Fourier Transform Infrared Microspectroscopy Combined with Principal Component Analysis and Artificial Neural Networks for the Study of the Effect of ?-Hydroxy-?-Methylbutyrate (HMB) Supplementation on Articular Cartilage
Abstract
:1. Introduction
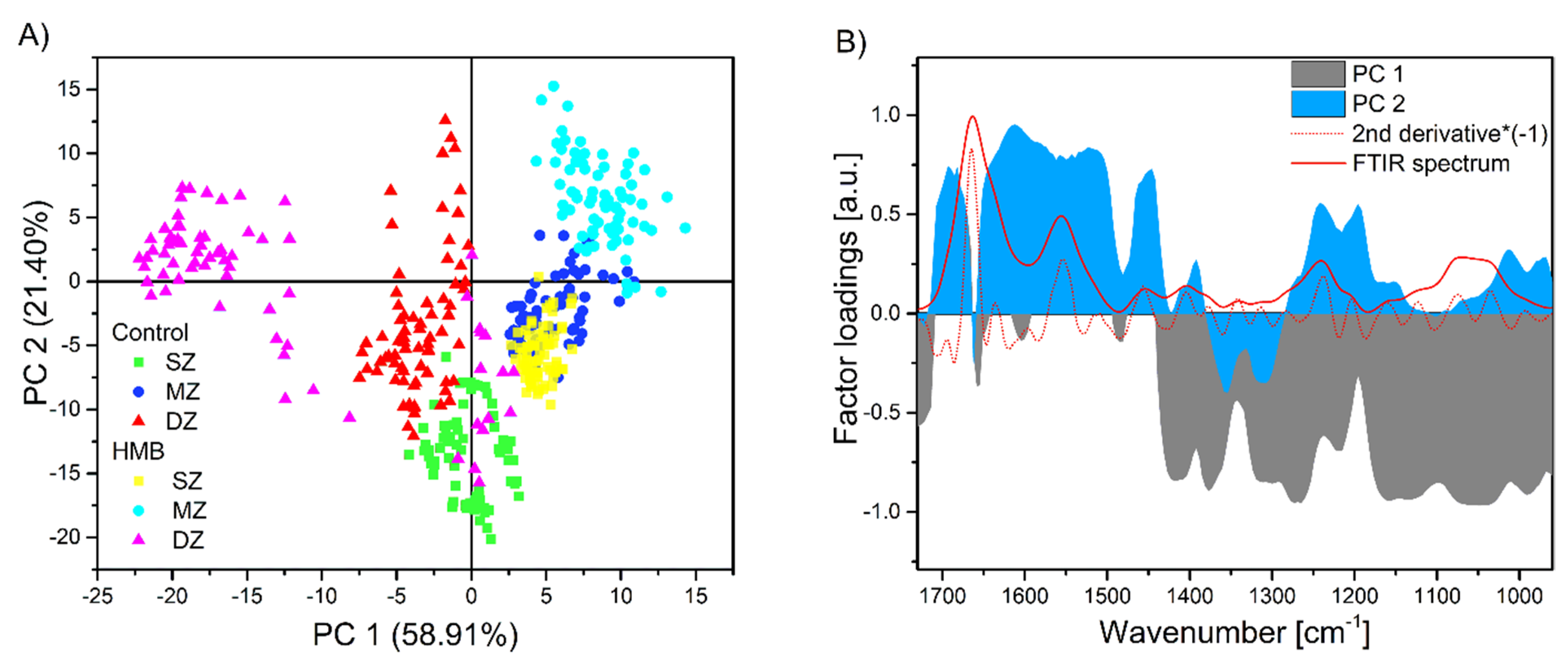
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Fourier Transform Infrared Microspectroscopy (FTIR Microspectroscopy) and Data Pre-Processing
3.3. Chemical Mapping
3.4. AFM Nanoindentation
3.5. Data Processing
3.6. Principal Component Analysis (PCA)
3.7. Artificial Neural Networks (ANNs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.; Wong, D.D.; Chao, P.H.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinnunen, J.; Saarakkala, S.; Hauta-Kasari, M.; Vahimaa, P.; Jurvelin, J.S. Optical spectral reflectance of human articular cartilage—Relationships with tissue structure, composition and mechanical properties. Biomed. Opt. Express 2011, 2, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Trattnig, S.; Lintner, F. Anatomy, biochemistry, and physiology of articular cartilage. Investig. Radiol. 2000, 35, 573–580. [Google Scholar] [CrossRef]
- Ramakrishnan, N.; Xia, Y.; Bidthanapally, A.; Lu, M. Determination of zonal boundaries in articular cartilage using infrared dichroism. Appl. Spectrosc. 2007, 61, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Oinas, J.; Ronkainen, A.P.; Rieppo, L.; Finnilä, M.A.J.; Iivarinen, J.T.; van Weeren, P.R.; Helminen, H.J.; Brama, P.A.J.; Korhonen, R.K.; Saarakkala, S. Composition, structure and tensile biomechanical properties of equine articular cartilage during growth and maturation. Sci. Rep. 2018, 8, 11357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayliss, M.T.; Venn, M.; Maroudas, A.; Ali, S.Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem. J. 1983, 209, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Ratcliffe, A.; Poole, A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials 1992, 13, 67–97. [Google Scholar] [CrossRef]
- van Turnhout, M.C.; Schipper, H.; Engel, B.; Buist, W.; Kranenbarg, S.; van Leeuwen, J.L. Postnatal development of collagen structure in ovine articular cartilage. BMC Dev. Biol. 2010, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, A.J.; Melrose, J. Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity. Biomolecules 2020, 10, 1244. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Slater, G.J.; Jenkins, D. Beta-hydroxy-beta-methylbutyrate (HMB) supplementation and the promotion of muscle growth and strength. Sports Med. (Auckl. N.Z.) 2000, 30, 105–116. [Google Scholar] [CrossRef]
- Kovarik, M.; Muthny, T.; Sispera, L.; Holecek, M. Effects of β-hydroxy-β-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J. Physiol. Biochem. 2010, 66, 311–319. [Google Scholar] [CrossRef]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Jackson, J.R.; Wang, Y.; Edens, N.; Pereira, S.L.; Alway, S.E. β-Hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R701–R715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelen, M.P.K.J.; Deutz, N.E.P. Is β-hydroxy β-methylbutyrate an effective anabolic agent to improve outcome in older diseased populations? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Santos-Fandila, A.; Zafra-Gómez, A.; Barranco, A.; Navalón, A.; Rueda, R.; Ramírez, M. Quantitative determination of β-hydroxymethylbutyrate and leucine in culture media and microdialysates from rat brain by UHPLC-tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Blicharski, T.; Tomaszewska, E.; Dobrowolski, P.; Hułas-Stasiak, M.; Muszyński, S. A metabolite of leucine (β-hydroxy-β-methylbutyrate) given to sows during pregnancy alters bone development of their newborn offspring by hormonal modulation. PLoS ONE 2017, 12, e0179693. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Wiącek, D.; Tomczyk-Warunek, A.; Świetlicka, I.; Pierzynowski, S.G. Maternal HMB treatment affects bone and hyaline cartilage development in their weaned piglets via the leptin/osteoprotegerin system. J. Anim. Physiol. Anim. Nutr. 2019, 103, 626–643. [Google Scholar] [CrossRef]
- Szcześniak, K.A.; Ostaszewski, P.; Fuller, J.C.; Ciecierska, A.; Sadkowski, T. Dietary supplementation of β-hydroxy-β-methylbutyrate in animals—A review. J. Anim. Physiol. Anim. Nutr. 2015, 99, 405–417. [Google Scholar] [CrossRef]
- Tomczyk-Warunek, A.; Blicharski, T.; Jarecki, J.; Dobrowolski, P.; Muszyński, S.; Tomaszewska, E.; Rovati, L.C. The effect of maternal HMB supplementation on bone mechanical and geometrical properties, as well as histomorphometry and immunolocalization of VEGF, TIMP2, MMP13, BMP2 in the bone and cartilage tissue of the humerus of their newborn piglets. PLoS ONE 2021, 16, e0240642. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.; Pleshko Camacho, N. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 2007, 28, 2465–2478. [Google Scholar] [CrossRef] [Green Version]
- Camacho, N.P.; West, P.; Torzilli, P.A.; Mendelsohn, R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers 2001, 62, 1–8. [Google Scholar] [CrossRef]
- Potter, K.; Kidder, L.H.; Levin, I.W.; Lewis, E.N.; Spencer, R.G.S. Imaging of collagen and proteoglycan in cartilage sections using Fourier transform infrared spectral imaging. Arthritis Rheum. 2001, 44, 846–855. [Google Scholar] [CrossRef]
- Rieppo, J.; Hyttinen, M.M.; Halmesmaki, E.; Ruotsalainen, H.; Vasara, A.; Kiviranta, I.; Jurvelin, J.S.; Helminen, H.J. Changes in spatial collagen content and collagen network architecture in porcine articular cartilage during growth and maturation. Osteoarthr. Cartil. 2009, 17, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieppo, L.; Saarakkala, S.; Närhi, T.; Holopainen, J.; Lammi, M.; Helminen, H.J.; Jurvelin, J.S.; Rieppo, J. Quantitative analysis of spatial proteoglycan content in articular cartilage with Fourier transform infrared imaging spectroscopy: Critical evaluation of analysis methods and specificity of the parameters. Microsc. Res. Tech. 2010, 73, 503–512. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, B.; Li, Y.; Zang, H.; Li, L. Vibrational Spectroscopy in Assessment of Early Osteoarthritis—A Narrative Review. Int. J. Mol. Sci. 2021, 22, 5235. [Google Scholar] [CrossRef]
- West, P.A.; Bostrom, M.P.; Torzilli, P.A.; Camacho, N.P. Fourier transform infrared spectral analysis of degenerative cartilage: An infrared fiber optic probe and imaging study. Appl. Spectrosc. 2004, 58, 376–381. [Google Scholar] [CrossRef]
- Rieppo, L.; Saarakkala, S.; Närhi, T.; Helminen, H.J.; Jurvelin, J.S.; Rieppo, J. Application of second derivative spectroscopy for increasing molecular specificity of Fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.-H.; Wu, Y.-C.; Zhang, X.-X.; Gao, H.; Yin, J.-H. Comparative study on identification of healthy and osteoarthritic articular cartilages by fourier transform infrared imaging and chemometrics methods. J. Innov. Opt. Health Sci. 2017, 10, 1650054. [Google Scholar] [CrossRef] [Green Version]
- Oinas, J.; Rieppo, L.; Finnilä, M.A.J.; Valkealahti, M.; Lehenkari, P.; Saarakkala, S. Imaging of Osteoarthritic Human Articular Cartilage using Fourier Transform Infrared Microspectroscopy Combined with Multivariate and Univariate Analysis. Sci. Rep. 2016, 6, 30008. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xia, Y.; Xiao, Z. Comparison of macromolecular component distributions in osteoarthritic and healthy cartilages by Fourier Transform Infrared imaging. J. Innov. Opt. Health Sci. 2013, 06, 1350048. [Google Scholar] [CrossRef] [Green Version]
- Bi, X.; Yang, X.; Bostrom, M.P.G.; Camacho, N.P. Fourier transform infrared imaging spectroscopy investigations in the pathogenesis and repair of cartilage. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 934–941. [Google Scholar] [CrossRef] [Green Version]
- Kobrina, Y.; Rieppo, L.; Saarakkala, S.; Pulkkinen, H.J.; Tiitu, V.; Valonen, P.; Kiviranta, I.; Jurvelin, J.S.; Isaksson, H. Cluster analysis of infrared spectra can differentiate intact and repaired articular cartilage. Osteoarthr. Cartil. 2013, 21, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, K.; Dicks, N.; Glanzner, W.; Agellon, L.; Bordignon, V. Efficacy of the porcine species in biomedical research. Front. Genet. 2015, 6, 293. [Google Scholar] [CrossRef] [Green Version]
- Humphray, S.J.; Scott, C.E.; Clark, R.; Marron, B.; Bender, C.; Camm, N.; Davis, J.; Jenks, A.; Noon, A.; Patel, M.; et al. A high utility integrated map of the pig genome. Genome Biol. 2007, 8, R139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, G.M.; Klisch, S.M.; Sah, R.L. Bioengineering Cartilage Growth, Maturation, and Form. Pediatric Res. 2008, 63, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Tatara, M.R.; Śliwa, E.; Krupski, W. Prenatal programming of skeletal development in the offspring: Effects of maternal treatment with β-hydroxy β-methylbutyrate (HMB) on femur properties in pigs at slaughter age. Bone 2007, 40, 1615–1622. [Google Scholar] [CrossRef]
- Flummer, C.; Kristensen, N.B.; Theil, P.K. Body composition of piglets from sows fed with the leucine metabolite β-hydroxy β-methylbutyrate in late gestation. J. Anim. Sci. 2012, 90, 442–444. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Kostro, K.; Jakubczak, A.; Taszkun, I.; Jaworska-Adamu, J.; Żmuda, A.; Rycerz, K.; Muszyński, S. Effect of HMB and 2-Ox administered during pregnancy on bone properties in primiparous and multiparous minks (Neivison vison). Bull. Vet. Inst. Pulawy 2015, 59, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Świetlicka, I.; Muszyński, S.; Tomaszewska, E.; Dobrowolski, P.; Kwaśniewska, A.; Świetlicki, M.; Skic, A.; Gołacki, K. Prenatally administered HMB modifies the enamel surface roughness in spiny mice offspring: An Atomic Force Microscopy study. Arch. Oral Biol. 2016, 70, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Dobrowolski, P.; Świetlicka, I.; Muszyński, S.; Kostro, K.; Jakubczak, A.; Taszkun, I.; Żmuda, A.; Rycerz, K.; Blicharski, T.; et al. Effects of maternal treatment with β-hydroxy-β-metylbutyrate and 2-oxoglutaric acid on femur development in offspring of minks of the standard dark brown type. J. Anim. Physiol. Anim. Nutr. 2018, 102, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eley, H.L.; Russel, S.T.; Baxter, J.H.; Mukerji, P.; Tisdale, M.J. Signaling pathways initiated by b-hydroxy-bmethylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 923–931. [Google Scholar] [CrossRef]
- Eley, H.L.; Russel, S.T.; Tisdale, M.J. Mechanism of attenuation of depression of muscle protein degradation induced by tumor necrosis factor-a and angiotensin II by b-hydroxy-bmethylbutyrate. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M.; Muthny, T.; Kovarlik, M.; Sispera, L. Effect of betahydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem. Toxicol. 2009, 47, 255–259. [Google Scholar] [CrossRef]
- Aversa, Z.; Bonetto, A.; Costelli, P.; Minero, V.G.; Penna, F.; Baccino, F.M.; Lucia, S.; Fanelli, F.R.; Muscaritoli, M. β-hydroxy-β-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int. J. Oncol. 2011, 38, 713–720. [Google Scholar] [PubMed] [Green Version]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite betahydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Mao, Z.-H.; Yin, J.-H.; Zhang, X.-X.; Wang, X.; Xia, Y. Discrimination of healthy and osteoarthritic articular cartilage by Fourier transform infrared imaging and Fishers discriminant analysis. Biomed. Opt. Express 2016, 7, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Xia, Y.; Lu, M. Concentration profiles of collagen and proteoglycan in articular cartilage by Fourier transform infrared imaging and principal component regression. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 88, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Susi, H.; Byler, D.M. Resolution-enhanced fourier transform infrared spectroscopy of enzymes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1986; Volume 130, pp. 290–311. [Google Scholar]
- de Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Croxford, A.M.; Crombie, D.; McNaughton, D.; Holmdahl, R.; Nandakumar, K.S.; Rowley, M.J. Specific antibody protection of the extracellular cartilage matrix against collagen antibody–induced damage. Arthritis Rheum. 2010, 62, 3374–3384. [Google Scholar] [CrossRef]
- Shah, N.B.; Wolkers, W.F.; Morrissey, M.; Sun, W.Q.; Bischof, J.C. Fourier Transform Infrared Spectroscopy Investigation of Native Tissue Matrix Modifications Using a Gamma Irradiation Process. Tissue Eng. Part C Methods 2008, 15, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, P.; Morris, M.D.; Ramamoorthy, A. Solid-State NMR Spectroscopy Provides Atomic-Level Insights Into the Dehydration of Cartilage. J. Phys. Chem. B 2011, 115, 9948–9954. [Google Scholar] [CrossRef] [Green Version]
- Kemp, A.D.; Harding, C.C.; Cabral, W.A.; Marini, J.C.; Wallace, J.M. Effects of tissue hydration on nanoscale structural morphology and mechanics of individual Type I collagen fibrils in the Brtl mouse model of Osteogenesis Imperfecta. J. Struct. Biol. 2012, 180, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Rieppo, L.; Töyräs, J.; Saarakkala, S. Vibrational spectroscopy of articular cartilage. Appl. Spectrosc. Rev. 2017, 52, 249–266. [Google Scholar] [CrossRef]
- Linka, K.; Thüring, J.; Rieppo, L.; Aydin, R.C.; Cyron, C.J.; Kuhl, C.; Merhof, D.; Truhn, D.; Nebelung, S. Machine learning-augmented and microspectroscopy-informed multiparametric MRI for the non-invasive prediction of articular cartilage composition. Osteoarthr. Cartil. 2021, 29, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Mantsch, H.H. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Pogoda, K.; Pięta, E.; Roman, M.; Piergies, N.; Liberda, D.; Wróbel, T.P.; Janmey, P.A.; Paluszkiewicz, C.; Kwiatek, W.M. In search of the correlation between nanomechanical and biomolecular properties of prostate cancer cells with different metastatic potential. Arch. Biochem. Biophys. 2021, 697, 108718. [Google Scholar] [CrossRef]
- Prein, C.; Warmbold, N.; Farkas, Z.; Schieker, M.; Aszodi, A.; Clausen-Schaumann, H. Structural and mechanical properties of the proliferative zone of the developing murine growth plate cartilage assessed by atomic force microscopy. Matrix Biol. J. Int. Soc. Matrix Biol. 2016, 50, 1–15. [Google Scholar] [CrossRef]
- Danalache, M.; Jacobi, L.F.; Schwitalle, M.; Hofmann, U.K. Assessment of biomechanical properties of the extracellular and pericellular matrix and their interconnection throughout the course of osteoarthritis. J. Biomech. 2019, 97, 109409. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fleischhauer, L.; Nicolae, C.; Prein, C.; Farkas, Z.; Saller, M.M.; Prall, W.C.; Wagener, R.; Heilig, J.; Niehoff, A.; et al. Mice Lacking the Matrilin Family of Extracellular Matrix Proteins Develop Mild Skeletal Abnormalities and Are Susceptible to Age-Associated Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 666. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, B.; Marchi, G.; Alberton, P.; Farkas, Z.; Aszodi, A.; Roths, J.; Clausen-Schaumann, H. Early Detection of Cartilage Degeneration: A Comparison of Histology, Fiber Bragg Grating-Based Micro-Indentation, and Atomic Force Microscopy-Based Nano-Indentation. Int. J. Mol. Sci. 2020, 21, 7384. [Google Scholar] [CrossRef] [PubMed]
- Loparic, M.; Wirz, D.; Daniels, A.U.; Raiteri, R.; Vanlandingham, M.R.; Guex, G.; Martin, I.; Aebi, U.; Stolz, M. Micro- and nanomechanical analysis of articular cartilage by indentation-type atomic force microscopy: Validation with a gel-microfiber composite. Biophys. J. 2010, 98, 2731–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saadat, E.; Lan, H.; Majumdar, S.; Rempel, D.M.; King, K.B. Long-term cyclical in vivoloading increases cartilage proteoglycan content in a spatially specific manner: An infrared microspectroscopic imaging and polarized light microscopy study. Arthritis Res. Ther. 2006, 8, R147. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, N.; Xia, Y. Fourier-transform infrared spectroscopic imaging of articular cartilage and biomaterials: A review. Trends Appl. Spectrosc. 2013, 10, 1–23. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis; Springer: New York, NY, USA, 2002. [Google Scholar]
- Wang, X.Z.; Yang, Y.; Li, R.; Mcguinnes, C.; Adamson, J.; Megson, I.L.; Donaldson, K. Principal component and causal analysis of structural and acute in vitro toxicity data for nanoparticles. Nanotoxicology 2014, 8, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Yao, F.; Coquery, J.; Lê Cao, K.-A. Independent Principal Component Analysis for biologically meaningful dimension reduction of large biological data sets. BMC Bioinform. 2012, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kinkiri, S.; Melis, W.J.C. Reducing data storage requirements for machine learning algorithms using principle component analysis. In Proceedings of the International Conference on Applied System Innovation (ICASI), Okinawa, Japan, 26–30 May 2016. [Google Scholar]
- Mow, V.C.; Guo, X.E. Mechano-electrochemical properties of articular cartilage: Their inhomogeneities and anisotropies. Annu. Rev. Biomed. Eng. 2002, 4, 175–209. [Google Scholar] [CrossRef]
- Bishop, C.M. Neural Networks for Pattern Recognition; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Hassoun, M.H. Fundamentals of Artificial Neural Networks; MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Kohonen, T. Self-Organizing Maps; Springer: Berlin, Germany, 2001. [Google Scholar]
- Friedrichs, J.; Legate, K.R.; Schubert, R.; Bharadwaj, M.; Werner, C.; Müller, D.J.; Benoit, M. A practical guide to quantify cell adhesion using single-cell force spectroscopy. Methods (San Diego Calif.) 2013, 60, 169–178. [Google Scholar] [CrossRef]
- Butt, H.J.; Jaschke, M. Calculation of thermal noise in atomic force microscopy. Nanotechnology 1995, 6, 1–7. [Google Scholar] [CrossRef]
- Muschter, D.; Fleischhauer, L.; Taheri, S.; Schilling, A.F.; Clausen-Schaumann, H.; Grässel, S. Sensory neuropeptides are required for bone and cartilage homeostasis in a murine destabilization-induced osteoarthritis model. Bone 2020, 133, 115181. [Google Scholar] [CrossRef]
- Reuten, R.; Zendehroud, S.; Nicolau, M.; Fleischhauer, L.; Laitala, A.; Kiderlen, S.; Nikodemus, D.; Wullkopf, L.; Nielsen, S.R.; McNeilly, S.; et al. Basement membrane stiffness determines metastases formation. Nat. Mater. 2021, 20, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.; Zhang, M.; Zhu, J.; Zheng, R.; Wu, Q. MPCE: A Maximum Probability Based Cross Entropy Loss Function for Neural Network Classification. IEEE Access 2019, 7, 146331–146341. [Google Scholar] [CrossRef]
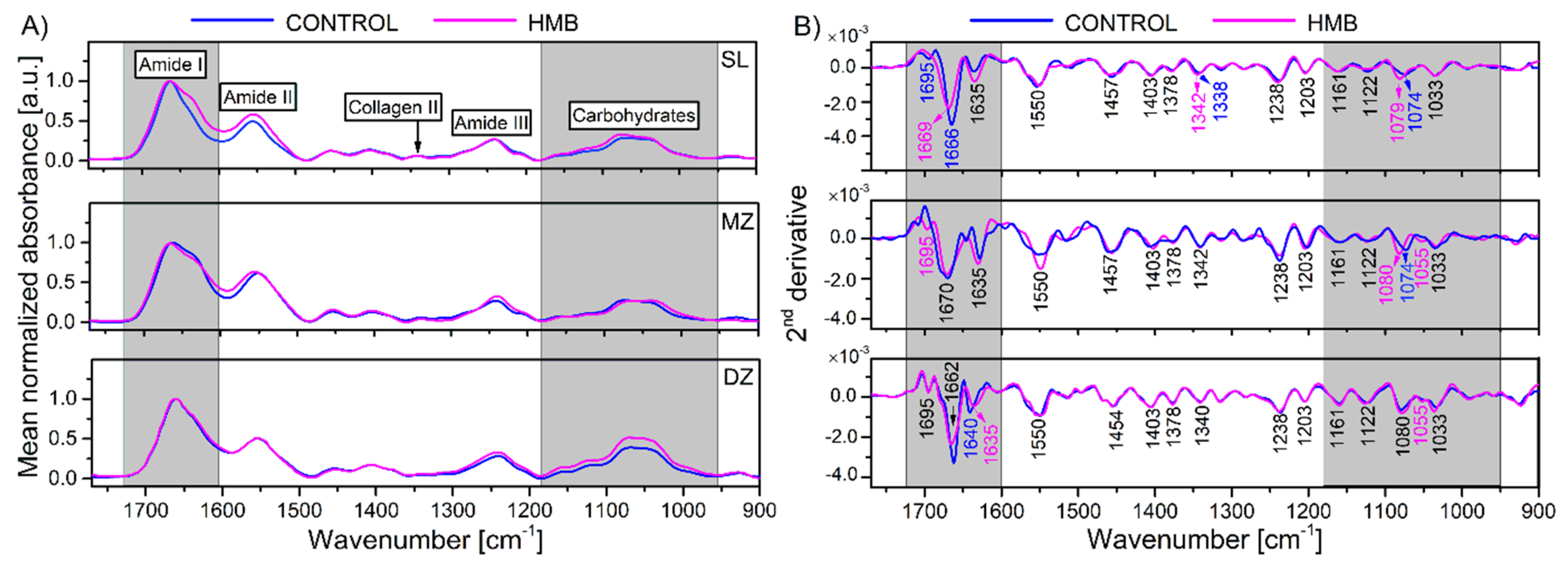





| Wavenumber [cm−1] | Assignment of Second Derivative FTIR Bands of AC |
|---|---|
| 1695, ~1660, 1635 | 80% ν(C=O), 20% ν(CN), τ(HOH), amide I from PGs, water |
| 1550 | 60% τ(N–H), 30% ν(C–N), 10% ν(C–C), amide II |
| 1457 | δas (CH3) |
| 1403 | νs(COO−) of GAGs |
| 1378 | δs (CH3) of GAGs |
| ~1340 | CH2 side-chain vibrations of collagen II |
| 1238 | νas SO3− of sulphated GAGs with CH2 wagging vibration from the glycine backbone and proline side-chain |
| 1204 | ν(C−N) of amide III, δ(N−H) of collagen |
| 1161 | ν(C–O) of the carbohydrate residues |
| 1122 | νas(C–O–S) |
| 1080–1060 | ν(C–O) of the carbohydrate residues in PGs, νsSO3− of sulphated GAGs |
| ~1050 | ν(C–O) of the carbohydrate residues in collagen and PGs |
| 1033 | ν(C–O) of the carbohydrate residues in collagen and PGs |
| Group | Zone | Young’s Modulus [kPa] | |
|---|---|---|---|
| Control | SZ | 33.88 (±0.58) a | |
| HMB | SZ | 88.82 (±1.18) b | |
| Control | MZ | 29.16 (±0.67) a | |
| HMB | MZ | 80.02 (±0.74) b | |
| Control | DZ | 1st peak | 40.02 (±1.03) a |
| 2nd peak | 77.58 (±0.79) b | ||
| HMB | DZ | 1st peak | 42.72 (±1.51) c |
| 2nd peak | 81.38 (±1.19) d | ||
| Network | Recognition Rate | Function | ||||
|---|---|---|---|---|---|---|
| Training | Validation | Testing | Error | Activation (Hidden) | Activation (Output) | |
| MLP BFGS 100 | 1.00 | 0.99 | 1.00 | Entropy | Logistic | Softmax |
| Network | QE (Teaching) | QE (Test) | QE (Validation) | Teaching Algorithm (Epochs) |
|---|---|---|---|---|
| SOFM 196-25 | 0.86 | 1.02 | 0.93 | Kohonen 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świetlicka, I.; Muszyński, S.; Prein, C.; Clausen-Schaumann, H.; Aszodi, A.; Arciszewski, M.B.; Blicharski, T.; Gagoś, M.; Świetlicki, M.; Dobrowolski, P.; et al. Fourier Transform Infrared Microspectroscopy Combined with Principal Component Analysis and Artificial Neural Networks for the Study of the Effect of ?-Hydroxy-?-Methylbutyrate (HMB) Supplementation on Articular Cartilage. Int. J. Mol. Sci. 2021, 22, 9189. https://doi.org/10.3390/ijms22179189
Świetlicka I, Muszyński S, Prein C, Clausen-Schaumann H, Aszodi A, Arciszewski MB, Blicharski T, Gagoś M, Świetlicki M, Dobrowolski P, et al. Fourier Transform Infrared Microspectroscopy Combined with Principal Component Analysis and Artificial Neural Networks for the Study of the Effect of ?-Hydroxy-?-Methylbutyrate (HMB) Supplementation on Articular Cartilage. International Journal of Molecular Sciences. 2021; 22(17):9189. https://doi.org/10.3390/ijms22179189
Chicago/Turabian StyleŚwietlicka, Izabela, Siemowit Muszyński, Carina Prein, Hauke Clausen-Schaumann, Attila Aszodi, Marcin B. Arciszewski, Tomasz Blicharski, Mariusz Gagoś, Michał Świetlicki, Piotr Dobrowolski, and et al. 2021. "Fourier Transform Infrared Microspectroscopy Combined with Principal Component Analysis and Artificial Neural Networks for the Study of the Effect of ?-Hydroxy-?-Methylbutyrate (HMB) Supplementation on Articular Cartilage" International Journal of Molecular Sciences 22, no. 17: 9189. https://doi.org/10.3390/ijms22179189
APA StyleŚwietlicka, I., Muszyński, S., Prein, C., Clausen-Schaumann, H., Aszodi, A., Arciszewski, M. B., Blicharski, T., Gagoś, M., Świetlicki, M., Dobrowolski, P., Kras, K., Tomaszewska, E., & Arczewska, M. (2021). Fourier Transform Infrared Microspectroscopy Combined with Principal Component Analysis and Artificial Neural Networks for the Study of the Effect of ?-Hydroxy-?-Methylbutyrate (HMB) Supplementation on Articular Cartilage. International Journal of Molecular Sciences, 22(17), 9189. https://doi.org/10.3390/ijms22179189








