Lignin/Carbohydrate Complex Isolated from Posidonia oceanica Sea Balls (Egagropili): Characterization and Antioxidant Reinforcement of Protein-Based Films
Abstract
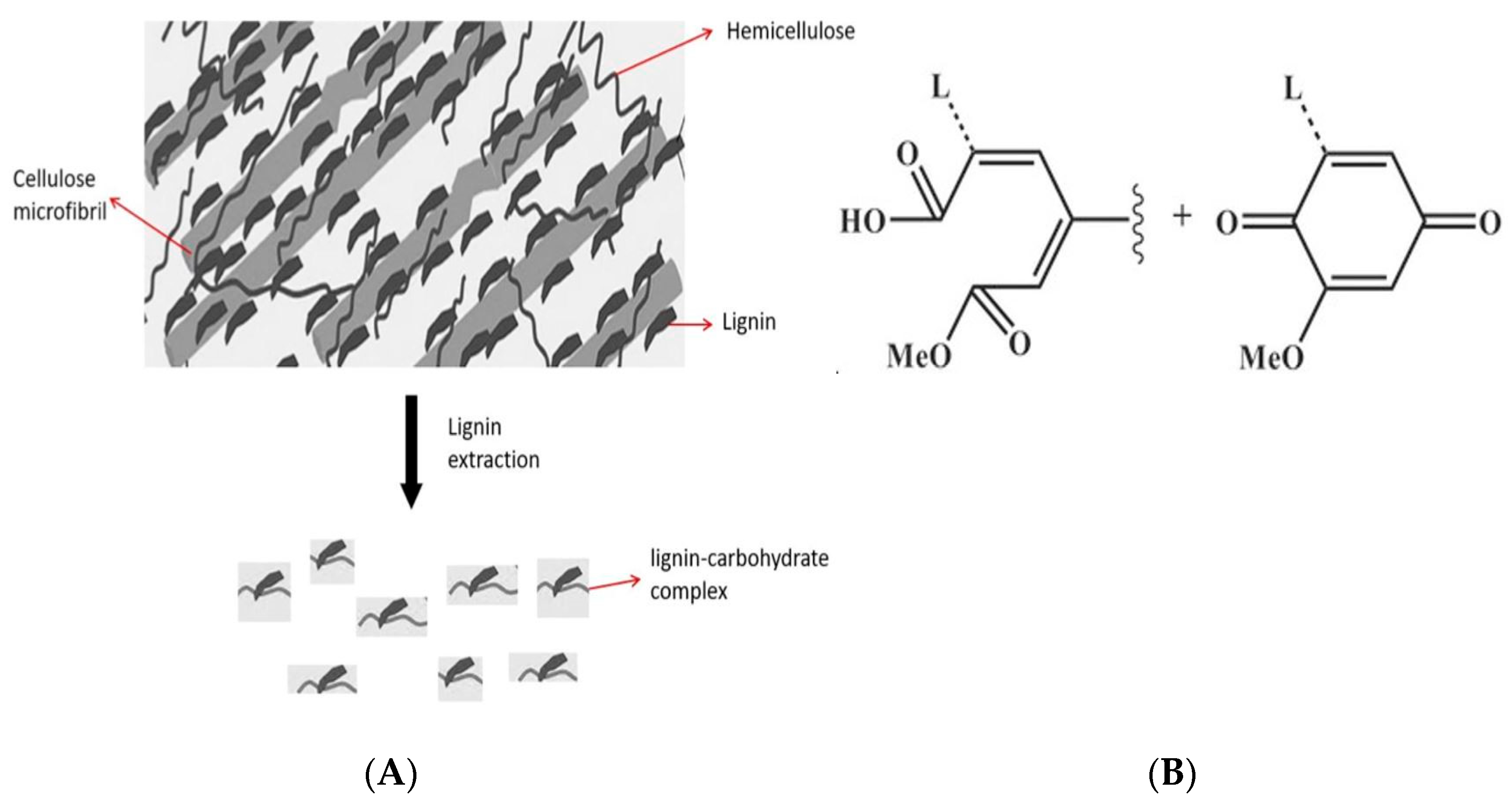
:1. Introduction
2. Results and Discussion
2.1. Egagropili Powder Fractionation and Fourier-Transform Infrared Spectroscopy Analysis
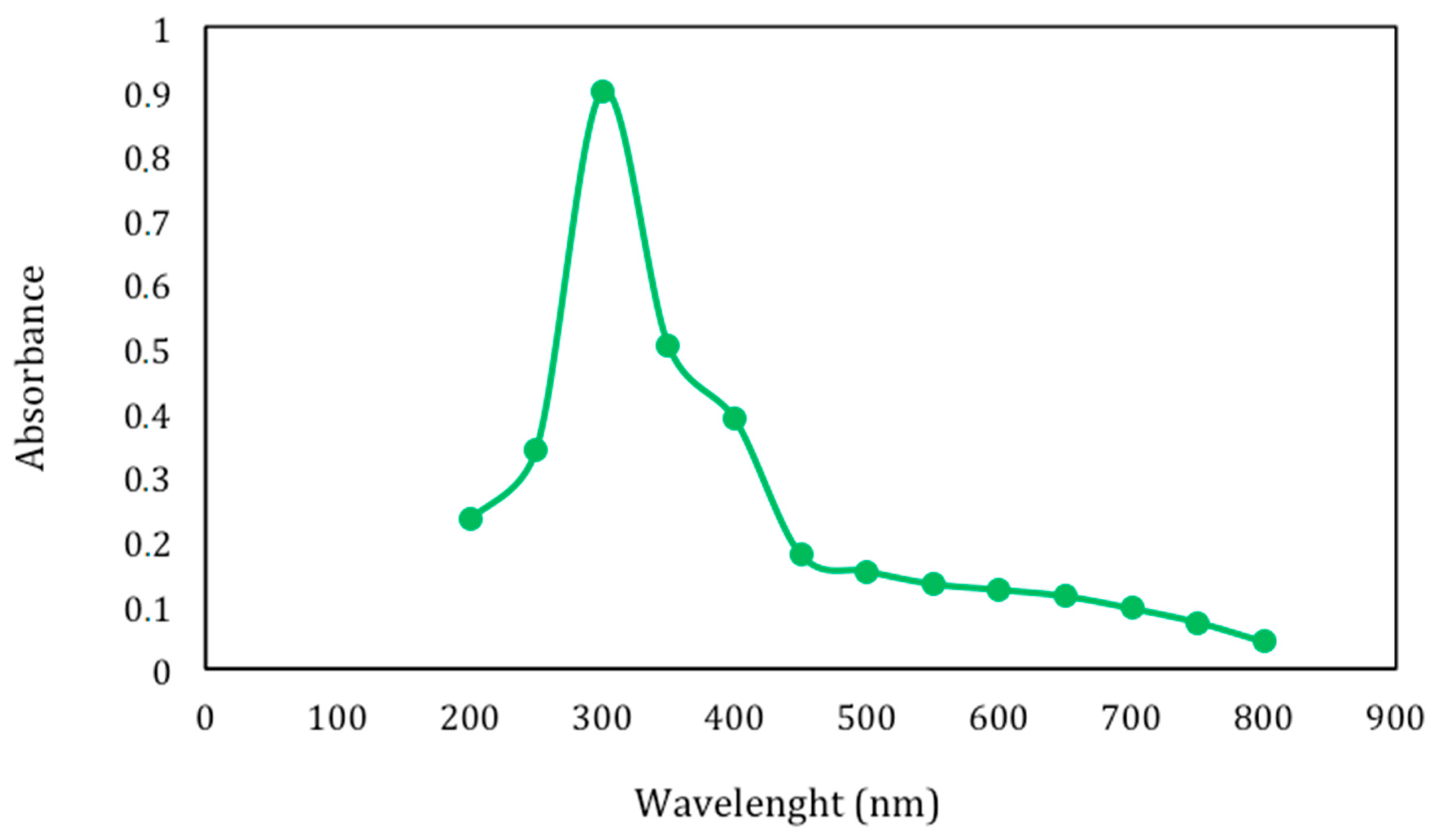
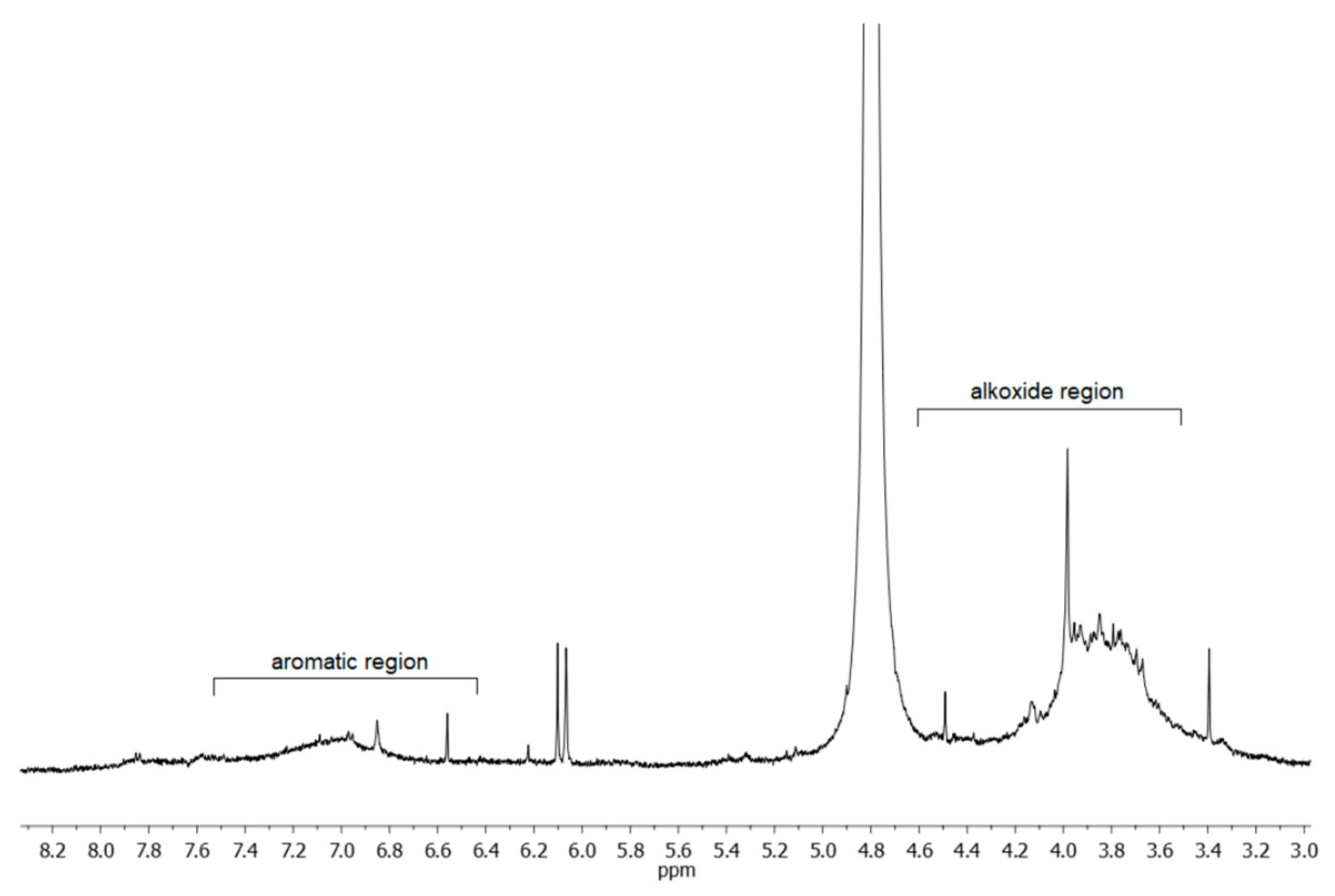
2.2. Egagropili Lignin Fraction Characterization
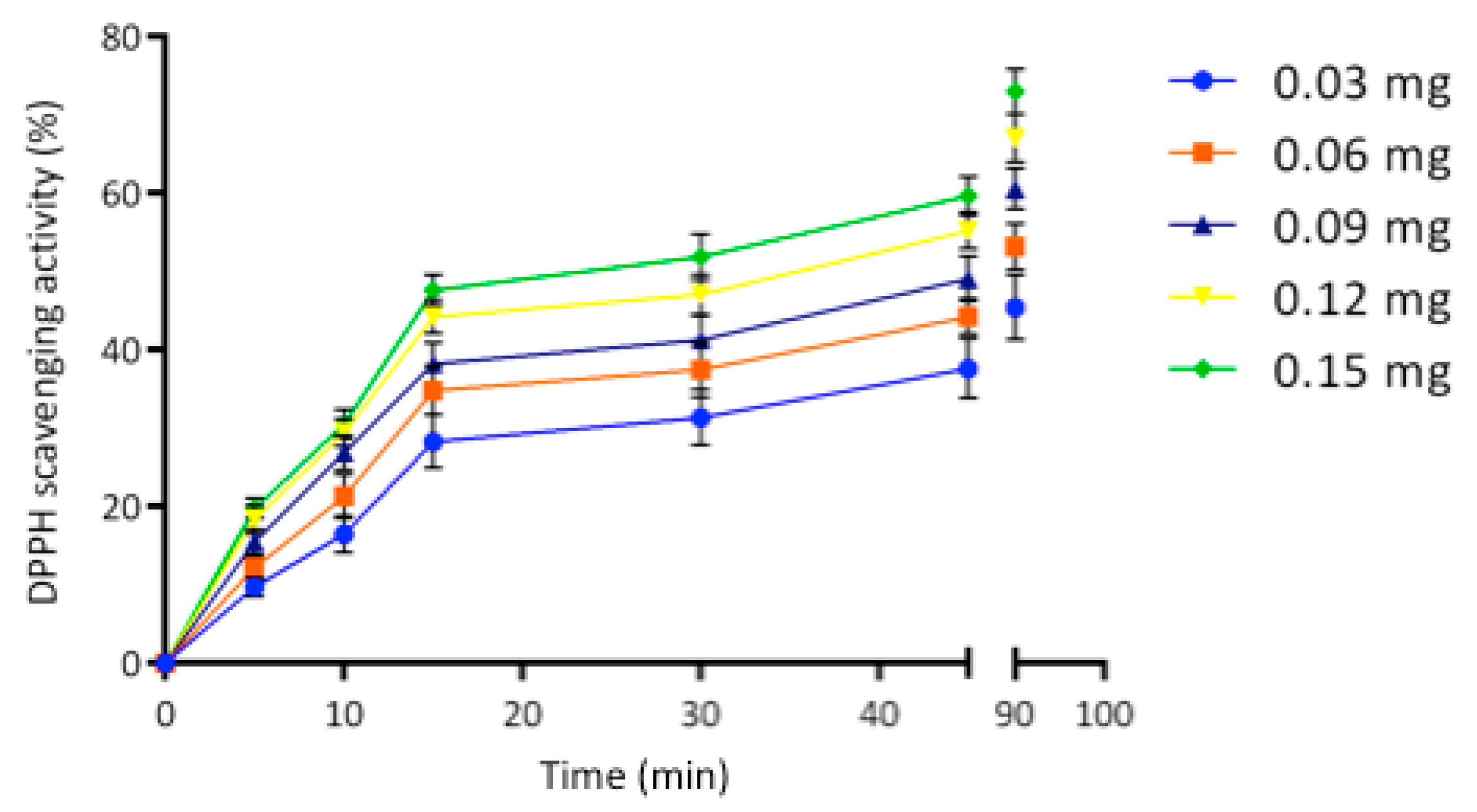
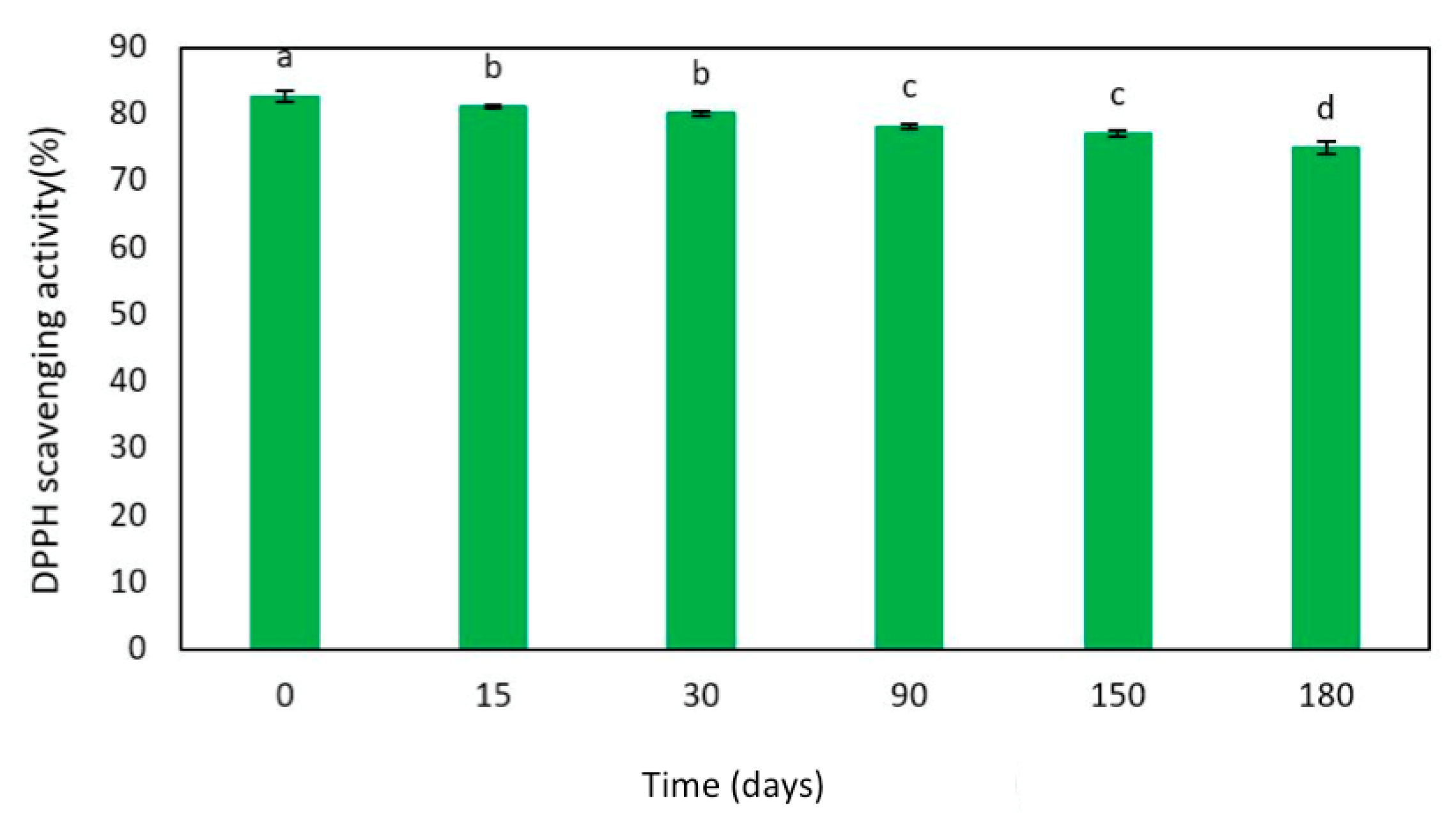
2.3. Egagropili Lignin Fraction Antioxidant Activity
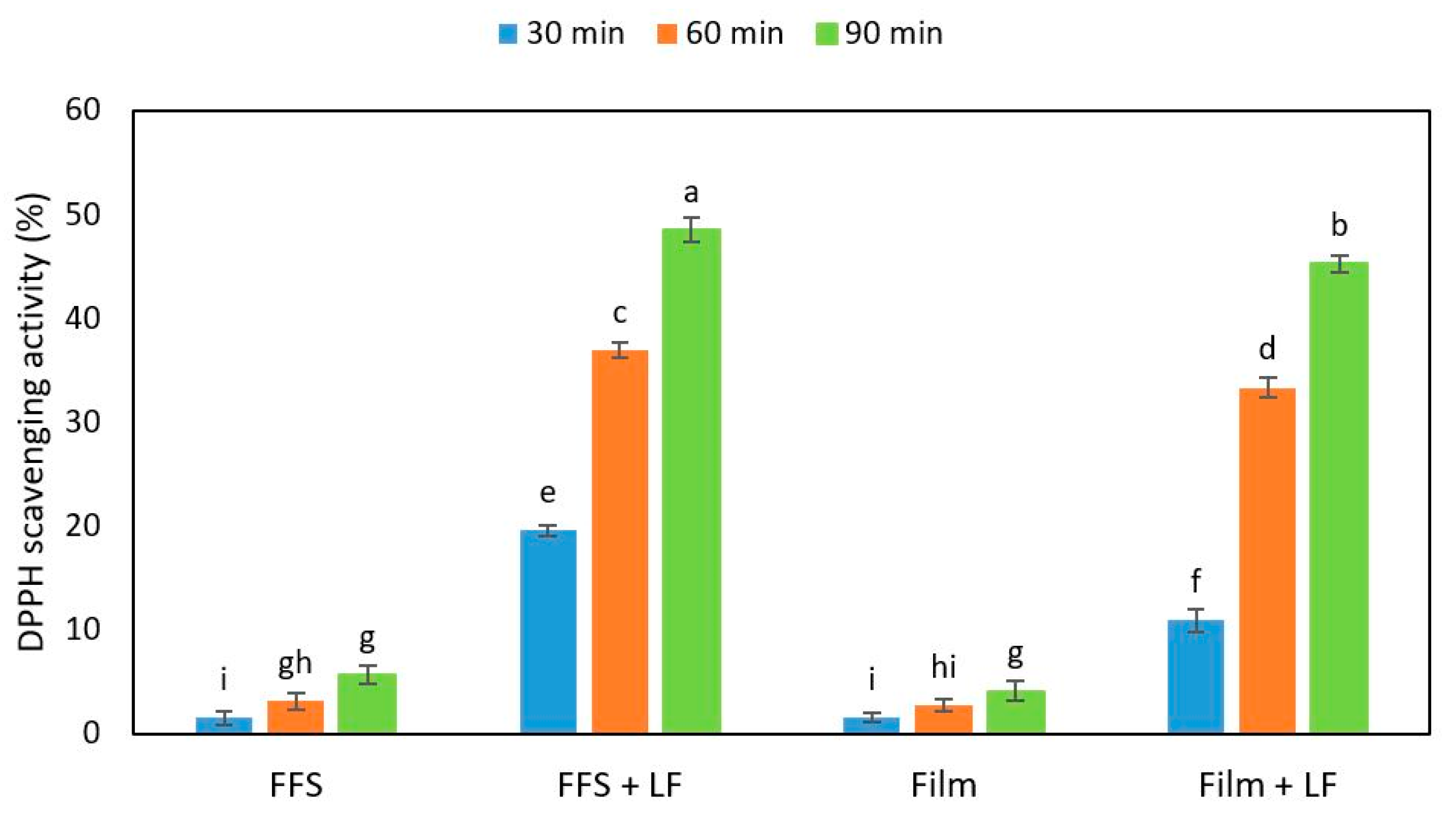
2.4. Antioxidant Activity of Hemp-Protein-Based Films Containing Egagropili Lignin Fraction
3. Materials and Methods
3.1. Materials
3.2. Egagropili Lignin Containing Fraction Preparation
3.3. Molecular Weight Analysis by Size-Exclusion Chromatography with Triple Detector Array
3.4. Monosaccharide Determination by High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection
3.5. Phenol, Flavonoid and Anthocyanin Determination
3.6. Antioxidant Activity Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Arab | arabinose |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FFSs | film forming solutions |
| FT-IR | fourier-transform infrared |
| Fuc | Fucose |
| Gal | galactose |
| Glc | glucose |
| GLcA | glucuronic acid |
| GlcN | glucosamine |
| GPC | gel permeation chromatography |
| HP | hemp protein |
| HPAE-PAD | anion-exchange chromatography with pulsed amperometric detection |
| LCC | lignin/carbohydrate complex |
| LF | lignin fraction |
| Mw | molecular weight |
| PEO | polyethylene oxide |
| Rham | rhamnose |
| SEC | size-exclusion chromatograph |
| SEC-TDA | size-exclusion chromatographic analysis with triple detector array system |
| TPC | total phenol content |
| Xyl | xylose |
References
- Doherty, W.O.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crop. Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Bruijnincx, P.C.; Weckhuysen, B.M. Biomass conversion: Lignin up for break-down. Nat. Chem. 2014, 6, 1035–1036. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Lin, C.L.; Wang, C.C.; Chang, S.C.; Stephen Inbaraj, B.; Chen, B.H. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int. J. Biol. Macromol. 2009, 45, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Hsu, B.Y.; Chen, B.H. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int. J. Biol. Macromol. 2010, 47, 445–453. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chen, B.Y.; Liao, C.W.; Chen, B.H. Green synthesis, characterization and evaluation of catalytic and antibacterial activities of chitosan, glycol chitosan and poly(γ-glutamic acid) capped gold nanoparticles. Int. J. Biol. Macromol. 2020, 161, 1484–1495. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Langan, P.; Petridis, L.; O’Neill, H.M.; Pingali, S.V.; Foston, M.; Nishiyama, Y.; Schulz, R.; Lindner, B.; Leif Hanson, B.; Harton, S.; et al. Common processes drive the thermochemical pretreatment of lignocellulosic biomass. Green Chem. 2014, 16, 63–68. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of lignocellulosic fibers and lignin in bioplastics: A review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [Green Version]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Sella Kapu, N.; Trajano, H.L. Review of hemicellulose hydrolysis in softwoods and bamboo. Biofuels Bioprod. Biorefin. 2014, 8, 857–870. [Google Scholar] [CrossRef]
- Balakshin, M.; Capanema, E.; Gracz, H.; Chang, H.M.; Jameel, H. Quantification of lignin-carbohydrate linkages with high-resolution NMR spectroscopy. Planta 2011, 233, 1097–1110. [Google Scholar] [CrossRef]
- Christopher, L.P. Adding value prior to pulping: Bioproducts from hemicellulose. Glob. Perspect. Sustain. For. Manag. 2012, 225–246. [Google Scholar] [CrossRef] [Green Version]
- Sjostrom, E. Wood Chemistry: Fundamentals and Applications; Academic Press: London, UK, 1993; pp. 1–293. [Google Scholar]
- Zhang, Y.; Zhou, S.; Fang, X.; Zhou, X.; Wang, J.; Bai, F.; Peng, S. Renewable and flexible UV-blocking film from poly(butylene succinate) and lignin. Eur. Polym. J. 2019, 116, 265–274. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Thermochemical conversion of lignin to functional materials: A review and future directions. Green Chem. 2015, 17, 4888–4907. [Google Scholar] [CrossRef]
- Lennartsson, P.R.; Niklasson, C.; Taherzadeh, M.J. A pilot study on lignocelluloses to ethanol and fish feed using NMMO pretreatment and cultivation with zygomycetes in an air-lift reactor. Bioresour. Technol. 2011, 102, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef]
- Holmgren, A.; Brunow, G.; Henriksson, G.; Zhang, L.; Ralph, J. Non-enzymatic reduction of quinone methides during oxidative coupling of monolignols: Implications for the origin of benzyl structures in lignins. Org. Biomol. Chem. 2006, 4, 3456–3461. [Google Scholar] [CrossRef]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Cline, S.P.; Smith, P.M. Opportunities for lignin valorization: An exploratory process. Energy Sustain. Soc. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.; Ungureanu, M.; Ignat, L.; Ungureanu, D.; Popa, V.I. Properties of lignin-polyurethane films prepared by casting method. Ind. Crop. Prod. 2004, 20, 231–241. [Google Scholar] [CrossRef]
- Kai, D.; Ren, W.; Tian, L.; Chee, P.L.; Liu, Y.; Ramakrishna, S.; Loh, X.J. Engineering poly(lactide)-lignin nanofibers with antioxidant activity for biomedical application. ACS Sustain. Chem. Eng. 2016, 4, 5268–5276. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Fang, X.; Bai, F.; Qiao, K.; Wang, L. Renewable high-performance polyurethane bioplastics derived from lignin-poly(ε-caprolactone). ACS Sustain. Chem. Eng. 2017, 5, 4276–4284. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Luo, B.; Ramakrishna, S.; Kai, D.; Loh, X.J.; Yang, H.I.; Deen, G.R.; Mo, X. Engineering PCL/lignin nanofibers as an antioxidant scaffold for the growth of neuron and Schwann cell. Colloids Surf. B Biointerfaces 2018, 169, 356–365. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S. Lignin valorization through catalytic lignocellulose fractionation: A fundamental platform for the future biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef]
- Saska, M.; Ozer, E. Aqueous extraction of sugarcane bagasse hemicellulose and production of xylose syrup. Biotechnol. Bioeng. 1995, 45, 517–523. [Google Scholar] [CrossRef]
- Al-Dajani, W.W.; Tschirner, U.W. Pre-extraction of hemicelluloses and subsequent kraft pulping, Part I: Alkaline extraction. Tappi J. 2008, 7, 3–8. [Google Scholar]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Di Pierro, P.; Di Girolamo, R.; Regalado-González, C.; Porta, R. Valorisation of Posidonia oceanica sea balls (egagropili) as a potential source of reinforcement agents in protein-based biocomposites. Polymers 2020, 12, 2788. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Q.; Rojas, R.; Yan, M.; Lawoko, M.; Berglund, L. Lignin-retaining transparent wood. ChemSusChem 2017, 10, 3445–3451. [Google Scholar] [CrossRef] [Green Version]
- Garside, P.; Wyeth, P. Identification of cellulosic fibres by FTIR spectroscopy-thread and single fibre analysis by attenuated total reflectance. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Neto, W.P.F.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue-soy hulls. Ind. Crop. Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Hinterstoisser, B.; Salmén, L. Two-dimensional step-scan FTIR: A tool to unravel the OH-valency-range of the spectrum of cellulose I. Cellulose 1999, 6, 251–263. [Google Scholar] [CrossRef]
- Silvério, H.A.; Neto, W.P.F.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind. Crop. Prod. 2013, 44, 427–436. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Abdullah, I. Isolation and characterization of cellulose nanocrystals from Agave angustifolia fibre. BioResources 2013, 8, 1893–1908. [Google Scholar] [CrossRef] [Green Version]
- Lun, L.W.; Gunny, A.A.N.; Kasim, F.H.; Arbain, D. Fourier transform infrared spectroscopy (FTIR) analysis of paddy straw pulp treated using deep eutectic solvent. AIP Conf. Proc. 2017, 1835, 20049. [Google Scholar] [CrossRef] [Green Version]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues–wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Costas, C.; Gouveia, S.; Sanromán, M.A.; Moldes, D. Structural characterization of kraft lignins from different spent cooking liquors by 1D and 2D nuclear magnetic resonance spectroscopy. Biomass Bioenergy 2014, 63, 156–166. [Google Scholar] [CrossRef]
- Polcin, J.; Rapson, W.H. Effects of bleaching agents on the absorption spectra of lignin in groundwood pulps, Part 2. Oxidative-Reductive Bleaching. Pulp Pap. Mag. Can. 1971, 72, 80–91. [Google Scholar]
- Paulsson, M.; Parkas, J. Review: Light-induced yellowing of ligninocellulosic pulps-mechanism and preventive methods. Bioresources 2012, 7, 5995–6040. [Google Scholar] [CrossRef] [Green Version]
- Rukmanikrishnan, B.; Ramalingam, S.; Rajasekharan, S.K.; Lee, J.; Lee, J. Binary and ternary sustainable composites of gellan gum, hydroxyethylcellulose and lignin for food packaging applications: Biocompatibility, antioxidant activity, UV and water barrier properties. Int. J. Biol. Macromol. 2020, 153, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Yoo, E.; Lee, S.H.; Won, K. Preparation and application of light-colored lignin nanoparticles for broad-spectrum sunscreens. Polymers 2020, 12, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito-González, I.; López-Rubio, A.; Martínez-Abad, A.; Ballester, A.-R.; Falcó, I.; González-Candelas, L.; Sánchez, G.; Lozano-Sánchez, J.; Borrás-Linares, I.; Segura-Carretero, A.; et al. In-depth characterization of bioactive extracts from Posidonia oceanica waste biomass. Mar. Drugs 2019, 17, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Tech. Metall. 2005, 40, 255–260. [Google Scholar]
- Okpuzor, J.; Ogbunugafor, H.; Kareem, G.K.; Igwo-Ezikpe, M.N. In vitro investigation of antioxidant phenolics compounds in extract of Senne alata. Res. J. Phytochem. 2009, 3, 68–76. [Google Scholar]
- Faustino, H.; Gil, N.; Baptista, C.; Duarte, A.P. Antioxidant activity of lignin phenolic compounds extracted from kraft and sulphite black liquors. Molecules 2010, 15, 9308–9322. [Google Scholar] [CrossRef] [Green Version]
- Alzagameem, A.; Khaldi-Hansen, B.E.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic biomass as source for lignin-based environmentally benign antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.V.; Bhat, S.V.; Nagasampagi, B.A.; Sivakumar, M. Chemistry of Natural Products; Narosa Publishing House: New Delhi, India, 2005. [Google Scholar]
- Hubbermann, E.M.; Heins, A.; Stockmann, H.; Schwarz, K. Influence of acids, salt, sugars and hydrocolloids on the colour stability of anthocyanin rich black currant and elderberry concentrates. Eur. Food Res. Technol. 2006, 223, 83–90. [Google Scholar] [CrossRef]
- Le, X.T.; Huynh, M.T.; Pham, T.N.; Than, V.T.; Toan, T.Q.; Bach, L.G.; Trung, N.Q. Optimization of total anthocyanin content, stability and antioxidant evaluation of the anthocyanin extract from Vietnamese Carissa carandas L. fruits. Processes 2019, 7, 468. [Google Scholar] [CrossRef] [Green Version]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod. Biorefin. 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Rencoret, J.; Marques, G.; Serrano, O.; Kaal, J.; Martínez, A.T.; del Río, J.C.; Gutíerrez, A. Deciphering the unique and acylation pattern of Posidonia oceanica lignin. ACS. Sustain. Chem. Eng. 2020, 8, 12521–12533. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Mohammad Zadeh, E.; O’Keefe, S.F.; Kim, Y.T. Utilization of lignin in biopolymeric packaging films. ACS Omega 2018, 3, 7388–7398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restaino, O.F.; D’Ambrosio, S.; Cassese, E.; Barbuto Ferraiuolo, S.; Alfano, A.; Ventriglia, R.; Marrazzo, A.; Schiraldi, C.; Cimini, D. Molecular weight determination of heparosan- and chondroitin-like capsular polysaccharides: Figuring out differences between wild-type and engineered Escherichia coli strains. Appl. Microbiol. Biotechnol. 2019, 103, 6771–6782. [Google Scholar] [CrossRef]
- Restaino, O.F.; Finamore, R.; Stellavato, A.; Diana, P.; Bedini, E.; Trifuoggi, M.; de Rosa, M.; Schiraldi, C. European chondroitin sulfate and glucosamine food supplements: A systematic quality and quantity assessment compared to pharmaceuticals. Carbohydr. Polym. 2019, 222, 114984. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; González-Aguilar, G.A.; Martín-Belloso, O. Interfacial activity of phenolic-rich extracts from avocado fruit waste: Influence on the colloidal and oxidative stability of emulsions and nanoemulsions. Innov. Food Sci. Emerg. Technol. 2021, 69, 102665. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Gonzalez-Aguilar, G.A. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: A comparative study of fifteen Indian cultivars. J. Food Sci. Technol. 2020, 57, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Teixé-Roig, J.; Oms-Oliu, G.; Velderrain-Rodríguez, G.R.; Odriozola-Serrano, I.; Martín-Belloso, O. The effect of sodium carboxymethylcellulose on the stability and bioaccessibility of anthocyanin water-in-oil-in-water emulsions. Food Bioprocess Technol. 2018, 11, 2229–2241. [Google Scholar] [CrossRef]
- Parveen, S.; Chaudhury, P.; Dasmahapatra, U.; Dasgupta, S. Biodegradable protein films from gallic acid and the cataractous eye protein isolate. Int. J. Biol. Macromol. 2019, 139, 12–20. [Google Scholar] [CrossRef]




| Total Phenols (mg gallic acid eqs./g extract) | 113.85 ± 5.87 |
| Hexan-extracted Phenols (mg gallic acid eqs./g extract) | 1.19 ± 0.63 |
| Flavonoids (mg quercetin eqs./g extract) | 24.5 ± 0.32 |
| Anthocyanins (mg cyanidin-3-glucoside/g extract) | 2.60 ± 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirpoor, S.F.; Restaino, O.F.; Schiraldi, C.; Giosafatto, C.V.L.; Ruffo, F.; Porta, R. Lignin/Carbohydrate Complex Isolated from Posidonia oceanica Sea Balls (Egagropili): Characterization and Antioxidant Reinforcement of Protein-Based Films. Int. J. Mol. Sci. 2021, 22, 9147. https://doi.org/10.3390/ijms22179147
Mirpoor SF, Restaino OF, Schiraldi C, Giosafatto CVL, Ruffo F, Porta R. Lignin/Carbohydrate Complex Isolated from Posidonia oceanica Sea Balls (Egagropili): Characterization and Antioxidant Reinforcement of Protein-Based Films. International Journal of Molecular Sciences. 2021; 22(17):9147. https://doi.org/10.3390/ijms22179147
Chicago/Turabian StyleMirpoor, Seyedeh Fatemeh, Odile Francesca Restaino, Chiara Schiraldi, Concetta Valeria L. Giosafatto, Francesco Ruffo, and Raffaele Porta. 2021. "Lignin/Carbohydrate Complex Isolated from Posidonia oceanica Sea Balls (Egagropili): Characterization and Antioxidant Reinforcement of Protein-Based Films" International Journal of Molecular Sciences 22, no. 17: 9147. https://doi.org/10.3390/ijms22179147
APA StyleMirpoor, S. F., Restaino, O. F., Schiraldi, C., Giosafatto, C. V. L., Ruffo, F., & Porta, R. (2021). Lignin/Carbohydrate Complex Isolated from Posidonia oceanica Sea Balls (Egagropili): Characterization and Antioxidant Reinforcement of Protein-Based Films. International Journal of Molecular Sciences, 22(17), 9147. https://doi.org/10.3390/ijms22179147










