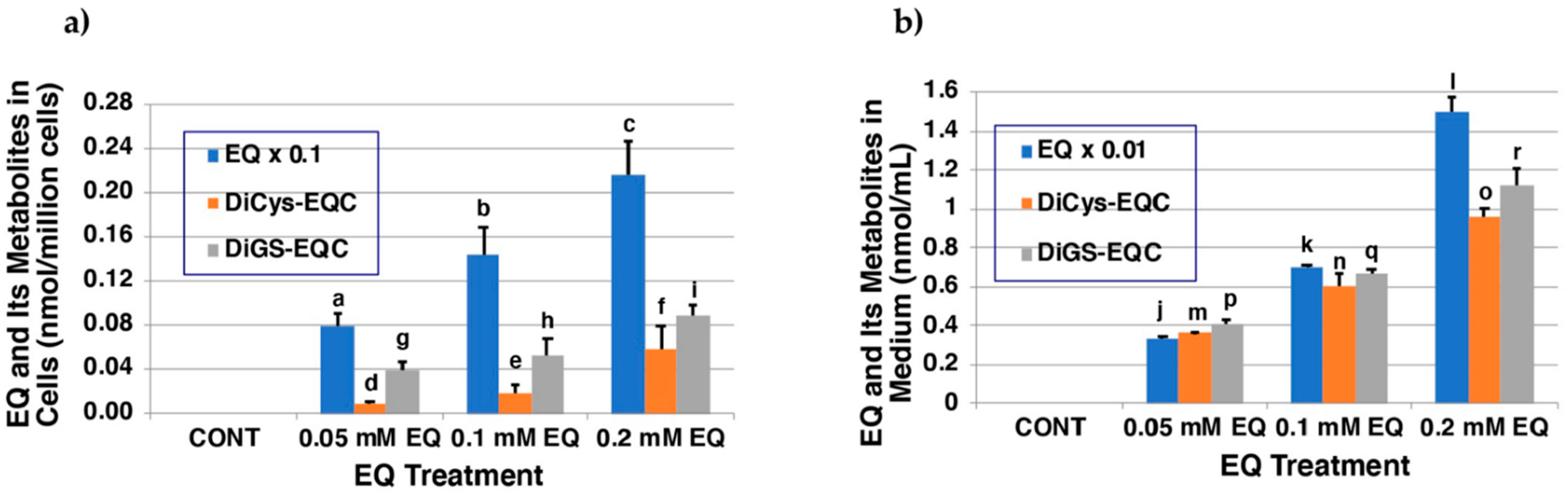
2.4. Metabolism of EQ 1 in Tyrosinase-Transfected Cells
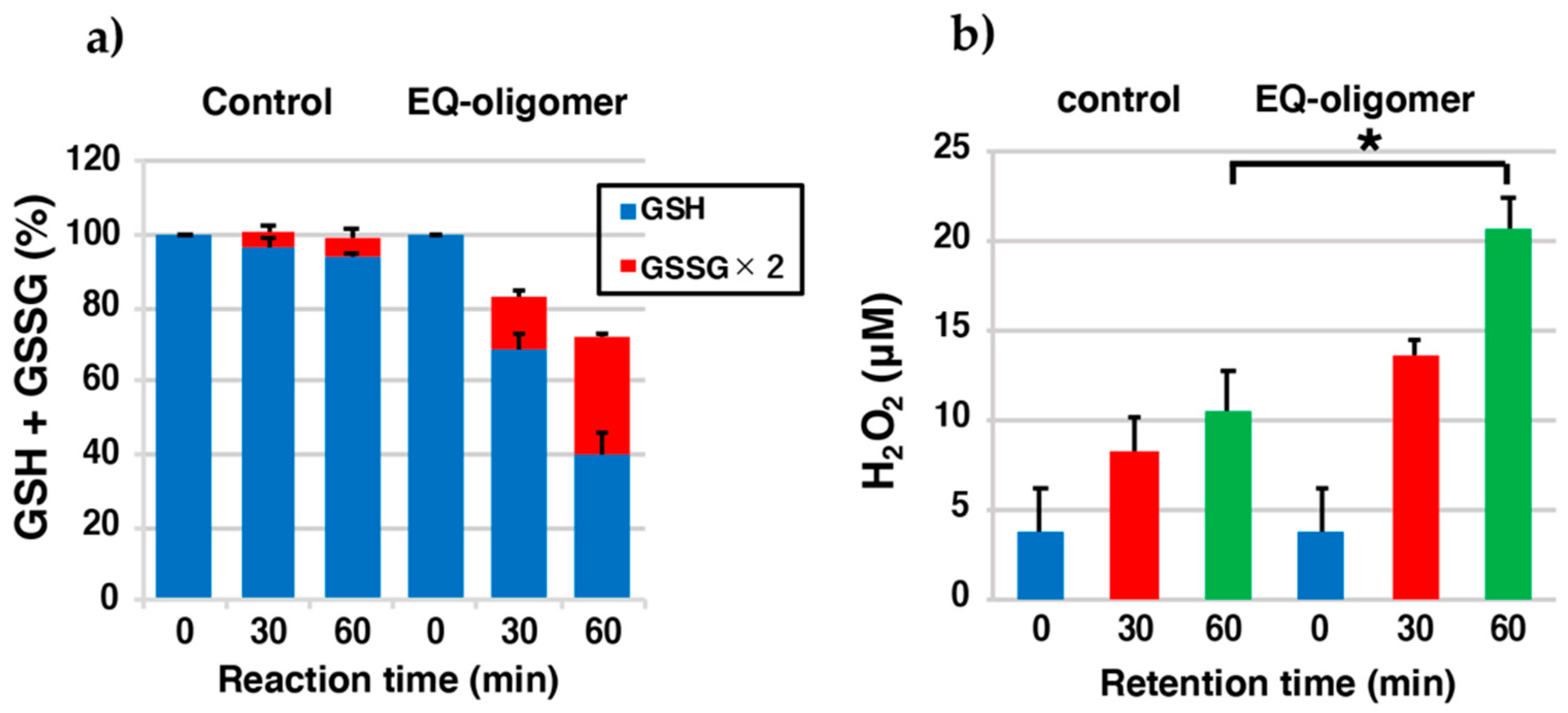
2.5. EQ-Oligomer Oxidizes GSH to GSSG (Pro-Oxidant Activity)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Instruments
4.3. Oxidation of EQ 1 by Tyrosinase in the Absence or Presence of L-Ascorbic Acid or a Thiol
4.4. Isolation of EQ-Catechols
Hydroxy-EQ (EQ-catechol-A, 2), 6-hydroxy-EQ (EQ-catechol-B, 3), and 6,3′-dihydroxy-EQ (EQ-catechol-C, 4)
4.5. Isolation of MonoNAC-EQ-Catechol 5 and DiNAC-EQ-Catechol 6
4.5.1. 5′-Mono-S-[(N-acetyl)cysteinyl]-3′-hydroxy-EQ (MonoNAC-EQ-catechol, 5)
4.5.2. 5,5′-Di-S-[(N-acetyl)cysteinyl]-6,3′-dihydroxy-EQ (DiNAC-EQ-catechol, 6)
4.6. Adduct Formation of EQ-Quinones with CySH and GSH and Isolation of GSH Adducts
4.7. Metabolism of EQ 1 in Tyrosinase-Transfected Cells
4.8. Pro-Oxidant Activity of the EQ 1 Oxidation Product, EQ-Oligomer
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AA | L-Ascorbic acid |
| Ampliflu™ Red reagent | 10-Acetyl-3,7-dihydroxyphenoxazine |
| BSA | Bovine serum albumin |
| COSY | Correlation spectroscopy |
| CySH | Cysteine |
| DBBQ | 3,5-di-tert.-butyl-1,2-benzoquinone |
| DMEM | Dulbecco’s modified eagle’s medium |
| DQF | Double quantum filtered |
| DTNB | 5.5′-Dithiobis (2-nitrobenzoic acid) |
| ER | Endoplasmic reticulum |
| EQ | Equol (7-hydroxy-3-(4′-hydroxyphenyl)-chroman) |
| ESI | Electrospray ionization |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| HMBC | Heteronuclear multiple-bond correlation |
| HPLC | High-performance liquid chromatography |
| HSQC | Heteronuclear single quantum correlation |
| NAC | N-Acetyl-L-cysteine |
| NEM | N-Ethylmaleimide |
| NMR | Nuclear magnetic resonance |
| SOD | Superoxide dismutase |
| TOF MS | Time of flight mass spectrometry |
| TRP-1 | Tyrosinase-related protein-1 |
| TRP-2 | Tyrosinase-related protein-2 |
| UV | Ultraviolet |
References
- Setchell, K.D.R.; Clerici, C. Equol: Pharmacokinetics and biological actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef] [Green Version]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [Green Version]
- Marrian, G.F.; Haslewood, G.A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares’ urine. Biochem. J. 1932, 26, 1227–1232. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef] [Green Version]
- Bennetau-Pelissero, C.; Arnal-Schnebelen, B.; Lamothe, V.; Sauvant, P.; Sagne, J.L.; Verbruggen, M.A.; Mathey, J.; Lavialle, O. ELISA as a new method to measure genistein and daidzein in food and human fluids. Food Chem. 2003, 82, 645–658. [Google Scholar] [CrossRef]
- Schwen, R.J.; Nguyen, L.; Charles, R.L. Elucidation of the metabolic pathway of S-equol in rat, monkey and man. Food Chem. Toxicol. 2012, 50, 2074–2083. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.I.; Han, J.; Wang, X.L.; Song, D.G.; Kim, S.U. Stereospecific biotransformation of dihydrodaidzein into (3S)-equol by the human intestinal bacterium Eggerthella strain Julong 732. Appl. Environ. Microbiol. 2009, 75, 3062–3068. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Lund, T.D.; Munson, D.J.; Haldy, M.E.; Setchell, K.D.; Lephart, E.D.; Handa, R.J. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol. Reprod. 2004, 70, 1188–1195. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Mol. Med. Rep. 2014, 9, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.J.; Wu, J.; Ni, Y.D.; Lu, L.Z.; Zhao, R.Q. Antioxidant effect of a phytoestrogen equol on cultured muscle cells of embryonic broilers. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 735–741. [Google Scholar] [CrossRef]
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr. Rev. 2011, 69, 432–448. [Google Scholar] [CrossRef]
- Nishigori, C.; Aoyama, Y.; Ito, A.; Suzuki, K.; Suzuki, T.; Tanemura, A.; Ito, M.; Katayama, I.; Oiso, N.; Kagohashi, Y.; et al. Guide for medical professionals (i.e. dermatologists) for the management of Rhododenol-induced leukoderma. J. Dermatol. 2015, 42, 113–128. [Google Scholar] [CrossRef]
- Sasaki, M.; Kondo, M.; Sato, K.; Umeda, M.; Kawabata, K.; Takahashi, Y.; Suzuki, T.; Matsunaga, K.; Inoue, S. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment. Cell Melanoma Res. 2014, 27, 754–763. [Google Scholar] [CrossRef]
- Ito, S.; Okura, M.; Nakanishi, Y.; Ojika, M.; Wakamatsu, K.; Yamashita, T. Tyrosinase-catalyzed metabolism of rhododendrol (RD) in B16 melanoma cells: Production of RD-pheomelanin and covalent binding with thiol proteins. Pigment. Cell Melanoma Res. 2015, 28, 295–306. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Biochemical mechanism of rhododendrol-induced leukoderma. Int. J. Mol. Sci. 2018, 19, e552. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Okura, M.; Wakamatsu, K.; Yamashita, T. The potent pro-oxidant activity of rhododendrol-eumelanin induces cysteine depletion in B16 melanoma cells. Pigment. Cell Melanoma Res. 2017, 30, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Colica, C.; Milanovi´c, M.; Mili´c, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A Systematic Review on Natural Antioxidant Properties of Resveratrol. Nat. Prod. Commun. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Na, J.I.; Shin, J.W.; Choi, H.R.; Kwon, S.H.; Park, K.C. Resveratrol as a multifunctional topical hypopigmenting agent. Int. J. Mol. Sci. 2019, 20, E956. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Park, J.H.; Suh, H.-J.; Lee, I.C.; Koh, J.; Boo, Y.C. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014, 306, 475–487. [Google Scholar] [CrossRef]
- Ito, S.; Fujiki, Y.; Matsui, N.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of resveratrol produces a highly reactive ortho-quinone: Implications for melanocyte toxicity. Pigment. Cell Melanoma Res. 2019, 32, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2019, 21, 2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortensen, A.; Kulling, S.E.; Schwartz, H.; Rowland, I.; Ruefer, C.E.; Rimbach, G.; Cassidy, A.; Magee, P.; Millar, J.; Hall, W.L.; et al. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol. Nutr. Food Res. 2009, 53, S266–S309. [Google Scholar] [CrossRef] [PubMed]
- Ishimi, Y. Soybean isoflavones in bone health. Forum Nutr. 2009, 61, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Arnould, S.; Scalbert, A.; Manach, C. Isoflavones and the prevention of breast and prostate cancer: New perspectives opened by nutrigenomics. Br. J. Nutr. 2008, 99, ES78–ES108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Ojika, M.; Yamashita, T.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of rhododendrol produces 2-methylchromane-6,7-dione, the putative ultimate toxic metabolite: Implications for melanocyte toxicity. Pigment. Cell Melanoma Res. 2014, 27, 744–753. [Google Scholar] [CrossRef]
- Lee, P.-G.; Lee, U.-J.; Song, H.; Choi, K.-Y.; Kim, B.-G. Recent advances in the microbioal hydroxylation and reduction of soy isoflavones. FEMS Micro. Lett. 2018, 365, fny195. [Google Scholar] [CrossRef]
- Ito, S.; Hinoshita, M.; Suzuki, E.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of the leukoderma-inducing agent Raspberry ketone produces (E)-4-(3-oxo-1-butenyl)-1,2-benzoquinone: Implications for melanocyte toxicity. Chem. Res. Toxicol. 2017, 30, 859–868. [Google Scholar] [CrossRef]
- Niwa, T.; Akiyama, H.; Echikawa, M.; Yokoyama, S.-I.; Mochizuki, M.; Osawa, T. Equol inhibits mushroom tyrosinase in vitro through tight binding. Biol. Pharm. Bull. 2020, 43, 550–553. [Google Scholar] [CrossRef] [Green Version]
- Shinkaruk, S.; Carreau, C.; Flouriot, G.; Bennetau-Pelissero, B.; Potier, M. Comparative effects of R- and S-equol and implication of transactivation functions (AF-1 and AF-2) in estrogen receptor-induced transcriptional activity. Nutrients 2010, 2, 340–354. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-L.; Hur, H.-G.; Lee, J.H.; Kim, K.T.; Kim, S.-I. Enantioselective synthesis of S-Equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. All. Environ. Microbiol. 2005, 71, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rüfer, E.; Glatt, H.; Kulling, S.E. Structural elucidation of hydroxylated metabolites of the isoflavan equol by gas chromatography-mass spectrometry and high-performance liquid chromatography-mass spectrometry. Amer. Soc. Pharmacol. Therap. 2006, 34, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manini, P.; Napolitano, A.; Westerhof, W.; Riley, P.A.; d’Ischia, M. A reactive ortho-quinone generated by tyrosinase-catalyzed oxidation of the skin depigmenting agent monobenzone: Self-coupling and thiol-conjugation reactions and possible implications for melanocyte toxicity. Chem. Res. Toxicol. 2009, 22, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Simonova, M.; Wall, A.; Weissleder, R.; Bogdanov, A., Jr. Tyrosinase mutants are capable of prodrug activation in transfected nonmelanotic cells. Cancer Res. 2000, 60, 6656–6662. [Google Scholar]
- Cordes, P.; Sun, W.; Wolber, R.; Kolbe, L.; Klebe, G.; Rohm, K.H. Expression in non-melanogenic systems and purification of soluble variants of human tyrosinase. Biol. Chem. 2013, 394, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Ito, S.; Fujita, K. Determination of natural thiols by liquid chromatography after derivatization with 3,5-di-tert.-butyl-1,2-benzoquinone. J. Chromatogr. 1987, 420, 404–410. [Google Scholar] [CrossRef]
- Chang, C.J.; Tsai, T.Y. Antimelanogenic effects of the novel melanogenic inhibitors daidzein and equol, derived from soymilk fermented with Lactobacillus plantarum strain TWK10, in B16F0 mouse melanoma cells. J. Funct. Foods 2016, 22, 211–223. [Google Scholar] [CrossRef]
- Ito, S.; Gerwat, W.; Kolbe, L.; Yamashita, T.; Ojika, M.; Wakamatsu, K. Human tyrosinase is able to oxidize both enantiomers of rhododendrol. Pigment. Cell Melanoma Res. 2014, 27, 1149–1153. [Google Scholar] [CrossRef]
- Yang, L.; Yang, F.; Wataya-Kaneda, M.; Tanemura, A.; Tsuruta, D.; Katayama, I. 4-(4-hydroroxyphenyl)-2-butanol (rhododendrol) activates the autophagy-lysosome pathway in melanocytes: Insights into the mechanisms of rhododendrol-induced leukoderma. J. Dermatol. Sci. 2015, 77, 182–185. [Google Scholar] [CrossRef]
- Zhou, M.; Panchuk-Voloshina, N. A one-step fluorometric method for the continuous measurement of monoamine oxidase activity. Anal. Biochem. 1997, 253, 169–174. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, H.; Ito, S.; Ojika, M.; Nishimaki-Mogami, T.; Kondo, K.; Wakamatsu, K. The Oxidation of Equol by Tyrosinase Produces a Unique Di-ortho-Quinone: Possible Implications for Melanocyte Toxicity. Int. J. Mol. Sci. 2021, 22, 9145. https://doi.org/10.3390/ijms22179145
Tanaka H, Ito S, Ojika M, Nishimaki-Mogami T, Kondo K, Wakamatsu K. The Oxidation of Equol by Tyrosinase Produces a Unique Di-ortho-Quinone: Possible Implications for Melanocyte Toxicity. International Journal of Molecular Sciences. 2021; 22(17):9145. https://doi.org/10.3390/ijms22179145
Chicago/Turabian StyleTanaka, Hitomi, Shosuke Ito, Makoto Ojika, Tomoko Nishimaki-Mogami, Kazunari Kondo, and Kazumasa Wakamatsu. 2021. "The Oxidation of Equol by Tyrosinase Produces a Unique Di-ortho-Quinone: Possible Implications for Melanocyte Toxicity" International Journal of Molecular Sciences 22, no. 17: 9145. https://doi.org/10.3390/ijms22179145
APA StyleTanaka, H., Ito, S., Ojika, M., Nishimaki-Mogami, T., Kondo, K., & Wakamatsu, K. (2021). The Oxidation of Equol by Tyrosinase Produces a Unique Di-ortho-Quinone: Possible Implications for Melanocyte Toxicity. International Journal of Molecular Sciences, 22(17), 9145. https://doi.org/10.3390/ijms22179145







