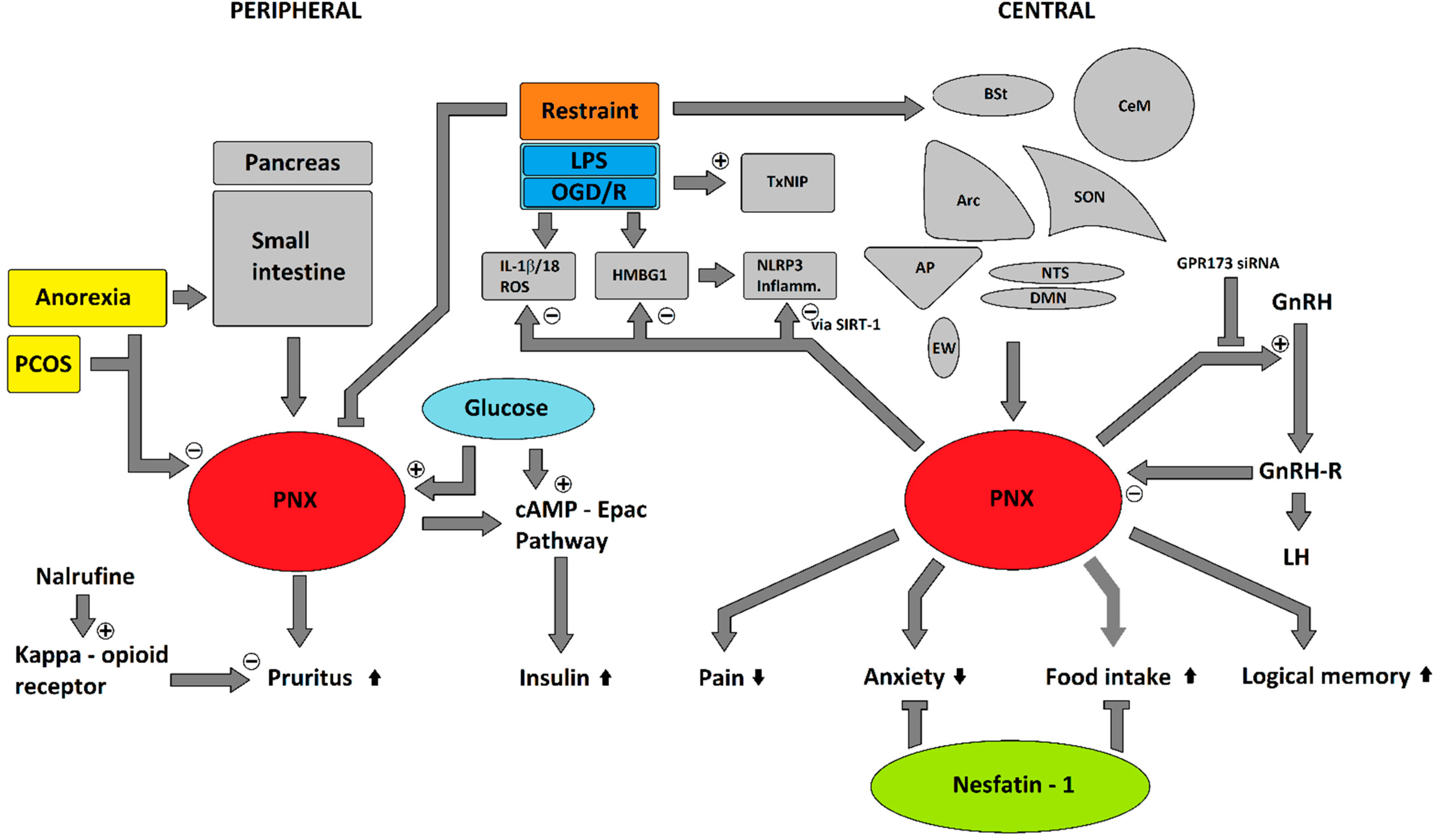
Role of the Novel Peptide Phoenixin in Stress Response and Possible Interactions with Nesfatin-1
Abstract
:1. Introduction
2. Expression Sites of Phoenixin and Nesfatin-1
3. Receptors of Phoenixin and Nesfatin-1
4. Pleiotropic Effects
5. Potential Involvement in Stress Response
6. Interactions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yosten, G.L.; Lyu, R.M.; Hsueh, A.J.; Avsian-Kretchmer, O.; Chang, J.K.; Tullock, C.W.; Dun, S.L.; Dun, N.; Samson, W.K. A novel reproductive peptide, phoenixin. J. Neuroendocrinol. 2013, 25, 206–215. [Google Scholar] [CrossRef]
- Cowan, A.; Lyu, R.M.; Chen, Y.H.; Dun, S.L.; Chang, J.K.; Dun, N.J. Phoenixin: A candidate pruritogen in the mouse. Neuroscience 2015, 310, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://phoenixpeptide.com/products/view/Peptides/079-02 (accessed on 22 July 2021).
- Suszka-Switek, A.; Palasz, A.; Filipczyk, L.; Menezes, I.C.; Mordecka-Chamera, K.; Angelone, T.; Bogus, K.; Bacopoulou, F.; Worthington, J.J.; Wiaderkiewicz, R. The GnRH analogues affect novel neuropeptide SMIM20/phoenixin and GPR173 receptor expressions in the female rat hypothalamic-pituitary-gonadal (HPG) axis. Clin. Exp. Pharmacol. Physiol. 2019, 46, 350–359. [Google Scholar] [CrossRef]
- Lyu, R.M.; Huang, X.F.; Zhang, Y.; Dun, S.L.; Luo, J.J.; Chang, J.K.; Dun, N.J. Phoenixin: A novel peptide in rodent sensory ganglia. Neuroscience 2013, 250, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Dennerlein, S.; Oeljeklaus, S.; Jans, D.; Hellwig, C.; Bareth, B.; Jakobs, S.; Deckers, M.; Warscheid, B.; Rehling, P. MITRAC7 Acts as a COX1-Specific Chaperone and Reveals a Checkpoint during Cytochrome c Oxidase Assembly. Cell Rep. 2015, 12, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.uniprot.org/uniprot/Q8N5G0 (accessed on 22 July 2021).
- Available online: https://www.omim.org/entry/617465#2 (accessed on 22 July 2021).
- Available online: https://www.phoenixpeptide.com/products/view/Peptides/079-01 (accessed on 22 July 2021).
- Oh, I.S.; Shimizu, H.; Satoh, T.; Okada, S.; Adachi, S.; Inoue, K.; Eguchi, H.; Yamamoto, M.; Imaki, T.; Hashimoto, K.; et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 2006, 443, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.phoenixpeptide.com/products/view/Peptides/003-26 (accessed on 22 July 2021).
- Prinz, P.; Scharner, S.; Friedrich, T.; Schalla, M.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem. Biophys. Res. Commun. 2017, 493, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Palasz, A.; Rojczyk, E.; Bogus, K.; Worthington, J.J.; Wiaderkiewicz, R. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical study. Neurosci. Lett. 2015, 592, 17–21. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Chen, J.; Brown, J.E.; Tripathi, G.; Hallschmid, M.; Patel, S.; Kern, W.; Hillhouse, E.W.; Lehnert, H.; Tan, B.K.; et al. Identification of nesfatin-1 in human and murine adipose tissue: A novel depot-specific adipokine with increased levels in obesity. Endocrinology 2010, 151, 3169–3180. [Google Scholar] [CrossRef] [Green Version]
- Stengel, A.; Hofmann, T.; Goebel-Stengel, M.; Lembke, V.; Ahnis, A.; Elbelt, U.; Lambrecht, N.W.; Ordemann, J.; Klapp, B.F.; Kobelt, P. Ghrelin and NUCB2/nesfatin-1 are expressed in the same gastric cell and differentially correlated with body mass index in obese subjects. Histochem. Cell Biol. 2013, 139, 909–918. [Google Scholar] [CrossRef]
- Stengel, A.; Goebel, M.; Yakubov, I.; Wang, L.; Witcher, D.; Coskun, T.; Tache, Y.; Sachs, G.; Lambrecht, N.W. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology 2009, 150, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, R.; Tiwari, A.; Unniappan, S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem. Biophys. Res. Commun. 2009, 381, 643–648. [Google Scholar] [CrossRef]
- Foo, K.S.; Brauner, H.; Ostenson, C.G.; Broberger, C. Nucleobindin-2/nesfatin in the endocrine pancreas: Distribution and relationship to glycaemic state. J. Endocrinol. 2010, 204, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Galiano, D.; Pineda, R.; Ilhan, T.; Castellano, J.M.; Ruiz-Pino, F.; Sanchez-Garrido, M.A.; Vazquez, M.J.; Sangiao-Alvarellos, S.; Romero-Ruiz, A.; Pinilla, L.; et al. Cellular distribution, regulated expression, and functional role of the anorexigenic peptide, NUCB2/nesfatin-1, in the testis. Endocrinology 2012, 153, 1959–1971. [Google Scholar] [CrossRef]
- Hui, J.; Aulakh, G.K.; Unniappan, S.; Singh, B. Localization of Nucleobindin2/Nesfatin-1-Like Immunoreactivity in Human Lungs and Neutrophils. Ann. Anat. 2021, 151774. [Google Scholar] [CrossRef]
- Goebel, M.; Stengel, A.; Wang, L.; Lambrecht, N.W.; Tache, Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci. Lett. 2009, 452, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brailoiu, G.C.; Dun, S.L.; Brailoiu, E.; Inan, S.; Yang, J.; Chang, J.K.; Dun, N.J. Nesfatin-1: Distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 2007, 148, 5088–5094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palasz, A.; Bogus, K.; Suszka-Switek, A.; Kaskosz, A.; Saint-Remy, S.; Piwowarczyk-Nowak, A.; Filipczyk, L.; Worthington, J.J.; Mordecka-Chamera, K.; Kostro, K.; et al. The first identification of nesfatin-1-expressing neurons in the human bed nucleus of the stria terminalis. J. Neural Transm. (Vienna) 2019, 126, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Lepiarczyk, E.; Bossowska, A.; Majewska, M.; Skowronska, A.; Kaleczyc, J.; Majewski, M. Distribution and chemical coding of phoenixin-immunoreactive nerve structures in the spinal cord of the pig. Ann. Anat. 2020, 232, 151559. [Google Scholar] [CrossRef]
- Foo, K.S.; Brismar, H.; Broberger, C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 2008, 156, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.M.; Tullock, C.W.; Mathews, S.K.; Garcia-Galiano, D.; Elias, C.F.; Samson, W.K.; Yosten, G.L. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: Importance of the orphan G protein-coupled receptor Gpr173. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R489–R496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerrant, Y.; Kottler, M.L.; Bergametti, F.; Moumni, M.; Blumberg-Tick, J.; Counis, R. Expression of gonadotropin-releasing hormone (GnRH) receptor gene is altered by GnRH agonist desensitization in a manner similar to that of gonadotropin beta-subunit genes in normal and castrated rat pituitary. Endocrinology 1995, 136, 2803–2808. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, U.B.; Jakubowiak, A.; Steinberger, A.; Chin, W.W. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology 1993, 133, 931–934. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Nakabayashi, H.; Kakei, M.; Shimizu, H.; Mori, M.; Yada, T. Nesfatin-1 evokes Ca2+ signaling in isolated vagal afferent neurons via Ca2+ influx through N-type channels. Biochem. Biophys. Res. Commun. 2009, 390, 958–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, E.; Hashimoto, K.; Shimizu, H.; Okada, S.; Satoh, T.; Kato, I.; Yamada, M.; Mori, M. Nesfatin-1 induces the phosphorylation levels of cAMP response element-binding protein for intracellular signaling in a neural cell line. PLoS ONE 2012, 7, e50918. [Google Scholar] [CrossRef]
- Prinz, P.; Goebel-Stengel, M.; Teuffel, P.; Rose, M.; Klapp, B.F.; Stengel, A. Peripheral and central localization of the nesfatin-1 receptor using autoradiography in rats. Biochem. Biophys. Res. Commun. 2016, 470, 521–527. [Google Scholar] [CrossRef]
- Clarke, I.J.; Cummins, J.T. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 1982, 111, 1737–1739. [Google Scholar] [CrossRef]
- Levine, J.E.; Ramirez, V.D. Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 1982, 111, 1439–1448. [Google Scholar] [CrossRef]
- Ullah, K.; Ur Rahman, T.; Wu, D.D.; Lin, X.H.; Liu, Y.; Guo, X.Y.; Leung, P.C.K.; Zhang, R.J.; Huang, H.F.; Sheng, J.Z. Phoenixin-14 concentrations are increased in association with luteinizing hormone and nesfatin-1 concentrations in women with polycystic ovary syndrome. Clin. Chim. Acta 2017, 471, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Ackerley, R.; Watkins, R.H. Microneurography as a tool to study the function of individual C-fiber afferents in humans: Responses from nociceptors, thermoreceptors, and mechanoreceptors. J. Neurophysiol. 2018, 120, 2834–2846. [Google Scholar] [CrossRef]
- Lefaucheur, J.P. Clinical neurophysiology of pain. Handb. Clin. Neurol. 2019, 161, 121–148. [Google Scholar] [CrossRef] [PubMed]
- Inan, S.; Dun, N.J.; Cowan, A. Investigation of gastrin-releasing peptide as a mediator for 5’-guanidinonaltrindole-induced compulsive scratching in mice. Peptides 2011, 32, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Schalla, M.; Prinz, P.; Friedrich, T.; Scharner, S.; Kobelt, P.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides 2017, 96, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Schalla, M.A.; Scharner, S.; Kuhne, S.G.; Goebel-Stengel, M.; Kobelt, P.; Rose, M.; Stengel, A. Intracerebroventricular injection of phoenixin alters feeding behavior and activates nesfatin-1 immunoreactive neurons in rats. Brain Res. 2019, 1715, 188–195. [Google Scholar] [CrossRef]
- Billert, M.; Kolodziejski, P.A.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Phoenixin-14 stimulates proliferation and insulin secretion in insulin producing INS-1E cells. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118533. [Google Scholar] [CrossRef]
- Hofmann, T.; Weibert, E.; Ahnis, A.; Elbelt, U.; Rose, M.; Klapp, B.F.; Stengel, A. Phoenixin is negatively associated with anxiety in obese men. Peptides 2017, 88, 32–36. [Google Scholar] [CrossRef]
- Jiang, J.H.; He, Z.; Peng, Y.L.; Jin, W.D.; Mu, J.; Xue, H.X.; Wang, Z.; Chang, M.; Wang, R. Effects of Phoenixin-14 on anxiolytic-like behavior in mice. Behav. Brain Res. 2015, 286, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; He, Z.; Peng, Y.L.; Jin, W.D.; Wang, Z.; Mu, L.Y.; Chang, M.; Wang, R. Phoenixin-14 enhances memory and mitigates memory impairment induced by Abeta1-42 and scopolamine in mice. Brain Res. 2015, 1629, 298–308. [Google Scholar] [CrossRef]
- Yuruyen, M.; Gultekin, G.; Batun, G.C.; Yavuzer, H.; Akcan, F.E.; Doventas, A.; Emul, M. Does plasma phoenixin level associate with cognition? Comparison between subjective memory complaint, mild cognitive impairment, and mild Alzheimer’s disease. Int. Psychogeriatr. 2017, 29, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Kinton, L.; Johnson, M.R.; Smith, S.J.; Farrell, F.; Stevens, J.; Rance, J.B.; Claudino, A.M.; Duncan, J.S.; Davis, M.B.; Wood, N.W.; et al. Partial epilepsy with pericentral spikes: A new familial epilepsy syndrome with evidence for linkage to chromosome 4p15. Ann. Neurol. 2002, 51, 740–749. [Google Scholar] [CrossRef]
- Stengel, A.; Goebel, M.; Wang, L.; Rivier, J.; Kobelt, P.; Monnikes, H.; Lambrecht, N.W.; Tache, Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: Differential role of corticotropin-releasing factor2 receptor. Endocrinology 2009, 150, 4911–4919. [Google Scholar] [CrossRef]
- Hofmann, T.; Ahnis, A.; Elbelt, U.; Rose, M.; Klapp, B.F.; Stengel, A. NUCB2/nesfatin-1 Is Associated with Elevated Levels of Anxiety in Anorexia Nervosa. PLoS ONE 2015, 10, e0132058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atsuchi, K.; Asakawa, A.; Ushikai, M.; Ataka, K.; Tsai, M.; Koyama, K.; Sato, Y.; Kato, I.; Fujimiya, M.; Inui, A. Centrally administered nesfatin-1 inhibits feeding behaviour and gastroduodenal motility in mice. Neuroreport 2010, 21, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galiano, D.; Navarro, V.M.; Roa, J.; Ruiz-Pino, F.; Sanchez-Garrido, M.A.; Pineda, R.; Castellano, J.M.; Romero, M.; Aguilar, E.; Gaytan, F.; et al. The anorexigenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J. Neurosci. 2010, 30, 7783–7792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catli, G.; Anik, A.; Kume, T.; Calan, O.G.; Dundar, B.N.; Bober, E.; Abaci, A. Serum nesfatin-1 and leptin levels in non-obese girls with premature thelarche. J. Endocrinol. Investig. 2015, 38, 909–913. [Google Scholar] [CrossRef]
- Kolgazi, M.; Cantali-Ozturk, C.; Deniz, R.; Ozdemir-Kumral, Z.N.; Yuksel, M.; Sirvanci, S.; Yegen, B.C. Nesfatin-1 alleviates gastric damage via direct antioxidant mechanisms. J. Surg. Res. 2015, 193, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, M.; Kocdor, M.A.; Kuloglu, T.; Sahin, I.; Sarac, M.; Aksoy, A.; Yardim, M.; Dalkilic, S.; Gursu, O.; Aydin, S.; et al. Comparison of the therapeutic effects of sildenafil citrate, heparin and neuropeptides in a rat model of acetic acid-induced gastric ulcer. Life Sci. 2017, 186, 102–110. [Google Scholar] [CrossRef]
- Kolgazi, M.; Demirci, E.K.; Ozdemir-Kumral, Z.N.; Yuksel, M.; Sirvanci, S.; Cantali-Ozturk, C.; Yegen, B.C. Anti-inflammatory effects of nesfatin-1 on acetic acid-induced gastric ulcer in rats: Involvement of cyclooxygenase pathway. J. Physiol. Pharmacol. 2017, 68, 765–777. [Google Scholar]
- Szlachcic, A.M.J.; Strzalka, M.; Szmyd, J.; Pajdo, R.; Ptak-Belowska, A.; Kwiecien, S.; Brzozowski, T. Experimental healing of preexisting gastric ulcers induced by hormones controlling food intake ghrelin, orexin-A and nesfatin-1 is impaired under diabetic conditions. A key to understanding the diabetic gastropathy? J. Physiol. Pharmacol. 2013, 64, 625–637. [Google Scholar]
- Gonzalez, R.; Reingold, B.K.; Gao, X.; Gaidhu, M.P.; Tsushima, R.G.; Unniappan, S. Nesfatin-1 exerts a direct, glucose-dependent insulinotropic action on mouse islet beta- and MIN6 cells. J. Endocrinol. 2011, 208, R9–R16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konczol, K.; Pinter, O.; Ferenczi, S.; Varga, J.; Kovacs, K.; Palkovits, M.; Zelena, D.; Toth, Z.E. Nesfatin-1 exerts long-term effect on food intake and body temperature. Int. J. Obes. (Lond.) 2012, 36, 1514–1521. [Google Scholar] [CrossRef] [Green Version]
- Wilz, A.M.; Wernecke, K.; Appel, L.; Kahrs, J.; Dore, R.; Johren, O.; Lehnert, H.; Schulz, C. Endogenous NUCB2/Nesfatin-1 Regulates Energy Homeostasis Under Physiological Conditions in Male Rats. Horm. Metab. Res. 2020, 52, 676–684. [Google Scholar] [CrossRef]
- Fan, X.T.; Tian, Z.; Li, S.Z.; Zhai, T.; Liu, J.L.; Wang, R.; Zhang, C.S.; Wang, L.X.; Yuan, J.H.; Zhou, Y.; et al. Ghrelin Receptor Is Required for the Effect of Nesfatin-1 on Glucose Metabolism. Front. Endocrinol. (Lausanne) 2018, 9, 633. [Google Scholar] [CrossRef]
- Mori, Y.; Shimizu, H.; Kushima, H.; Hiromura, M.; Terasaki, M.; Tanaka, M.; Osaki, A.; Hirano, T. Increased blood pressure in nesfatin/nuclebindin-2-transgenic mice. Hypertens. Res. 2017, 40, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Aydin, B.; Guvenc, G.; Altinbas, B.; Niaz, N.; Yalcin, M. Modulation of nesfatin-1-induced cardiovascular effects by the central cholinergic system. Neuropeptides 2018, 70, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Merali, Z.; Cayer, C.; Kent, P.; Anisman, H. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology (Berl.) 2008, 201, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.F.; Xu, Y.Y.; Qin, G.; Pan, X.Y.; Cheng, J.Q.; Chen, F.H. Nesfatin-1, a potent anorexic agent, decreases exploration and induces anxiety-like behavior in rats without altering learning or memory. Brain Res. 2015, 1629, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Hsuchou, H.; Kastin, A.J. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides 2007, 28, 2223–2228. [Google Scholar] [CrossRef]
- Friedrich, T.; Schalla, M.A.; Lommel, R.; Goebel-Stengel, M.; Kobelt, P.; Rose, M.; Stengel, A. Restraint stress increases the expression of phoenixin immunoreactivity in rat brain nuclei. Brain Res. 2020, 1743, 146904. [Google Scholar] [CrossRef] [PubMed]
- Schalla, M.A.; Goebel-Stengel, M.; Friedrich, T.; Kuhne, S.G.; Kobelt, P.; Rose, M.; Stengel, A. Restraint stress affects circulating NUCB2/nesfatin-1 and phoenixin levels in male rats. Psychoneuroendocrinology 2020, 122, 104906. [Google Scholar] [CrossRef]
- Zeng, X.; Li, Y.; Ma, S.; Tang, Y.; Li, H. Phoenixin-20 Ameliorates Lipopolysaccharide-Induced Activation of Microglial NLRP3 Inflammasome. Neurotox. Res. 2020, 38, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Nasoohi, S.; Ismael, S.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) in Cerebrovascular and Neurodegenerative Diseases: Regulation and Implication. Mol. Neurobiol. 2018, 55, 7900–7920. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, B.; Yang, S.; Tang, X.; Wang, J.; Wei, D. The protective effects of phoenixin-14 against lipopolysaccharide-induced inflammation and inflammasome activation in astrocytes. Inflamm. Res. 2020, 69, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Weber, M.D.; Fonken, L.K.; Hershman, S.A.; Watkins, L.R.; Maier, S.F. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome. Brain Behav. Immun. 2016, 55, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Li, J. Phoenixin-14 protects human brain vascular endothelial cells against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced inflammation and permeability. Arch. Biochem. Biophys. 2020, 682, 108275. [Google Scholar] [CrossRef]
- Grover, H.M.; Smith, P.M.; Ferguson, A.V. Phoenixin influences the excitability of nucleus of the solitary tract neurones, effects which are modified by environmental and glucocorticoid stress. J. Neuroendocrinol. 2020, 32, e12855. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutierrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Palasz, A.; Tyszkiewicz-Nwafor, M.; Suszka-Switek, A.; Bacopoulou, F.; Dmitrzak-Weglarz, M.; Dutkiewicz, A.; Slopien, A.; Janas-Kozik, M.; Wilczynski, K.M.; Filipczyk, L.; et al. Longitudinal study on novel neuropeptides phoenixin, spexin and kisspeptin in adolescent inpatients with anorexia nervosa—Association with psychiatric symptoms. Nutr. Neurosci. 2019, 1–11. [Google Scholar] [CrossRef]
- Paszynska, E.; Dmitrzak-Weglarz, M.; Tyszkiewicz-Nwafor, M.; Slopien, A. Salivary alpha-amylase, secretory IgA and free cortisol as neurobiological components of the stress response in the acute phase of anorexia nervosa. World J. Biol. Psychiatry 2016, 17, 266–273. [Google Scholar] [CrossRef]
- Goebel, M.; Stengel, A.; Wang, L.; Tache, Y. Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009, 1300, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Stengel, A.; Goebel, M.; Wang, L.; Tache, Y. Abdominal surgery activates nesfatin-1 immunoreactive brain nuclei in rats. Peptides 2010, 31, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Zhao, H.; Yan, Z.; Liu, X.; Yin, P.; Zhang, J. Protective role and molecular mechanism of action of Nesfatin-1 against high glucose-induced inflammation, oxidative stress and apoptosis in retinal epithelial cells. Exp. Ther. Med. 2021, 22, 833. [Google Scholar] [CrossRef]
- Konczol, K.; Bodnar, I.; Zelena, D.; Pinter, O.; Papp, R.S.; Palkovits, M.; Nagy, G.M.; Toth, Z.E. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic-pituitary-adrenal axis in rats. Neurochem. Int. 2010, 57, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Vermetten, E.; Bremner, J.D. Circuits and systems in stress. I. Preclinical studies. Depress. Anxiety 2002, 15, 126–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, X.; Sanford, L.D. Stressor controllability and Fos expression in stress regulatory regions in mice. Physiol. Behav. 2009, 97, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daviu, N.; Fuzesi, T.; Rosenegger, D.G.; Rasiah, N.P.; Sterley, T.L.; Peringod, G.; Bains, J.S. Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat. Neurosci. 2020, 23, 398–410. [Google Scholar] [CrossRef]
- Yoshida, N.; Maejima, Y.; Sedbazar, U.; Ando, A.; Kurita, H.; Damdindorj, B.; Takano, E.; Gantulga, D.; Iwasaki, Y.; Kurashina, T.; et al. Stressor-responsive central nesfatin-1 activates corticotropin-releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamic-pituitary-adrenal axis. Aging (Albany NY) 2010, 2, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Okere, B.; Xu, L.; Roubos, E.W.; Sonetti, D.; Kozicz, T. Restraint stress alters the secretory activity of neurons co-expressing urocortin-1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 in the mouse Edinger-Westphal nucleus. Brain Res. 2010, 1317, 92–99. [Google Scholar] [CrossRef]
- Pacak, K.; Palkovits, M.; Kvetnansky, R.; Kopin, I.J.; Goldstein, D.S. Stress-induced norepinephrine release in the paraventricular nucleus of rats with brainstem hemisections: A microdialysis study. Neuroendocrinology 1993, 58, 196–201. [Google Scholar] [CrossRef]
- Ekizceli, G.; Halk, K.Z.; Minbay, Z.; Eyigor, O. Nesfatin-1 and neuronostatin neurons are co-expressed with glucocorticoid receptors in the hypothalamus. Biotech. Histochem. 2020, 1–7. [Google Scholar] [CrossRef]
- Harris, N.A.; Isaac, A.T.; Gunther, A.; Merkel, K.; Melchior, J.; Xu, M.; Eguakun, E.; Perez, R.; Nabit, B.P.; Flavin, S.; et al. Dorsal BNST alpha2A-Adrenergic Receptors Produce HCN-Dependent Excitatory Actions That Initiate Anxiogenic Behaviors. J. Neurosci. 2018, 38, 8922–8942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Sierra, O.E.; Goswami, S.; Turesson, H.K.; Pare, D. Altered responsiveness of BNST and amygdala neurons in trauma-induced anxiety. Transl. Psychiatry 2016, 6, e857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelrine, E.; Pasik, S.D.; Bayat, L.; Goldschmiedt, D.; Bauer, E.P. 5-HT2C receptors in the BNST are necessary for the enhancement of fear learning by selective serotonin reuptake inhibitors. Neurobiol. Learn Mem. 2016, 136, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Swaab, D.F. Sexual differentiation of the brain and behavior. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.S.; Gorski, R.A. Sex difference in the bed nucleus of the stria terminalis of the human brain. J. Comp. Neurol. 1990, 302, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Elbelt, U.; Ahnis, A.; Rose, M.; Klapp, B.F.; Stengel, A. Sex-specific regulation of NUCB2/nesfatin-1: Differential implication in anxiety in obese men and women. Psychoneuroendocrinology 2015, 60, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Weibert, E.; Hofmann, T.; Stengel, A. Role of nesfatin-1 in anxiety, depression and the response to stress. Psychoneuroendocrinology 2019, 100, 58–66. [Google Scholar] [CrossRef]
- Ge, J.F.; Xu, Y.Y.; Qin, G.; Peng, Y.N.; Zhang, C.F.; Liu, X.R.; Liang, L.C.; Wang, Z.Z.; Chen, F.H. Depression-like Behavior Induced by Nesfatin-1 in Rats: Involvement of Increased Immune Activation and Imbalance of Synaptic Vesicle Proteins. Front. Neurosci. 2015, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Maejima, Y.; Sedbazar, U.; Suyama, S.; Kohno, D.; Onaka, T.; Takano, E.; Yoshida, N.; Koike, M.; Uchiyama, Y.; Fujiwara, K.; et al. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009, 10, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Iovino, M.; Giagulli, V.A.; Licchelli, B.; Iovino, E.; Guastamacchia, E.; Triggiani, V. Synaptic Inputs of Neural Afferent Pathways to Vasopressin- and Oxytocin-Secreting Neurons of Supraoptic and Paraventricular Hypothalamic Nuclei. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Blanch, G.T.; Freiria-Oliveira, A.H.; Murphy, D.; Paulin, R.F.; Antunes-Rodrigues, J.; Colombari, E.; Menani, J.V.; Colombari, D.S. Inhibitory mechanism of the nucleus of the solitary tract involved in the control of cardiovascular, dipsogenic, hormonal, and renal responses to hyperosmolality. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R531–R542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palasz, A.; Zarczynski, P.; Bogus, K.; Mordecka-Chamera, K.; Della Vecchia, A.; Skalbania, J.; Worthington, J.J.; Krzystanek, M.; Zarczynska, M. Modulatory effect of olanzapine on SMIM20/phoenixin, NPQ/spexin and NUCB2/nesfatin-1 gene expressions in the rat brainstem. Pharmacol. Rep. 2021, 1–7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedrich, T.; Stengel, A. Role of the Novel Peptide Phoenixin in Stress Response and Possible Interactions with Nesfatin-1. Int. J. Mol. Sci. 2021, 22, 9156. https://doi.org/10.3390/ijms22179156
Friedrich T, Stengel A. Role of the Novel Peptide Phoenixin in Stress Response and Possible Interactions with Nesfatin-1. International Journal of Molecular Sciences. 2021; 22(17):9156. https://doi.org/10.3390/ijms22179156
Chicago/Turabian StyleFriedrich, Tiemo, and Andreas Stengel. 2021. "Role of the Novel Peptide Phoenixin in Stress Response and Possible Interactions with Nesfatin-1" International Journal of Molecular Sciences 22, no. 17: 9156. https://doi.org/10.3390/ijms22179156
APA StyleFriedrich, T., & Stengel, A. (2021). Role of the Novel Peptide Phoenixin in Stress Response and Possible Interactions with Nesfatin-1. International Journal of Molecular Sciences, 22(17), 9156. https://doi.org/10.3390/ijms22179156




