Enzyme Therapy: Current Challenges and Future Perspectives
Abstract
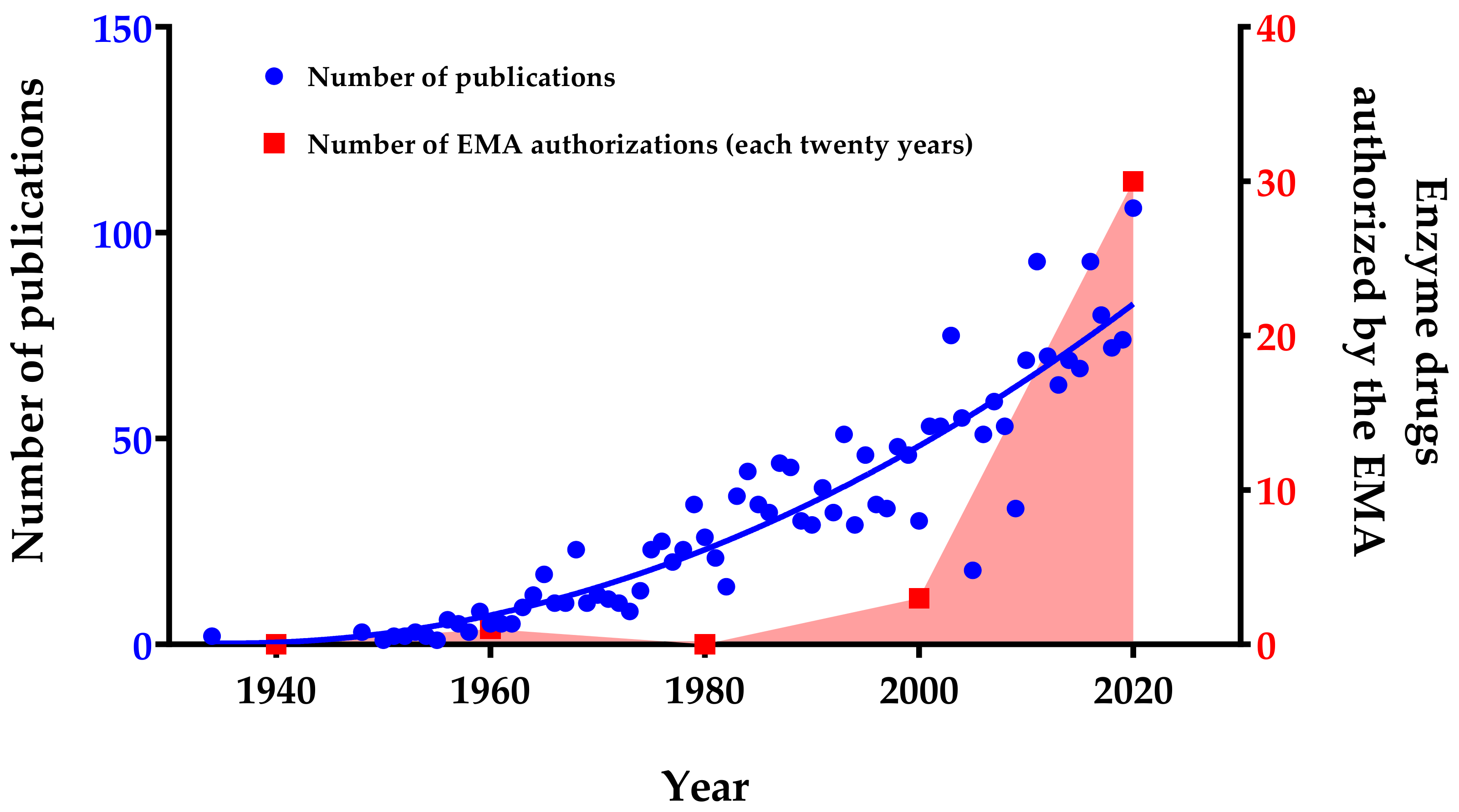
1. Introduction
2. Methodology
3. Enzyme Therapies for Different Pathologies
3.1. Metabolic Deficiencies
3.1.1. Lysosomal Storage Diseases (LSD)
3.1.2. Further Metabolic Deficiencies
3.2. Fibrosis Conditions
3.3. Ocular Affections
3.4. Joint Problems
3.5. Cancer
3.6. Cardiovascular Diseases
3.7. Extracellular Matrix Disorders
3.8. Reactive Oxygen Species Damage
3.9. Other Applications
4. Current Challenges of Enzyme Therapies
5. Enzyme Therapies Troubleshooting
5.1. Encapsulation of Enzymes
5.2. Modification of Enzymes
5.3. Monitorization of Patients’ Immunoresponses
6. Future Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- A09AA02 Cerezyme (Imiglucerase) 1997.
- A16AB10 Vprip (Velaglucerase alpha) 2010.
- A16AB11 Taliglucerase alpha (glucocerebrosidase) 2010.
- A16AB09 Elaprase (Idursulfase) 2007.
- A16AB04 Fabrazyme (Agalsidase beta) 2001.
- fA16AB03 Replagal (Agalsidase alpha) 2001.
- A16AB05 Aldurazyme (Lanoridase) 2003.
- A16AB12 Vimizim (Elosulfase alpha) 2014.
- A16AB08 Naglazyme (Galsulfase) 2006.
- A16AB18 Mepsevii (Vestronidase Alfa) 2018.
- A16AB15 Lamzede (Velmanase alpha) 2018.
- A16AB17 Brineura (Cerliponase alpha) 2017.
- A16AB07 Myozyme (Alglucosidase alpha) 2006.
- A09AA02 Enzepi (Multienzymes) 2016.
- A16AB19 Palynziq (PEGvaliace) 2010.
- A16AB14 Kanuma (Sevelipase alpha) 2010.
- A16AB13 Strensiq (Arfotase alpha) 2015.
- B01AD12 Ceprotin (Protein C) 2001.
- M09AB02 Xiapex (Collagenase Clostridium Histolyticum) 2011.
- R05CB13 Pulmozyme (Dornase alpha) 2017.
- S01XA22 Jetrea (Ocriplasmin) 2013.
- - Voraxaze (Carboxypeptidase G2) 2003.
- B06AA03 PEG hyaluronidase PH20 (pegvorhyaluronidase alpha) 2014.
- - PEGarginine deaminase 2005.
- L01XX02 Spectrila, Kidrolase, Erwinase (L-asparaginase) 2016.
- L01XX24 Oncaspar (PEGasparginase) 2016.
- V03AF07 Fasturtec (Rasburicase) 2001.
- B01AD01 Streptase (Streptokinase) 1960.
- B01AD04 Syner-Kinase, Kinclytic (Urokinase) 2019.
- B01AD07 Rapilsyn (Reteplase) 1996.
- B01AD02 Actilyse (Alteplase) 1999.
- B01AD11 Metalyse (Tenecteplase) 2001.
- D03BA03 // M09AB03 NexoBrid (Proteolytic enzymes enriched in bromelain) 2012.
References
- Tjhung, K.F.; Shokhirev, M.N.; Horning, D.P.; Joyce, G.F. An RNA polymerase ribozyme that synthesizes its own ancestor. Proc. Natl. Acad. Sci. USA 2020, 117, 2906–2913. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.T.; Lawrence, D.S.; Rokita, S.E. The chemical modification of enzymatic specificity. Annu. Rev. Biochem. 1985, 54, 565–595. [Google Scholar] [CrossRef] [PubMed]
- Adamson, C.; Kanai, M. Integrating abiotic chemical catalysis and enzymatic catalysis in living cells. Org. Biomol. Chem. 2021, 19, 37–45. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Singh, A.K.; Keshari, A.K.; Maity, S.; Sarkar, S.; Saha, S. Human metabolic enzymes deficiency: A genetic mutation based approach. Scientifica 2016. [Google Scholar] [CrossRef]
- Robertson, J.G. Enzymes as a special class of therapeutic target: Clinical drugs and modes of action. Curr. Opin. Struct. Biol. 2007, 17, 674–679. [Google Scholar] [CrossRef]
- Petersen, K.-U. Pepsin and its importance for functional dyspepsia: Relic, regulator or remedy? Dig. Dis. 2018, 36, 98–105. [Google Scholar] [CrossRef]
- [No authors listed]. Very early thrombolytic therapy in suspected acute myocardial infarction. The Thrombolysis Early in Acute Heart Attack Trial Study Group. Am. J. Cardiol. 1990, 65, 401–407. [Google Scholar] [CrossRef]
- Collen, D. Molecular mechanism of action of newer thrombolytic agents. J. Am. Coll. Cardiol. 1987, 10, 11–15. [Google Scholar] [CrossRef]
- Demain, A.; Vaishnav, P. Production of Recombinant Enzymes. Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081005965. [Google Scholar]
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake venom: From deadly toxins to life-saving therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef]
- Frangieh, J.; Rima, M.; Fajloun, Z.; Henrion, D.; Sabatier, J.-M.; Legros, C.; Mattei, C. Snake venom components: Tools and cures to target cardiovascular diseases. Molecules 2021, 26, 2223. [Google Scholar] [CrossRef] [PubMed]
- Mordor Intelligence. Industrial Enzymes Market—Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026); Mordor Intelligence: Hyderabad, India, 2021. [Google Scholar]
- Global Market Insights. Enzymes Market Size by Product (Proteases, Lipases, Carbohydrases [Amylases, Xylanases, Cellulases, Pectinases, Lactases], Polymerases & Nucleases, Phytases, Catalyses), by Application (Food & Beverage, Processed Food, Diary, Bakery, Confectionary), Industry Analysis Report, Regional Outlook, Growth Potential, Price Trends, Competitive Market Share & Forecast, 2018–2024; Global Market Insights: Pune, India, 2019. [Google Scholar]
- Triplett, T.A.; Garrison, K.C.; Marshall, N.; Donkor, M.; Blazeck, J.; Lamb, C.; Qerqez, A.; Dekker, J.D.; Tanno, Y.; Lu, W.-C.; et al. Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat. Biotechnol. 2018, 36, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, R.H. Enzyme replacement therapy for lysosomal storage diseases. Curr. Opin. Pediatriatic 2011, 23, 588–593. [Google Scholar] [CrossRef]
- Radadiya, A.; Zhu, W.; Coricello, A.; Alcaro, S.; Richards, N.G.J. Improving the treatment of acute lymphoblastic leukemia. Biochemistry 2020, 59, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; McGowan, E.M.; Ren, N.; Lal, S.; Nassif, N.; Shad-Kaneez, F.; Qu, X.; Lin, Y. Nattokinase: A promising alternative in prevention and treatment of cardiovascular diseases. Biomark. Insights 2018, 13. [Google Scholar] [CrossRef]
- Lenders, M.; Brand, E. Effects of enzyme replacement therapy and antidrug antibodies in patients with Fabry disease. J. Am. Soc. Nephrol. 2018, 29, 2265–2278. [Google Scholar] [CrossRef] [PubMed]
- Lapuhs, P.; Fuhrmann, G. Engineering strategies for oral therapeutic enzymes to enhance their stability and activity. Adv. Exp. Med. Biol. 2019, 1148, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, K.; Shukla, C.J. Non-invasive treatment in the management of Peyronie’s disease. Ther. Adv. Urol. 2019, 11. [Google Scholar] [CrossRef]
- Robinson, P.J. Dornase alfa in early cystic fibrosis lung disease. Pediatr. Pulmonol. 2002, 34, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Badalamente, M.A.; Hurst, L.C. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J. Hand Surg. Am. 2000, 25, 629–636. [Google Scholar] [CrossRef]
- De Frutos, L.L.; García-González, E.; García-Rodríguez, B.; González-Irazabal, Y.; Lahoz, C.; Irún, P.; Cebolla, J.J.; Giraldo, P. Serum protein profile analysis in lysosomal storage disorders patients. Clin. Chim. Acta 2020, 510, 430–436. [Google Scholar] [CrossRef]
- Giraldo, P.; de Frutos, L.L.; Cebolla, J.J. Biomarker combination is necessary for the assessment of Gaucher disease? Ann. Transl. Med. 2018, 6, S81. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, E.; Deroma, L.; Bembi, B.; Deegan, P.; Hollak, C.; Weinreb, N.J.; Cox, T.M. Enzyme replacement and substrate reduction therapy for Gaucher disease. Cochrane Database Syst. Rev. 2015, 27, CD010324. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, D.A.; Kimura, A. Development of idursulfase therapy for mucopolysaccharidosis type II (Hunter syndrome): The past, the present and the future. Drug Des. Dev. Ther. 2017, 11, 2467–2480. [Google Scholar] [CrossRef]
- Chan, B.; Adam, D.N. A Review of Fabry Disease. Skin Ther. Lett. 2018, 23, 4–6. [Google Scholar]
- El Dib, R.; Gomaa, H.; Carvalho, R.P.; Camargo, S.E.; Bazan, R.; Barretti, P.; Barreto, F.C. Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst. Rev. 2016, 7, CD006663. [Google Scholar] [CrossRef]
- Brooks, D.A. Alpha-L-iduronidase and enzyme replacement therapy for mucopolysaccharidosis I. Expert Opin. Biol. Ther. 2002, 2, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K.A. Elosulfase Alfa: A review of its use in patients with mucopolysaccharidosis type IVA (Morquio A syndrome). BioDrugs 2014, 28, 465–475. [Google Scholar] [CrossRef]
- Harmatz, P.; Hendriksz, C.J.; Lampe, C.; McGill, J.J.; Parini, R.; Leão-Teles, E.; Valayannopoulos, V.; Cole, T.J.; Matousek, R.; Graham, S.; et al. The effect of galsulfase enzyme replacement therapy on the growth of patients with mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). Mol. Genet. Metab. 2017, 122, 107–112. [Google Scholar] [CrossRef]
- McCafferty, E.H.; Scott, L.J. Vestronidase alfa: A review in mucopolysaccharidosis VII. BioDrugs 2019, 33, 233–240. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Codini, M.; Conte, C.; Patria, F.; Cataldi, S.; Bertelli, M.; Albi, E.; Beccari, T. Alpha-mannosidosis: Therapeutic strategies. Int. J. Mol. Sci. 2018, 19, 1500. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.B.; Cain, J.T.; White, K.A.; Ramirez-Montealegre, D.; Pearce, D.A.; Weimer, J.M. Therapeutic landscape for Batten disease: Current treatments and future prospects. Nat. Rev. Neurol. 2019, 15, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Kishnani, P.S.; Beckemeyer, A.A. New therapeutic approaches for Pompe disease: Enzyme replacement therapy and beyond. Pediatr. Endocrinol. Rev. 2014, 12, 114–124. [Google Scholar] [PubMed]
- Somaraju, U.R.R.; Solis-Moya, A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. Cochrane Database Syst. Rev. 2020, 8, CD008227. [Google Scholar] [CrossRef]
- Dominguez-Muñoz, J.E. Management of pancreatic exocrine insufficiency. Curr. Opin. Gastroenterol. 2019, 35, 455–459. [Google Scholar] [CrossRef]
- Carroccio, A.; Guarino, A.; Zuin, G.; Verghi, F.; Berni Canani, R.; Fontana, M.; Bruzzese, E.; Montalto, G.; Notarbartolo, A. Efficacy of oral pancreatic enzyme therapy for the treatment of fat malabsorption in HIV-infected patients. Aliment. Pharmacol. Ther. 2001, 15, 1619–1625. [Google Scholar] [CrossRef]
- Kim, W.; Erlandsen, H.; Surendran, S.; Stevens, R.C.; Gamez, A.; Michols-Matalon, K.; Tyring, S.K.; Matalon, R. Trends in enzyme therapy for phenylketonuria. Mol. Ther. 2004, 10, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Tartibi, H.M.; Hershfield, M.S.; Bahna, S.L. A 24-year enzyme replacement therapy in an adenosine-deaminase-deficient patient. Pediatrics 2016, 137. [Google Scholar] [CrossRef]
- Bax, B.E.; Bain, M.D.; Fairbanks, L.D.; Webster, A.D.; Chalmers, R.A. In vitro and in vivo studies with human carrier erythrocytes loaded with polyethylene glycol-conjugated and native adenosine deaminase. Br. J. Haematol. 2000, 109, 549–554. [Google Scholar] [CrossRef]
- Pastores, G.M.; Hughes, D.A. Lysosomal acid lipase deficiency: Therapeutic options. Drug Des. Devel. Ther. 2020, 14, 591–601. [Google Scholar] [CrossRef]
- Fontanellas, A.; Ávila, M.A.; Berraondo, P. Emerging therapies for acute intermittent porphyria. Expert Rev. Mol. Med. 2016, 18, e17. [Google Scholar] [CrossRef] [PubMed]
- Puntis, J.W.L.; Zamvar, V. Congenital sucrase-isomaltase deficiency: Diagnostic challenges and response to enzyme replacement therapy. Arch. Dis. Child. 2015, 100, 869–871. [Google Scholar] [CrossRef]
- Hofmann, C.; Seefried, L.; Jakob, F. Asfotase alfa: Enzyme replacement for the treatment of bone disease in hypophosphatasia. Drugs Today 2016, 52, 271–285. [Google Scholar] [CrossRef]
- Dinarvand, P.; Moser, K.A. Protein C deficiency. Arch. Pathol. Lab. Med. 2019, 143, 1281–1285. [Google Scholar] [CrossRef]
- Catanzaro, R.; Sciuto, M.; Marotta, F. Lactose intolerance: An update on its pathogenesis, diagnosis, and treatment. Nutr. Res. 2021, 89, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Strauss, B.H.; Goldman, L.; Qiang, B.; Nili, N.; Segev, A.; Butany, J.; Sparkes, J.D.; Jackson, Z.S.; Eskandarian, M.R.; Virmani, R. Collagenase plaque digestion for facilitating guide wire crossing in chronic total occlusions. Circulation 2003, 108, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Degreef, I. Collagenase treatment in dupuytren contractures: A review of the current state versus future needs. Rheumatol. Ther. 2016, 3, 43–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, D.K.; Leppert, P.C. Treatment for uterine fibroids: Searching for effective drug therapies. Drug Discov. Today. Ther. Strateg. 2012, 9, e41–e49. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Trowbridge, R.M.; Ayoub, N.T.; Agrawal, D.K. High-mobility group box protein-1, matrix metalloproteinases, and vitamin D in keloids and hypertrophic scars. Plast. Reconstr. Surg. Glob. Open 2015, 3, e425. [Google Scholar] [CrossRef]
- Bae-Harboe, Y.-S.C.; Harboe-Schmidt, J.E.; Graber, E.; Gilchrest, B.A. Collagenase followed by compression for the treatment of earlobe keloids. Dermatologic Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2014, 40, 519–524. [Google Scholar] [CrossRef]
- Yang, C.; Chilvers, M.; Montgomery, M.; Nolan, S.J. Dornase alfa for cystic fibrosis. Cochrane Database Syst. Rev. 2016, 4, CD001127. [Google Scholar] [CrossRef]
- Honkanen, R. Use of Collagenase to Treat Glaucoma. Patent US20150273028A1, 24 October 2013. [Google Scholar]
- Shah, A.R.; Trese, M.T. Enzymatic vitrectomy and pharmacologic vitreodynamics. Dev. Ophthalmol. 2016, 55, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Iwai, H.; Koga, H. Chemonucleolysis with chondroitin sulfate ABC endolyase as a novel minimally invasive treatment for patients with lumbar intervertebral disc herniation. J. Spine Surg. 2019, 5, S115–S121. [Google Scholar] [CrossRef]
- Naeem, H.; Naqvi, S.N.-U.-H.; Perveen, R.; Ishaque, F.; Bano, R.; Abrar, H.; Arsalan, A.; Malik, N. Efficiency of proteolytic enzymes in treating lumbar spine osteoarthritis (low back pain) patients and its effects on liver and kidney enzymes. Pak. J. Pharm. Sci. 2020, 33, 371–378. [Google Scholar]
- Hochberg, M.C. New directions in symptomatic therapy for patients with osteoarthritis and rheumatoid arthritis. Semin. Arthritis Rheum. 2002, 32, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, Q.; Zhou, H.; Zhang, M.; Shen, J.; Ju, D.; Triplett, T.A.; Garrison, K.C.; Marshall, N.; Donkor, M.; et al. Amino acid degrading enzymes and autophagy in cancer therapy. Nat. Biotechnol. 2020, 11, 758–764. [Google Scholar] [CrossRef]
- Cammalleri, L.; Malaguarnera, M. Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int. J. Med. Sci. 2007, 4, 83–93. [Google Scholar] [CrossRef]
- Kunamneni, A.; Ravuri, B.; Ellaiah, P.; Prabhakhar, T.; Vinjamuri, S. Urokinase-A strong plasminogen activator. Biotechnol. Mol. Biol. Rev. 2008, 3, 58–70. [Google Scholar]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Pham, C.H.; Collier, Z.J.; Fang, M.; Howell, A.; Gillenwater, T.J. The role of collagenase ointment in acute burns: A systematic review and meta-analysis. J. Wound Care 2019, 28, S9–S15. [Google Scholar] [CrossRef]
- Hansbrough, J.F.; Achauer, B.; Dawson, J.; Himel, H.; Luterman, A.; Slater, H.; Levenson, S.; Salzberg, C.A.; Hansbrough, W.B.; Doré, C. Wound healing in partial-thickness burn wounds treated with collagenase ointment versus silver sulfadiazine cream. J. Burn Care Rehabil. 1995, 16, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Sadick, N. Treatment for cellulite. Int. J. Women Dermatol. 2019, 5, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Izumi, M.; McDonald, M.C.; Sharpe, M.A.; Chatterjee, P.K.; Thiemermann, C. Superoxide dismutase mimetics with catalase activity reduce the organ injury in hemorrhagic shock. Shock 2002, 18, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-D.; Zhao, X.; Li, Y.; Li, G.-R.; Liu, X.-L. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review). Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Mao, Y.; Xu, E.; Jia, H.; Zhang, S.; Dawson, V.; Dawson, T.; Li, Y.-M.; Zheng, Z.; He, W.; et al. Nanozyme scavenging ROS for prevention of pathologic α-synuclein transmission in Parkinson’s disease. Nano Today 2021, 36, 101027. [Google Scholar] [CrossRef]
- Wei, G.; Helmerhorst, E.J.; Darwish, G.; Blumenkranz, G.; Schuppan, D. Gluten degrading enzymes for treatment of celiac disease. Nutrients 2020, 12, 2095. [Google Scholar] [CrossRef]
- Li, X.-H.; Lee, J.-H. Antibiofilm agents: A new perspective for antimicrobial strategy. J. Microbiol. 2017, 55, 753–766. [Google Scholar] [CrossRef]
- Viswanatha Swamy, A.H.M.; Patil, P.A. Effect of some clinically used proteolytic enzymes on inflammation in rats. Indian J. Pharm. Sci. 2008, 70, 114–117. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Shah, N.; Rathi, A.; Rathi, V.; Rathi, A. Serratiopeptidase: Insights into the therapeutic applications. Biotechnol. Rep. 2020, 28, e00544. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, X.; Zhou, Z.; Chen, X.; Jin, Z.; Deng, J.; Zhan, C.-G.; Zheng, F. Clinical potential of an enzyme-based novel therapy for cocaine overdose. Sci. Rep. 2017, 7, 15303. [Google Scholar] [CrossRef]
- Werle, M.; Bernkop-Schnürch, A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 2006, 30, 351–367. [Google Scholar] [CrossRef]
- Eng, C.M.; Banikazemi, M.; Gordon, R.E.; Goldman, M.; Phelps, R.; Kim, L.; Gass, A.; Winston, J.; Dikman, S.; Fallon, J.T.; et al. A phase 1/2 clinical trial of enzyme replacement in fabry disease: Pharmacokinetic, substrate clearance, and safety studies. Am. J. Hum. Genet. 2001, 68, 711–722. [Google Scholar] [CrossRef]
- Ashworth, J.L.; Biswas, S.; Wraith, E.; Lloyd, I.C. Mucopolysaccharidoses and the eye. Surv. Ophthalmol. 2006, 51, 1–17. [Google Scholar] [CrossRef]
- Yamanishi, R.; Nakamura, N.; Tsunoda, K. Recovery of vision following enzyme replacement therapy in a patient with mucopolysaccharidosis type II, hunter syndrome. Case Rep. Ophthalmol. 2019, 10, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Kingma, W.; Fitzpatrick, M.A.; Richards, S.M. Immunosurveillance of alglucerase enzyme therapy for Gaucher patients: Induction of humoral tolerance in seroconverted patients after repeat administration. Blood 1999, 93, 2081–2088. [Google Scholar] [CrossRef]
- Kakavanos, R.; Turner, C.T.; Hopwood, J.J.; Kakkis, E.D.; Brooks, D.A. Immune tolerance after long-term enzyme-replacement therapy among patients who have mucopolysaccharidosis I. Lancet 2003, 361, 1608–1613. [Google Scholar] [CrossRef]
- Amalfitano, A.; Bengur, A.R.; Morse, R.P.; Majure, J.M.; Case, L.E.; Veerling, D.L.; Mackey, J.; Kishnani, P.; Smith, W.; McVie-Wylie, A.; et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: Results of a phase I/II clinical trial. Genet. Med. 2001, 3, 132–138. [Google Scholar]
- Eng, C.M.; Guffon, N.; Wilcox, W.R.; Germain, D.P.; Lee, P.; Waldek, S.; Caplan, L.; Linthorst, G.E.; Desnick, R.J. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N. Engl. J. Med. 2001, 345, 9–16. [Google Scholar] [CrossRef]
- Mok, C.C.; van der Kleij, D.; Wolbink, G.J. Drug levels, anti-drug antibodies, and clinical efficacy of the anti-TNFα biologics in rheumatic diseases. Clin. Rheumatol. 2013, 32, 1429–1435. [Google Scholar] [CrossRef]
- Harmatz, P. Enzyme replacement therapies and immunogenicity in lysosomal storage diseases: Is there a pattern? Clin. Ther. 2015, 37, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-Y.; Suen, C.-S.; Hwang, M.-J.; Roffler, S.R. Toward reducing immunogenicity of enzyme replacement therapy: Altering the specificity of human β-glucuronidase to compensate for α-iduronidase deficiency. Protein Eng. Des. Sel. 2015, 28, 519–529. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devonshire, A.L.; Makhija, M. Approach to primary immunodeficiency. Allergy Asthma Proc. 2019, 40, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Fuentes, M.; Alarcón, M.; Palomo, I. Immune system dysfunction in the elderly. An. Acad. Bras. Cienc. 2017, 89, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Guideline on Immunogenicity Assessment of Therapeutic Proteins. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-immunogenicity-assessment-therapeutic-proteins-revision-1_en.pdf (accessed on 31 July 2021).
- Abbas, M.; Moussa, M.; Akel, H. Type I Hypersensitivity Reaction; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar] [PubMed]
- De Duve, C.; Wattiaux, R. Functions of lysosomes. Annu. Rev. Physiol. 1966, 28, 435–492. [Google Scholar] [CrossRef] [PubMed]
- Vellard, M. The enzyme as drug: Application of enzymes as pharmaceuticals. Curr. Opin. Biotechnol. 2003, 14, 444–450. [Google Scholar] [CrossRef]
- Dean, S.N.; Turner, K.B.; Medintz, I.L.; Walper, S.A. Targeting and delivery of therapeutic enzymes. Ther. Deliv. 2017, 8, 577–595. [Google Scholar] [CrossRef]
- Bigger, B.W.; Saif, M.; Linthorst, G.E. The role of antibodies in enzyme treatments and therapeutic strategies. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Mitchel, J.F.; Shwedick, M.; Alberghini, T.A.; Knibbs, D.; McKay, R.G. Catheter-based local thrombolysis with urokinase: Comparative efficacy of intraluminal clot lysis with conventional urokinase infusion techniques in an in vivo porcine thrombus model. Cathet. Cardiovasc. Diagn. 1997, 41, 293–302. [Google Scholar] [CrossRef]
- Mun, C.; Gulati, S.; Tibrewal, S.; Chen, Y.-F.; An, S.; Surenkhuu, B.; Raju, I.; Buwick, M.; Ahn, A.; Kwon, J.-E.; et al. A phase I/II placebo-controlled randomized pilot clinical trial of recombinant deoxyribonuclease (DNase) eye drops use in patients with dry eye disease. Transl. Vis. Sci. Technol. 2019, 8, 10. [Google Scholar] [CrossRef]
- Karimi, M.; Mirshekari, H.; Moosavi Basri, S.M.; Bahrami, S.; Moghoofei, M.; Hamblin, M.R. Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. Adv. Drug Deliv. Rev. 2016, 106, 45–62. [Google Scholar] [CrossRef]
- Turrens, J.F.; Crapo, J.D.; Freeman, B.A. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J. Clin. Investig. 1984, 73, 87–95. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Rodriguez-Torres, M.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Goodwine, J.; Romero, N.; Steck, K.S.; Sauer, K.; Doiron, A. Enzyme-encapsulating polymeric nanoparticles: A potential adjunctive therapy in Pseudomonas aeruginosa biofilm-associated infection treatment. Colloids Surfaces B Biointerfaces 2019, 184, 110512. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Abad, D.; Kickhoefer, V.A.; Rome, L.H.; Mahendra, S. Vault nanoparticles packaged with enzymes as an efficient pollutant biodegradation technology. ACS Nano 2015, 9, 10931–10940. [Google Scholar] [CrossRef] [PubMed]
- Lothe, A.G.; Kalra, S.S.; Wang, M.; Mack, E.E.; Walecka-Hutchison, C.; Kickhoefer, V.A.; Rome, L.H.; Mahendra, S. Vault packaged enzyme mediated degradation of amino-aromatic energetic compounds. Chemosphere 2020, 242, 125117. [Google Scholar] [CrossRef]
- Muñoz-Juan, A.; Carreño, A.; Mendoza, R.; Corchero, J.L. Latest advances in the development of eukaryotic vaults as targeted drug delivery systems. Pharmaceutics 2019, 11, 300. [Google Scholar] [CrossRef]
- Liu, H.; Tu, Z.; Feng, F.; Shi, H.; Chen, K.; Xu, X. Virosome, a hybrid vehicle for efficient and safe drug delivery and its emerging application in cancer treatment. Acta Pharm. 2015, 65, 105–116. [Google Scholar] [CrossRef]
- Kim, E.-M.; Jeong, H.-J. Liposomes: Biomedical applications. Chonnam Med. J. 2021, 57, 27–35. [Google Scholar] [CrossRef]
- Santi, M.; Finamore, F.; Cecchettini, A.; Santorelli, F.M.; Doccini, S.; Rocchiccioli, S.; Signore, G. Protein delivery by peptide-based stealth liposomes: A biomolecular insight into enzyme replacement therapy. Mol. Pharm. 2020, 17, 4510–4521. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.; Kooijmans, S.; Murphy, D.; Linglei, J.; Evers, M.; Sluijter, J.; Vader, P.; Schiffelers, R. Drug delivery with extracellular vesicles: From imagination to innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Q.; Zi, Z.; Liu, Z.; Wan, C.; Crisman, L.; Shen, J.; Liu, X. Programmable extracellular vesicles for macromolecule delivery and genome modifications. Dev. Cell 2020, 55, 784–801. [Google Scholar] [CrossRef] [PubMed]
- Murciano, J.-C.; Medinilla, S.; Eslin, D.; Atochina, E.; Cines, D.B.; Muzykantov, V.R. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat. Biotechnol. 2003, 21, 891–896. [Google Scholar] [CrossRef]
- Rossi, L.; Pierigè, F.; Aliano, M.P.; Magnani, M. Ongoing Developments and Clinical Progress in Drug-Loaded Red Blood Cell Technologies. BioDrugs 2020, 34, 265–272. [Google Scholar] [CrossRef]
- Rossi, L.; Pierigè, F.; Bregalda, A.; Magnani, M. Preclinical developments of enzyme-loaded red blood cells. Expert Opin. Drug Deliv. 2021, 18, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Koleva, L.; Bovt, E.; Ataullakhanov, F.; Sinauridze, E. Erythrocytes as carriers: From drug delivery to biosensors. Pharmaceutics 2020, 12, 276. [Google Scholar] [CrossRef]
- Arslan, F.B.; Ozturk Atar, K.; Calis, S. Antibody-mediated drug delivery. Int. J. Pharm. 2021, 596, 120268. [Google Scholar] [CrossRef]
- Yata, V.K.; Banerjee, S.; Ghosh, S. Folic acid conjugated-bio polymeric nanocarriers: Synthesis, characterization and in vitro delivery of prodrug converting enzyme. Adv. Sci. Eng. Med. 2014, 6, 388–392. [Google Scholar] [CrossRef]
- Matsushima, A.; Kodera, Y.; Hiroto, M.; Nishimura, H.; Inada, Y. Polyethylene Glycol-Modified Enzymes in Hydrophobic Media. Enzymes in Nonaqueous Solvents; Humana Press: Totowa, NJ, USA, 2001; pp. 49–64. ISBN 1-59259-112-4. [Google Scholar]
- Heo, Y.-A.; Syed, Y.Y.; Keam, S.J. Pegaspargase: A review in acute lymphoblastic leukaemia. Drugs 2019, 79, 767–777. [Google Scholar] [CrossRef]
- Chandrasekaran, A.R.; Levchenko, O. DNA nanocages. Chem. Mater. 2016, 28, 5569–5581. [Google Scholar] [CrossRef]
- Jiang, D.; England, C.G.; Cai, W. DNA nanomaterials for preclinical imaging and drug delivery. J. Control Release 2016, 239, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fu, J.; Dhakal, S.; Johnson-Buck, A.; Liu, M.; Zhang, T.; Woodbury, N.W.; Liu, Y.; Walter, N.G.; Yan, H. Nanocaged enzymes with enhanced catalytic activity and increased stability against protease digestion. Nat. Commun. 2016, 7, 10619. [Google Scholar] [CrossRef] [PubMed]
- Maximov, V.; Reukov, V.; Vertegel, A.A. Targeted delivery of therapeutic enzymes. J. Drug Deliv. Sci. Technol. 2009, 19, 311–320. [Google Scholar] [CrossRef]
- Meirow, Y.; Baniyash, M. Immune biomarkers for chronic inflammation related complications in non-cancerous and cancerous diseases. Cancer Immunol. Immunother. 2017, 66, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Del-Castillo, J.M.; Soria, J.; Acera, A.; Muñoz, A.M.; Rodríguez, S.; Suárez, T. Quantification of a panel for dry-eye protein biomarkers in tears: A comparative pilot study using standard ELISA and customized microarrays. Mol. Vis. 2021, 27, 243–261. [Google Scholar]
- Manuel, I.; Barreda-Gómez, G.; de San Román, E.G.; Veloso, A.; Fernández, J.A.; Giralt, M.T.; Rodríguez-Puertas, R. Neurotransmitter receptor localization: From autoradiography to imaging mass spectrometry. ACS Chem. Neurosci. 2015, 6, 362–373. [Google Scholar] [CrossRef]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A.C.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef]
- Rienda, B.; Elexpe, A.; Tolentino-Cortez, T.; Gulak, M.; Bruzos-Cidón, C.; Torrecilla, M.; Astigarraga, E.; Barreda-Gómez, G. Analysis of acetylcholinesterase activity in cell membrane microarrays of brain areas as a screening tool to identify tissue specific inhibitors. Analytica 2021, 2, 25–36. [Google Scholar] [CrossRef]
- Fernández, R.; Garate, J.; Tolentino-Cortez, T.; Herraiz, A.; Lombardero, L.; Ducrocq, F.; Rodríguez-Puertas, R.; Trifilieff, P.; Astigarraga, E.; Barreda-Gómez, G.; et al. Microarray and mass spectrometry-based methodology for lipid profiling of tissues and cell cultures. Anal. Chem. 2019, 91, 15967–15973. [Google Scholar] [CrossRef]
- Soria, J.; Acera, A.; Durán, J.A.; Boto-de-Los-Bueis, A.; Del-Hierro-Zarzuelo, A.; González, N.; Reigada, R.; Suárez, T. The analysis of human conjunctival epithelium proteome in ocular surface diseases using impression cytology and 2D-DIGE. Exp. Eye Res. 2018, 167, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Ruano-Gallego, D.; García-Villadangos, M.; Moreno-Paz, M.; Gómez-Elvira, J.; Postigo, M.; Simón-Sacristán, M.; Reyburn, H.T.; Carolis, C.; Rodrigo, N.; Codeseira, Y.B.; et al. A multiplex antigen microarray for simultaneous IgG and IgM detection against SARS-CoV-2 reveals higher seroprevalence than reported. Microb. Biotechnol. 2021, 14, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Negm, O.H.; Hamed, M.; Monaghan, T.M. A protein microarray assay for serological determination of antigen-specific antibody responses following clostridium difficile infection. J. Vis. Exp. 2018, 15, 57399. [Google Scholar] [CrossRef] [PubMed]
- Dotsey, E.Y.; Gorlani, A.; Ingale, S.; Achenbach, C.J.; Forthal, D.N.; Felgner, P.L.; Gach, J.S. A high throughput protein microarray approach to classify HIV monoclonal antibodies and variant antigens. PLoS ONE 2015, 10, e0125581. [Google Scholar] [CrossRef][Green Version]
- Moser, K.L.; Gaffney, P.M.; Grandits, M.E.; Emamian, E.S.; Machado, D.B.; Baechler, E.C.; Rhodus, N.L.; Behrens, T.W. The use of microarrays to study autoimmunity. J. Investig. Dermatol. Symp. Proc. 2004, 9, 18–22. [Google Scholar] [CrossRef]
- Davies, D.H.; Liang, X.; Hernandez, J.E.; Randall, A.; Hirst, S.; Mu, Y.; Romero, K.M.; Nguyen, T.T.; Kalantari-Dehaghi, M.; Crotty, S.; et al. Profiling the humoral immune response to infection by using proteome microarrays: High-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA 2005, 102, 547–552. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Wiese, O.; Zemlin, A.E.; Pillay, T.S. Molecules in pathogenesis: Angiotensin converting enzyme 2 (ACE2). J. Clin. Pathol. 2021, 74, 285–290. [Google Scholar] [CrossRef]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C.; et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; del Pozo, C.H.; Prosper, F.; et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef]
- El-aziz, T.M.A.; Al-sabi, A.; Stockand, J.D. Human recombinant soluble ACE2 (hrsACE2) shows promise for treating severe COVID19. Signal Transduct. Target. Ther. 2020, 2, 3–4. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Lockey, R.F.; Kolliputi, N. Soluble ACE2 as a potential therapy for COVID-19. Am. J. Physiol. Cell Physiol. 2021, 320, C279–C281. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Boix, E. Host defence rnases as antiviral agents against enveloped single stranded RNA viruses. Virulence 2021, 12, 444–469. [Google Scholar] [CrossRef]
- Müller, C.; Ulyanova, V.; Ilinskaya, O.; Pleschka, S.; Shah Mahmud, R. A novel antiviral strategy against MERS-CoV and HCoV-229E using binase to target viral genome replication. Bionanoscience 2017, 7, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Cao, Z.; Wen, J.; Yu, Q.; Liu, C.; Wang, F.; Zhang, J.; Yang, F.; Li, Y.; Fishbein, G.; et al. An antioxidant enzyme therapeutic for COVID-19. Adv. Mater. 2020, 32, 2004901. [Google Scholar] [CrossRef] [PubMed]
- Christopher, M.; Kooloth-Valappil, P.; Sreeja-Raju, A.; Sukumaran, R.K. Repurposing proteases: An in-silico analysis of the binding potential of extracellular fungal proteases with selected viral proteins. Bioresour. Technol. Rep. 2021, 15, 100756. [Google Scholar] [CrossRef]
- Collier, D.A.; Monit, C.; Gupta, R.K. The impact of HIV-1 drug escape on the global treatment landscape. Cell Host Microbe 2019, 26, 48–60. [Google Scholar] [CrossRef]
- Monteil, V.; Dyczynski, M.; Lauschke, V.M.; Kwon, H.; Wirnsberger, G.; Youhanna, S.; Zhang, H.; Slutsky, A.S.; Hurtado Del Pozo, C.; Horn, M.; et al. Human soluble ACE2 improves the effect of remdesivir in SARS-CoV-2 infection. EMBO Mol. Med. 2021, 13, e13426. [Google Scholar] [CrossRef]

| Disease/ Condition | Cause/Pathology | Therapeutic Enzymes * | Ref. |
|---|---|---|---|
| Lysosomal storage diseases | |||
| Gaucher’s disease | Deficiency of glucocerebrosidase | Glucocerebrosidase [Cerezyme, Vprip, Taliglucerase alpha] | [25], (a,b,c) |
| Hunter’s syndrome | Deficiency of iduronate-2-sulfatase | Iduronate-2-sulfatase [Elaprase] | [26], (d) |
| Fabry’s disease | Deficiency of α-galactosidase A | α, β-galactosidase A [Replagal, Fabrazyme] | [28], (e,f) |
| Hurler’s syndrome | Deficiency of α-L-iduronidase | α-L-iduronidase [Aldurazyme] | [29], (g) |
| Morquio syndrome type A | Deficiency of N-acetylgalactosamine-6-sulfate sulfatase | N-acetylgalactosamine-6-sulfate sulfatase [Vimizim] | [30], (h) |
| Maroteaux-Lamy syndrome | Deficiency of arylsulfatase B | N-acetylgalactosamine-4-sulfatase [Naglazyme] | [31], (i) |
| Sly syndrome | Deficiency of β-glucuronidase | β-glucuronidase [Mepsevii] | [32], (j) |
| α-Mannosidosis | Deficiency of α-D-mannosidase | Velmanase α [Lamzede] | [33], (k) |
| Batten disease | Deficiency of tripeptidyl peptidase 1 | Cerliponase α [Brineura] | [34], (l) |
| Pompe’s disease | Deficiency of acid α-glucosidase | α-glucosidase [Myozyme] | [35], (m) |
| Metabolic deficiencies | |||
| Exocrine pancreatic insufficiency (EPI) | Insufficient secretion of pancreatic enzymes | Pancreatic enzymes [Enzepi] | [36,37,38], (n) |
| Phenylketonuria (PKU) | Deficiency of phenylalanine hydroxylase (PAH) | PAH and phenylalanine ammonia-lyase PAH [Palynziq] | [39], (o) |
| Severe combined immunodeficiency (SCID) | Deficiency of adenosine deaminase (ADA) | Polyethylene glycol-conjugated ADA | [40,41] |
| Wolman disease | Deficiency of lysosomal acid lipase | Lysosomal acid lipase [Kanuma] | [42], (p) |
| Acute intermittent porphyria (AIP) | Deficiency of hydroxymethylbilane synthase | Hydroxymethylbilane synthase and porphobilinogen deaminase | [43] |
| Congenital sucrase-isomaltase deficiency (CSID) | Deficiency of sucrase and isomaltase | Sacrosidase | [44] |
| Hypophosphatasia | Deficiency of tissue-nonspecific isoenzyme of alkaline phosphatase (TNSALP) | TNSALP [Strensiq] | [45], (q) |
| Protein C deficiency | Deficiency of Protein C | Protein C [Ceprotin] | [46], (r) |
| Lactose intolerance | Reduction or loss of the activity of lactase-phlorizin hydrolase | Lactase | [47] |
| Fibrosis conditions | |||
| Chronic total occlusions | Fibrous plaques obstructing coronary arteries | Collagenase Clostridium histolyticum (CCH) | [48] |
| Dupuytren’s disease | Thickening of the fascia tissue in the hands | Collagenase Clostridium histolyticum (CCH) [Xiapex] | [22,49], (s) |
| Peyronie’s disease | Fibrous plaques formation in the penis | Collagenase Clostridium histolyticum (CCH) | [20] |
| Uterine fibroid | Fibroid tissue growth around the uterus | Collagenase Clostridium histolyticum (CCH) | [50] |
| Keloid disease | Overgrowth of granulation scar tissue | Collagenases and matrix metallopeptidases | [51,52] |
| Lung cystic fibrosis | Viscose secretions in the lungs | Deoxyribonuclease I [Pulmozyme] | [21], (t) |
| Glaucoma | Fibrous formations at the trabecular meshwork of the eye | Collagenases | [54] |
| Ocular affections | |||
| Different ocular diseases treated with vitrectomy | Malfunction of the vitreous humor of the eye solved by its enzymatic removal | Chondroitinase, hyaluronidase, nattokinase and ocriplasmin [Jetrea] | [55], (u) |
| Joint problems | |||
| Intervertebral disc herniation | Disc material penetrating the spinal dura | Chondroitin sulfate ABC endolyase | [56] |
| Arthritis | Osteophytes formation and inflammation | Proteolytic enzymes | [57,58] |
| Cancer | |||
| Different types of cancer | Increased amino acid metabolism in the tumor microenvironment | PEGylated arginine deaminase and kynureninase [Voraxaze, PEG hyaluronidase PH20] | [14,59], (v,w,x) |
| Leukemia | Increased amino acid metabolism in the tumor microenvironment | L-asparaginase [Spectrila, Kidrolase, Erwinase, Oncaspar] | [16,59], (y,z) |
| Chemotherapy-induced hyperuricemia | Increase in uric acid due to tumor lysis syndrome | Urate oxidase and rasburicase [Fasturtec] | [60], (aa) |
| Cardiovascular diseases | |||
| Cardiovascular disease | Formation of fibrin clots degraded by plasmin | Nattokinase and urokinase [Streptase, Syner-Kinase, Kinclytic, Rapilsyn, Actilyse, Metalyse] | [17], (ab,ac,ad,ae,af) |
| Extracellular matrix disorders | |||
| Burns | Denatured collagen in necrotic tissue | Collagenase Clostridium histolyticum (CCH) [Nexobrid] | [63,64], (ag) |
| Cellulite | Accumulation of subdermal collagen in the dermal septa | Collagenases | [65] |
| Reactive oxygen species damage | |||
| Organ injury in hemorrhagic shock | Reactive oxygen species (ROS) tissue damage | Superoxide dismutase | [66] |
| Parkinson’s | Reactive oxygen species (ROS) tissue damage | Nanozyme (PtCu nanoalloys) | [68] |
| Other applications | |||
| Celiac disease | Gluten intolerance | Gluten-degrading enzymes | [69] |
| Microbial infections | Microbial biofilm formation during infection | Matrix-degrading enzymes (polysaccharide-degrading enzymes, nucleases and proteases) | [70] |
| Inflammation | Inflammation of overexpressed pathways disrupting physiological homeostasis | Proteolytic enzymes (trypsin or serratiopeptidase) | [71,72] |
| Cocaine overdose | Cocaine toxicity | Human butyrylcholinesterase (BChE) or Bacterial cocaine esterase (CocE) | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Fuente, M.; Lombardero, L.; Gómez-González, A.; Solari, C.; Angulo-Barturen, I.; Acera, A.; Vecino, E.; Astigarraga, E.; Barreda-Gómez, G. Enzyme Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9181. https://doi.org/10.3390/ijms22179181
de la Fuente M, Lombardero L, Gómez-González A, Solari C, Angulo-Barturen I, Acera A, Vecino E, Astigarraga E, Barreda-Gómez G. Enzyme Therapy: Current Challenges and Future Perspectives. International Journal of Molecular Sciences. 2021; 22(17):9181. https://doi.org/10.3390/ijms22179181
Chicago/Turabian Stylede la Fuente, Miguel, Laura Lombardero, Alfonso Gómez-González, Cristina Solari, Iñigo Angulo-Barturen, Arantxa Acera, Elena Vecino, Egoitz Astigarraga, and Gabriel Barreda-Gómez. 2021. "Enzyme Therapy: Current Challenges and Future Perspectives" International Journal of Molecular Sciences 22, no. 17: 9181. https://doi.org/10.3390/ijms22179181
APA Stylede la Fuente, M., Lombardero, L., Gómez-González, A., Solari, C., Angulo-Barturen, I., Acera, A., Vecino, E., Astigarraga, E., & Barreda-Gómez, G. (2021). Enzyme Therapy: Current Challenges and Future Perspectives. International Journal of Molecular Sciences, 22(17), 9181. https://doi.org/10.3390/ijms22179181








