Establishment of Canine Transitional Cell Carcinoma Cell Lines Harboring BRAF V595E Mutation as a Therapeutic Target
Abstract
:1. Introduction
2. Results
2.1. Novel Cell Lines Were Established from Canine TCC Patients
2.2. All Cell Lines Have BRAF V595E Mutations and MAPK Pathway Activation
2.3. In Vitro Evaluation of General Growth Characteristics
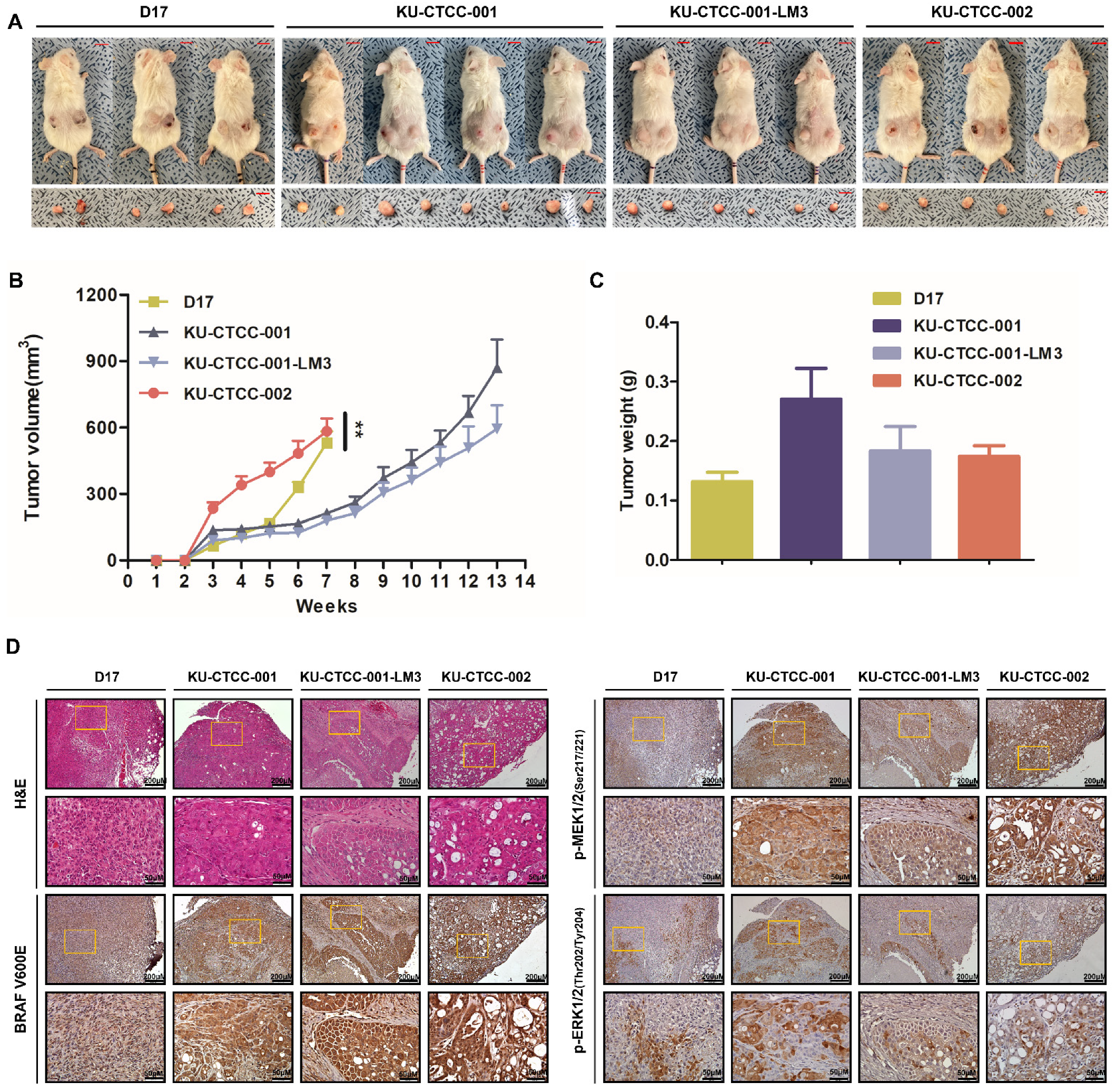
2.4. Tumorigenicity Is Confirmed in an In Vivo Xenograft Mouse Model
2.5. Canine TCC Cell Lines Are More Sensitive to Sorafenib than Vemurafenib
3. Discussion
4. Materials and Methods
4.1. Patient Information and Primary Cell Culture
4.2. Sequencing and STR Analysis
4.3. Colony-Forming Assay and Migration Assay (Wound-Healing Assay)
4.4. Western Blot Analysis
4.5. In Vivo Mouse Xenograft
4.6. Hematoxylin and Eosin Staining and Immunohistochemistry
4.7. MTT Assay and IC50
4.8. Cell Cycle Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mutsaers, A.J.; Widmer, W.R.; Knapp, D.W. Canine transitional cell carcinoma. J. Vet. Intern. Med. 2003, 17, 136–144. [Google Scholar] [CrossRef]
- Griffin, M.A.; Culp, W.T.N.; Rebhun, R.B. Lower urinary tract neoplasia. Vet. Sci. 2018, 5, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decker, B.; Parker, H.G.; Dhawan, D.; Kwon, E.M.; Karlins, E.; Davis, B.W.; Ramos-Vara, J.A.; Bonney, P.L.; McNiel, E.A.; Knapp, D.W.; et al. Homologous mutation to human BRAF V600E is common in naturally occurring canine bladder Cancer—Evidence for a relevant model system and urine-based diagnostic test. Mol. Cancer Res. 2015, 13, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochizuki, H.; Kennedy, K.; Shapiro, S.G.; Breen, M. BRAF Mutations in canine cancers. PLoS ONE 2015, 10, e0129534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagawa, M.; Tambo, N.; Maezawa, M.; Tomihari, M.; Watanabe, K.I.; Inokuma, H.; Miyahara, K. Quantitative analysis of the BRAF V595E mutation in plasma cell-free DNA from dogs with urothelial carcinoma. PLoS ONE 2020, 15, e0232365. [Google Scholar] [CrossRef]
- Gollob, J.A.; Wilhelm, S.; Carter, C.; Kelley, S.L. Role of Raf kinase in cancer: Therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin. Oncol. 2006, 33, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.; Wu, W.; Bivona, T.G. Targeting oncogenic BRAF: Past, present, and future. Cancers 2019, 11, 1197. [Google Scholar] [CrossRef] [Green Version]
- Cohen, Y.; Xing, M.; Mambo, E.; Guo, Z.; Wu, G.; Trink, B.; Beller, U.; Westra, W.H.; Ladenson, P.W.; Sidransky, D. BRAF mutation in papillary thyroid carcinoma. J. Natl. Cancer Inst. 2003, 95, 625–627. [Google Scholar] [CrossRef] [Green Version]
- The Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Boulalas, I.; Zaravinos, A.; Delakas, D.; Spandidos, D.A. Mutational analysis of the BRAF gene in transitional cell carcinoma of the bladder. Int. J. Biol. Markers 2009, 24, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Guan, K.L. Activation of B-Raf kinase requires phosphorylation of the conserved residues Thr598 and Ser601. EMBO J. 2000, 19, 5429–5439. [Google Scholar] [CrossRef] [Green Version]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Breen, M. Comparative aspects of BRAF mutations in canine cancers. Vet. Sci. 2015, 2, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Cronise, K.E.; Hernandez, B.G.; Gustafson, D.L.; Duval, D.L. Identifying the ErbB/MAPK signaling cascade as a therapeutic target in canine bladder cancer. Mol. Pharmacol. 2019, 96, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Higgins, B.; Kolinsky, K.; Packman, K.; Go, Z.; Iyer, R.; Kolis, S.; Zhao, S.; Lee, R.; Grippo, J.F.; et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010, 70, 5518–5527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollag, G.; Tsai, J.; Zhang, J.; Zhang, C.; Ibrahim, P.; Nolop, K.; Hirth, P. Vemurafenib: The first drug approved for BRAF-mutant cancer. Nat. Rev. Drug Discov. 2012, 11, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Cohen, M.S. The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma. Exp. Opin. Drug Discov. 2016, 11, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [Green Version]
- Kolar, G.R.; Miller-Thomas, M.M.; Schmidt, R.E.; Simpson, J.R.; Rich, K.M.; Linette, G.P. Neoadjuvant treatment of a solitary melanoma brain metastasis with vemurafenib. J. Clin. Oncol. 2013, 31, e40–e43. [Google Scholar] [CrossRef]
- Luebker, S.A.; Koepsell, S.A. Diverse mechanisms of BRAF inhibitor resistance in melanoma identified in clinical and preclinical studies. Front. Oncol. 2019, 9, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trunzer, K.; Pavlick, A.C.; Schuchter, L.; Gonzalez, R.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; Kim, K.B.; Weber, J.S.; et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J. Clin. Oncol. 2013, 31, 1767–1774. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Atreya, C.E.; Falchook, G.S.; Kwak, E.L.; Ryan, D.P.; Bendell, J.C.; Hamid, O.; Messersmith, W.A.; Daud, A.; Kurzrock, R.; et al. Combined BRAF and MEK Inhibition with dabrafenib and trametinib in BRAF V600—Mutant colorectal cancer. J. Clin. Oncol. 2015, 33, 4023–4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Spevak, W.; Zhang, Y.; Burton, E.A.; Ma, Y.; Habets, G.; Zhang, J.; Lin, J.; Ewing, T.; Matusow, B.; et al. RAF inhibitors that evade paradoxical MAPK pathway activation. Nature 2015, 526, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.M.; Yang, I.S.; Seung, B.J.; Lee, S.; Kim, D.; Ha, Y.J.; Seo, M.K.; Kim, K.K.; Kim, H.S.; Cheong, J.H.; et al. Cross-Species oncogenic signatures of breast cancer in canine mammary tumors. Nat. Commun. 2020, 11, 3616. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xiong, H.; Ellis, A.E.; Northrup, N.C.; Rodriguez, C.O., Jr.; O’Regan, R.M.; Dalton, S.; Zhao, S. Molecular homology and difference between spontaneous canine mammary cancer and human breast cancer. Cancer Res. 2014, 74, 5045–5056. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Li, Y.; Lyon, K.; Camps, J.; Dalton, S.; Ried, T.; Zhao, S. Cancer driver-passenger distinction via sporadic human and dog cancer comparison: A proof-of-principle study with colorectal cancer. Oncogene 2014, 33, 814–822. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, D.; Ramos-Vara, J.A.; Stewart, J.C.; Zheng, R.; Knapp, D.W. Canine invasive transitional cell carcinoma cell lines: In vitro tools to complement a relevant animal model of invasive urinary bladder cancer. Urol. Oncol. 2009, 27, 284–292. [Google Scholar] [CrossRef]
- Elbadawy, M.; Usui, T.; Mori, T.; Tsunedomi, R.; Hazama, S.; Nabeta, R.; Uchide, T.; Fukushima, R.; Yoshida, T.; Shibutani, M.; et al. Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture. Cancer Sci. 2019, 110, 2806–2821. [Google Scholar] [CrossRef]
- Fulkerson, C.M.; Knapp, D.W. Management of transitional cell carcinoma of the urinary bladder in dogs: A review. Vet. J. 2015, 205, 217–225. [Google Scholar] [CrossRef]
- Knapp, D.W.; Glickman, N.W.; Denicola, D.B.; Bonney, P.L.; Lin, T.L.; Glickman, L.T. Naturally-Occurring canine transitional cell carcinoma of the urinary bladder a relevant model of human invasive bladder cancer. Urol. Oncol. 2000, 5, 47–59. [Google Scholar] [CrossRef]
- Knapp, D.W.; Ramos-Vara, J.A.; Moore, G.E.; Dhawan, D.; Bonney, P.L.; Young, K.E. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014, 55, 100–118. [Google Scholar] [CrossRef]
- Henry, C.J.; McCaw, D.L.; Turnquist, S.E.; Tyler, J.W.; Bravo, L.; Sheafor, S.; Straw, R.C.; Dernell, W.S.; Madewell, B.R.; Jorgensen, L.; et al. Clinical evaluation of mitoxantrone and piroxicam in a canine model of human invasive urinary bladder carcinoma. Clin. Cancer Res. 2003, 9, 906–911. [Google Scholar]
- Walters, L.; Martin, O.; Price, J.; Sula, M.M. Expression of receptor tyrosine kinase targets PDGFR-β, VEGFR2 and KIT in canine transitional cell carcinoma. Vet. Comp. Oncol. 2018, 16, E117–E122. [Google Scholar] [CrossRef]
- Lee, J.T.; McCubrey, J.A. BAY-43-9006 Bayer/Onyx. Curr. Opin. Investig. Drugs 2003, 4, 757–763. [Google Scholar] [PubMed]
- Arozarena, I.; Wellbrock, C. Overcoming resistance to BRAF inhibitors. Ann. Transl. Med. 2017, 5, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulkerson, C.M.; Dhawan, D.; Ratliff, T.L.; Hahn, N.M.; Knapp, D.W. Naturally occurring canine invasive urinary bladder cancer: A complementary animal model to improve the success rate in human clinical trials of new cancer drugs. Int. J. Genom. 2017, 2017, 6589529. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.; Ramos-Vara, J.A.; Hahn, N.M.; Waddell, J.; Olbricht, G.R.; Zheng, R.; Stewart, J.C.; Knapp, D.W. DNMT1: An emerging target in the treatment of invasive urinary bladder cancer. Urol. Oncol. 2013, 31, 1761–1769. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, D.H.; Moon, J.S.; Han, H.J.; Bae, K.; Yoon, K.A. Longitudinal assessment of B-RAF V595E levels in the peripheral cell-free tumor DNA of a 10-year-old spayed female Korean Jindo dog with unresectable metastatic urethral transitional cell carcinoma for monitoring the treatment response to a RAF inhibitor (sorafenib). Vet. Q. 2021, 41, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Petit, V.; Massonnet, G.; Maciorowski, Z.; Touhami, J.; Thuleau, A.; Nemati, F.; Laval, J.; Chateau-Joubert, S.; Servely, J.L.; Vallerand, D.; et al. Optimization of tumor xenograft dissociation for the profiling of cell surface markers and nutrient transporters. Lab. Investig. 2013, 93, 611–621. [Google Scholar] [CrossRef] [Green Version]
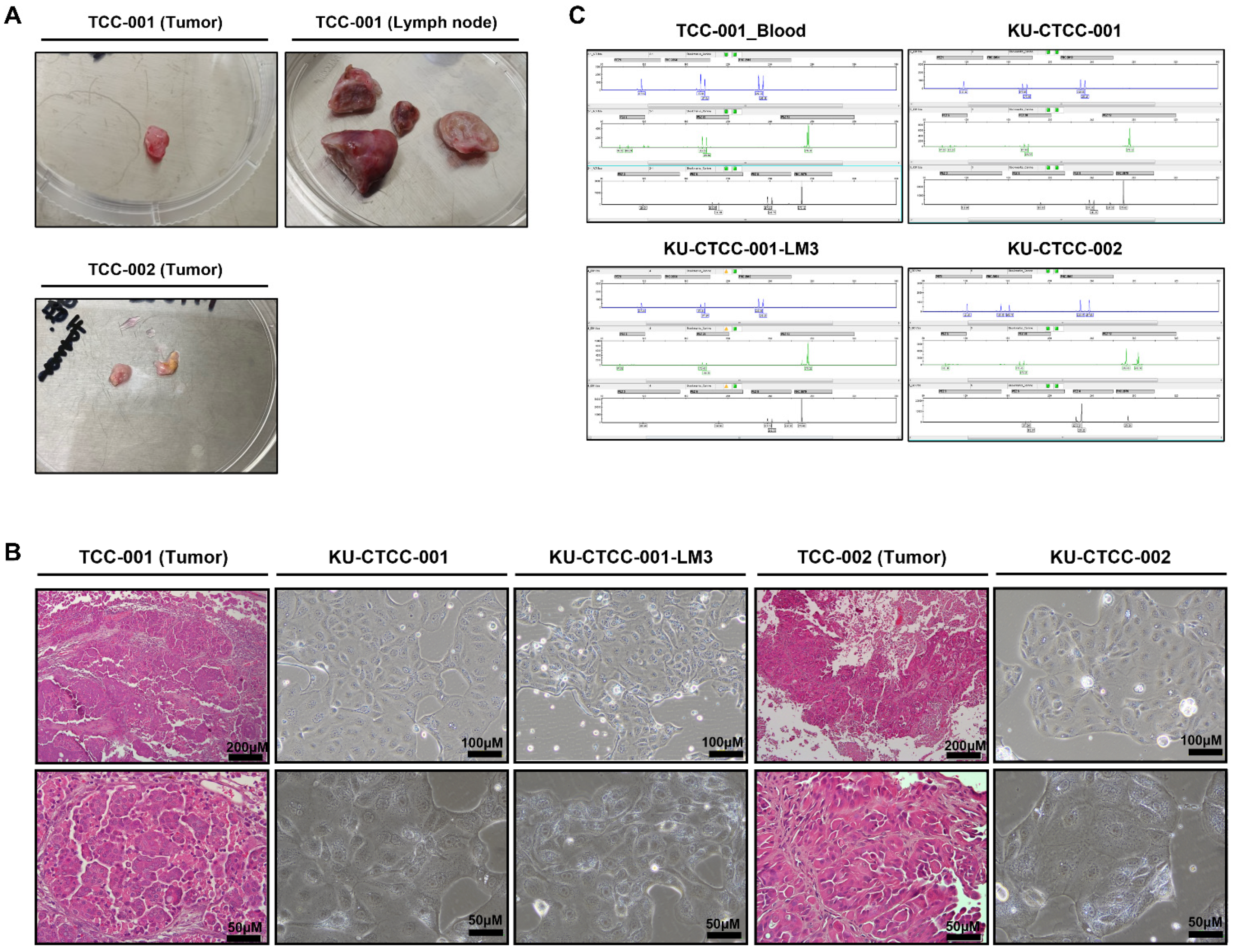



| Patient ID | Body Weight | Sex 1 | Age | Breed | Tumor Location | Histologic Diagnosis | Cell Lines |
|---|---|---|---|---|---|---|---|
| TCC-001 | 22.8 kg (BCS 5/9) | SF | 10y | Jindo dog | Urethra (extending to trigone region of bladder)/Sublumbar lymph nodes | Urothelial Transitional cell carcinoma, non-papillary/infiltrative subtype | KU-CTCC-001/KU-CTCC-001-LM3 |
| TCC-002 | 3.2 kg (BCS 4/9) | NM | 11y | Maltese | Urinary bladder (trigone region) extending to urethra including prostate | Urothelial Transitional cell carcinoma | KU-CTCC-002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.; Bae, K.; Lee, J.Y.; Kim, J.-H.; Han, H.-J.; Yoon, H.-Y.; Yoon, K.-A. Establishment of Canine Transitional Cell Carcinoma Cell Lines Harboring BRAF V595E Mutation as a Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 9151. https://doi.org/10.3390/ijms22179151
Jung H, Bae K, Lee JY, Kim J-H, Han H-J, Yoon H-Y, Yoon K-A. Establishment of Canine Transitional Cell Carcinoma Cell Lines Harboring BRAF V595E Mutation as a Therapeutic Target. International Journal of Molecular Sciences. 2021; 22(17):9151. https://doi.org/10.3390/ijms22179151
Chicago/Turabian StyleJung, Hyojik, Kieun Bae, Ja Young Lee, Jung-Hyun Kim, Hyun-Jung Han, Hun-Young Yoon, and Kyong-Ah Yoon. 2021. "Establishment of Canine Transitional Cell Carcinoma Cell Lines Harboring BRAF V595E Mutation as a Therapeutic Target" International Journal of Molecular Sciences 22, no. 17: 9151. https://doi.org/10.3390/ijms22179151
APA StyleJung, H., Bae, K., Lee, J. Y., Kim, J.-H., Han, H.-J., Yoon, H.-Y., & Yoon, K.-A. (2021). Establishment of Canine Transitional Cell Carcinoma Cell Lines Harboring BRAF V595E Mutation as a Therapeutic Target. International Journal of Molecular Sciences, 22(17), 9151. https://doi.org/10.3390/ijms22179151






