Study on the Introduction of Solid Fat with a High Content of Unsaturated Fatty Acids to Gluten-Free Muffins as a Basis for Designing Food with Higher Health Value
Abstract
:1. Introduction
2. Results
2.1. Specific Gravity of Muffin Dough
2.2. Analysis of the Microstructure of Dough at Room Temperature
2.3. Physical Parameters of Gluten-Free Muffins
2.4. Texture Profile Analysis
2.5. Color Analysis of Gluten-Free Muffins
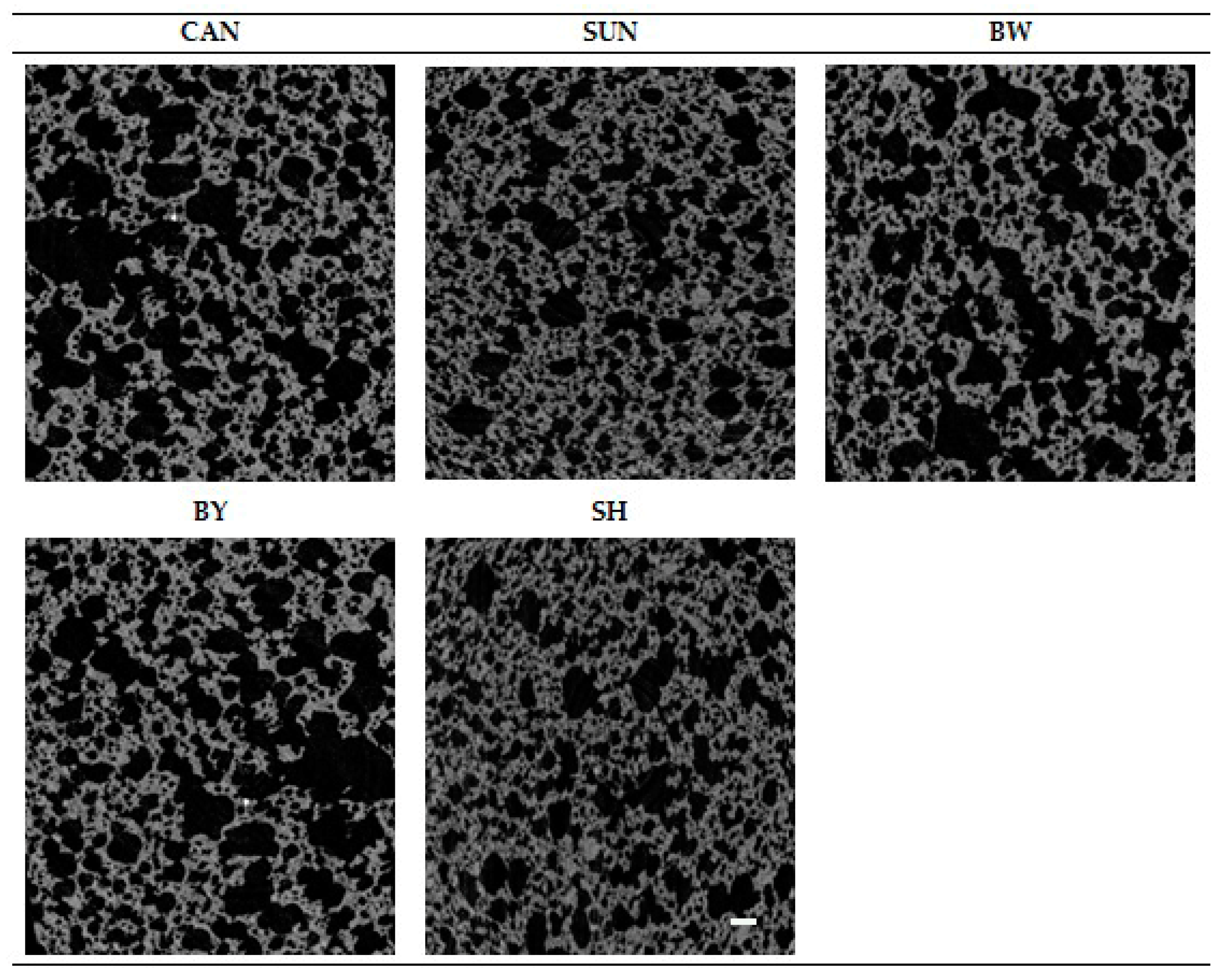
2.6. Analysis of Gluten-Free Muffin Structure
2.7. Fatty Acids Composition of the Lipid Fraction Extracted from Gluten-Free Muffins
2.8. Quality of Fats Extracted from Muffins
3. Discussion
3.1. Physical Properties of Raw and Baked Dough
3.2. Profile of Fatty Acids and Chemical Quality of the Lipid Fraction
4. Materials and Methods
4.1. Materials
4.1.1. Preparation of Gluten-Free Muffins
4.1.2. Preparation of Oleogels
4.2. Methods
4.2.1. Specific Gravity of Dough
4.2.2. Pictures of the Microstructure of Raw Cakes
4.2.3. Geometric Dimensions, Weight, and Volume of Gluten-Free Muffins
4.2.4. Texture Profile Analysis (TPA) of Crumb of Muffins
4.2.5. Water Content of Gluten-Free Muffins
4.2.6. Color of the Crust of Baked Goods
- , , —color coordinates of a muffin variant with oleogel;
- , , —color coordinates obtained for the control variant.
4.2.7. Microtomographic Analysis of Muffins
4.2.8. Extraction of Lipid Fraction of Gluten-Free Muffins
Fatty Acids Profile and the Atherosclerotic Index
- Σ—summatory, MUFA—monounsaturated fatty acids, PUFA—polyunsaturated fatty acids.
Quality Determination of Lipid Fraction
4.2.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witkamp, R.F.; van Norren, K. Let thy food be thy medicine….when possible. Eur. J. Pharmacol. 2018, 836, 102–114. [Google Scholar] [CrossRef]
- Castro-Gomez, P.; Garcia-Serrano, A.; Visioli, F.; Fontecha, J. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot. Essent. Fat. Acids 2015, 101, 41–51. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A. Fatty acids and cardiovascular risk. Evidence, lack of evidence, and diligence. Nutrients 2020, 12, 3782. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, H. Recent practical researches in the development of gluten-free breads. Npj Sci. Food 2019, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codex Alimentarius International Food Standards: Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten. No. CXS 118-1979. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B118-1979%252FCXS_118e_2015.pdf (accessed on 2 July 2021).
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Barbaro, M.R.; Cremon, C.; Stanghellini, V.; Barbara, G. Recent advances in understanding non-celiac gluten sensitivity. F1000Research 2018, 7, 1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christoph, M.J.; Larson, N.; Hootman, K.C.; Miller, J.M.; Neumark-Sztainer, D. Who values gluten-free? Dietary intake, behaviors, and sociodemographic characteristics of young adults who value gluten-free food. J. Acad. Nutr. Diet. 2018, 118, 1389–1398. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada: “Gluten Free” Claims in the Marketplace. 2014, pp. 1–8. Available online: https://publications.gc.ca/collections/collection_2014/aac-aafc/A72-123-2014-eng.pdf (accessed on 2 July 2021).
- Michota-Katulska, E.; Zegan, M.; Leydy, K. The legitimacy of gluten-free diet. Med. Rodz. 2017, 4, 259–264. [Google Scholar] [CrossRef]
- Blicharz-Kania, A. The Future of Dietary Fairs. Machines and Technologies 2019. Available online: https://informatormasarski.pl/maszyny-i-technologie/przyszlosc-dietetycznych-parowek/ (accessed on 2 July 2021).
- Zbikowska, A.; Rutkowska, J.; Kowalska, M. Consumption safety of pastries, confectioneries, and potato products as related to fat content. J. Am. Coll. Nutr. 2015, 34, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Zbikowska, A.; Onacik-Gür, S.; Kowalska, M.; Rutkowska, J. Trans fatty acids in polish pastry. J. Food Prot. 2019, 82, 1028–1033. [Google Scholar] [CrossRef]
- Lim, J.; Jeong, S.; Lee, J.H.; Park, S.; Lee, J.; Lee, S. Effect of shortening replacement with oleogels on the rheological and tomographic characteristics of aerated baked goods. J. Sci. Food Agric. 2017, 97, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- EFSA-European Food Safety Authority. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to nutrition claims concerning omega-3 fatty acids, monounsaturated fat, polyunsaturated fat and unsaturated fat. EFSA J. 2005, 253, 1–29. [Google Scholar] [CrossRef] [Green Version]
- EU REGULATION: Commission Regulation (EU) 2019/649 of 24 April 2019 Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Trans Fat, Other than Trans Fat Naturally Occurring in Fat of Animal. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32019R0649 (accessed on 2 July 2021).
- Krygier, K.; Żbikowska, A. The influence of fatty acids composition on biscuit-fat cake quality. Żywność 2002, 3, 47–57. Available online: https://wydawnictwo.pttz.org/wp-content/uploads/2018/01/23_Zbikowska.pdf (accessed on 2 July 2021).
- Żbikowska, A.; Kowalska, M.; Stauffer, C.E. Fats and oils in bakery products. In Bailey’s Industrial Oil and Fat Products; Fereidoon, S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 181–208. ISBN 978-1-119-25788-2. [Google Scholar] [CrossRef]
- Rogers, M.A. Self-assembled fibrillar networks of low molecular weight oleogelators. In Edible Nanostructures: A Bottom-Up Approach; Marangoni, A.G., Pink, D., Eds.; RSC: London, UK, 2015; pp. 144–154. [Google Scholar]
- Codex Alimentarius International Food Standards: Standard for Named Vegetable Oils CXS 210-1999. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf (accessed on 2 July 2021).
- WHO: World and Health Organization; Global Health Observatory (GHO). Overweight and Obesity. Available online: https://www.who.int/gho/ncd/risk_factors/overweight/en (accessed on 2 July 2021).
- Berski, W. Selected physical-chemical properties of starches extracted from Polish varieties of naked oats. Food Sci. Technol. Qual. 2010, 3, 76–87. [Google Scholar]
- Kasapis, S.; Bannikova, A. Rheology and food microstructure. In Woodhead Publishing Series in Food Science, Technology and Nutrition. Advances in Food Rheology and Its Applications; Ahmed, J., Ptaszek, P., Basu, S., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 7–46. [Google Scholar] [CrossRef]
- Żbikowska, A.; Kowalska, M.; Rutkowska, J. Solid fat content versus quality and technologiical usefulness of shortenings in making shortcrust pastries. Food Sci. Technol. Qual. 2012, 2, 173–185. [Google Scholar]
- Devi, A.; Khatkar, B. Physicochemical, rheological and functional properties of fats and oils in relation to cookie quality: A review. J. Food Sci. Technol. 2016, 53, 3633–3641. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cervera, S.; Salvador, A.; Sanz, T. Comparison of different polyols as total sucrose replacers in muffins: Thermal, rheological, texture and acceptability properties. Food Hydrocoll. 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Ghotra, B.S.; Dyal, S.D.; Narine, S.S. Lipid shortenings: A review. Int. Food Res. J. 2002, 35, 1015–1048. [Google Scholar] [CrossRef]
- Föste, M.; Jekle, M.; Becker, T. Structure stabilization in starch-quinoa bran doughs: The role of water availability and gelatinization. Carbohydr. Polym. 2017, 174, 1018–1025. [Google Scholar] [CrossRef]
- TBIRT: The Baking Industry Research Trust; New Zealand Association of Bakers. Available online: https://www.bakeinfo.co.nz/Facts/Biscuit-making (accessed on 19 May 2020).
- Onacik-Gür, S.; Żbikowska, A.; Kapler, E.; Kowalska, H. Effect of barley β-glucan addition as a fat replacer on muffin quality. Acta Sci. Pol. Technol. Aliment. 2016, 15, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesso, N.; Garnier, C.; Loisel, C.; Chevallier, S.; Bouchet, B.; Le-Bail, A. Formulation effect study on batter and cake microstructure: Correlation with rheology and texture. Food Struct. 2015, 5, 31–41. [Google Scholar] [CrossRef]
- Fennema, O.R. Food Chemistry, 3rd ed.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 1–1059. [Google Scholar]
- Purlis, E. Browning development in bakery products–A review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Ureta, M.M.; Olivera, D.F.; Salvadori, V.O. Influence of baking conditions on the quality attributes of sponge cake. Food Sci. Technol. Int. 2016, 23, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Tomasevic, I.; Tomovic, V.; Milovanovic, B.; Lorenzo, J.; Đorđević, V.; Karabasil, N.; Djekic, I. Comparison of a computer vision system vs. traditional colorimeter for color evaluation of meat products with various physical properties. Meat Sci. 2019, 148, 5–12. [Google Scholar] [CrossRef]
- Lucciardello, F.; Frisullo, P.; Laverse, J.; Muratore, G.; Nobile, M. Effect of sugar, citric acid and egg white type on microstructural and mechanical properties of meringues. J. Food Eng. 2011, 108, 453–462. [Google Scholar] [CrossRef]
- Żbikowska, A.; Kowalska, M. Influence of trans unsaturated fatty acids content on chemical changes in the shortening during baking and storage of cakes. Pol. J. Food Nutr. Sci. 2007, 57, 451–455. [Google Scholar]
- PN-A86902. Animal and Vegetable Fats and Oils-Confectionery and Bakery Fats; Polish Committee for Standardization: Warsaw, Poland, 1997.
- PN-A-86908. Animal and Vegetable Fats and Oils–Rafined Vegetable Oils; Polish Committee for Standardization: Warsaw, Poland, 2000.
- Kafel, S. Significance of unsaturated fats in carcinogenesis. Żyw. Człow. Metab. 1987, 14, 117–120. [Google Scholar]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Rapeseed oil in new application: Assessment of structure of oleogels based on their physicochemical properties and microscopic observations. Agriculture 2020, 10, 211. [Google Scholar] [CrossRef]
- Matsuda, T. Rice flour: A promising food material for nutrition and global health. J. Nutr. Sci. Vitaminol. 2019, 65, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Omri, B.; Chalghoumi, R.; Izzo, L.; Ritieni, A.; Lucarini, M.; Durazzo, A.; Abdouli, H.; Santini, A. Effect of dietary incorporation of linseed alone or together with tomato-red pepper mix on laying hens′ egg yolk fatty acids profile and health lipid indexes. Nutrients 2019, 11, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 660. Animal and Vegetable Fats and Oils-Determination of Acid Value and Acidity; Polish Committee for Standardization: Warsaw, Poland, 2010.
- ISO 3960. Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination; Polish Committee for Standardization: Warsaw, Poland, 2012.
- PN-93/A-86926. Edible Vegetable Fats-Determination of the Anisidine Value and Calculation of the Totox Fat Oxidation Index; Polish Committee for Standardization, Measures and Quality: Warsaw, Poland, 1993.



| Parameter | Type of Wax | |||
|---|---|---|---|---|
| Candelilla | Sunflower | Beeswax White | Beeswax Yellow | |
| Acid value [mg KOH/g] | 12–22 | 2–8 | 17–24 | |
| Ester value [mg/g] | - | 67–93 | 70–80 | |
| Melting temperature [°C] | 68.5–72.5 | 74–80 | 62–65 | |
| Color | Yellow | Pale-yellowish | Off-White | Yellow |
| Variant of Muffin | Parameter | ||||
|---|---|---|---|---|---|
| Weight [g] | Diameter [mm] | Height [mm] | Volume [cm3] | Water Content [%] | |
| CAN | 25.97 ± 0.16 a | 50.53 ± 0.41 a | 32.47 ± 0.44 a | 53.70 ± 2.78 a | 25.60 + 1.59 a |
| SUN | 25.69 ± 0.06 a | 51.27 ± 0.54 a | 32.44 ± 0.53 a | 54.81 ± 3.35 a | 23.48 + 0.46 a |
| BW | 25.78 ± 0.30 a | 51.33 ± 025 a | 32.50 ± 0.82 a | 55.44 ± 4.22 a | 23.25 + 0.38 a |
| BY | 25.74 ± 0.12 a | 51.24 ± 0.33 a | 32.49 ± 0.46 a | 55.55 ± 3.89 a | 24.49 + 0.76 a |
| SH | 25.70 ± 0.05 a | 51.54 ± 0.79 a | 33.18 ± 0.17 a | 53.00 ± 0.91 a | 23.25 + 0.38 a |
| Weight * | Diameter * | Height (y) | Volume * | |
|---|---|---|---|---|
| r = −0.54 | ||||
| Specific gravity (x) | - | - | R2 = 0.30 | - |
| Y = 45.53 − 10.97x |
| Variant of Muffin | Hardness [N] | Springiness [–] | Cohesiveness [–] | Chewiness [N] |
|---|---|---|---|---|
| CAN | 8.94 ± 2.47 a | 1.18 ± 0.36 a | 0.76 ± 0.06 a | 7.60 ± 2.01 a |
| SUN | 11.88 ± 0.79 a | 0.95 ± 0.02 a | 0.73 ± 0.06 a | 8.22 ± 0.21 a |
| BY | 11.89 ± 1.20 a | 0.97 ± 0.03 a | 0.63 ± 0.06 a | 7.13 ± 0.59 a |
| BW | 9.75 ± 1.49 a | 1.57 ± 0.55 a | 0.68 ± 0.03 a | 9.95 ± 3.85 a |
| SH | 8.36 ± 1.06 a | 1.14 ± 0.30 a | 0.77 ± 0.08 a | 7.49 ± 2.13 a |
| Color Parameters | |||||
|---|---|---|---|---|---|
| Variant of Muffin | L* | a* | b* | ∆E | BI |
| CAN | 66.24 ± 0.06 a | 10.95 ± 0.35 c | 31.78 ± 0.56 bc | 5.33 ± 0.92 b | 74.87 ± 1.81 b |
| SUN | 68.11 ± 0.37 b | 7.99 ± 0.67 a | 31.99 ± 0.27 bc | 3.03 ± 0.42 a | 69.48 ± 1.27 ab |
| BW | 66.00 ± 0.56 a | 9.82 ± 0.21 bc | 30.55 ± 0.07 ab | 5.15 ± 0.55 b | 70.70 ± 0.72 b |
| BY | 67.13 ± 0.21 ab | 8.95 ± 0.01 ab | 29.12 ± 1.36 a | 4.69 ± 0.65 b | 64.66 ± 3.33 a |
| SH | 69.87 ± 1.19 c | 9.11 ± 0.66 ab | 32.46 ± 0.21 c | - | 69.62 ± 2.66 ab |
| Variant of Muffin Crumb | Total Porosity [%] |
|---|---|
| CAN | 53.64 ± 0.69 cd |
| SUN | 42.96 ± 0.00 a |
| BW | 52.46 ± 0.00 c |
| BY | 55.07 ± 0.79 d |
| SH | 49.49 ± 0.10 b |
| CAN * | SUN * | BW * | BY * | SH * | |
|---|---|---|---|---|---|
| C12:0 | - | - | - | - | 0.71 |
| C14:0 | - | - | - | - | 1.07 |
| C16:0 | 6.46 | 6.20 | 6.66 | 6.38 | 43.31 |
| C18:0 | 1.75 | 2.05 | 2.32 | 2.38 | 5.11 |
| C18:1 (trans-9) | - | - | - | - | - |
| C18:1 (cis-9) | 62.32 | 63.15 | 62.98 | 61.22 | 37.88 |
| C18:2 (cis-9,12) | 19.34 | 20.20 | 18.94 | 19.29 | 10.50 |
| C18:3 (cis-9,12,15) | 6.58 | 6.52 | 6.16 | 7.32 | 0.70 |
| C20:0 | 0.54 | 0.53 | 0.53 | 0.31 | 0.41 |
| C20:1 | 1.19 | 0.88 | 1.26 | 1.17 | - |
| C22:0 | 0.37 | 0.48 | 0.27 | 0.49 | - |
| Others (<0,3) | 1.45 | - | 0.88 | 1.44 | 0.31 |
| Σ TFA | - | - | - | - | - |
| Σ SFA | 9.12 | 9.26 | 9.78 | 9.56 | 50.61 |
| Σ MUFA | 63.51 | 64.03 | 64.24 | 62.39 | 37.88 |
| Σ PUFA | 25.92 | 26.72 | 25.10 | 26.61 | 11.20 |
| ΣPUFA-ω-6 | 19.34 | 20.20 | 18.94 | 19.29 | 10.50 |
| ΣPUFA-ω-3 | 6.58 | 6.52 | 6.16 | 7.32 | 0.70 |
| IA | 0.09 | 0.31 | 0.10 | 0.33 | 1.07 |
| AV [mg KOH/g] | PV [meq O2/kg] | AnV [absorb.·100] | |
|---|---|---|---|
| Rafined rapeseed oil | 0.25 ± 0.01 | 2.50 ± 0.52 | 1.40 ± 0.14 |
| Shortening | 0.37 ± 0.06 | 2.68 ± 0.08 | 3.75 ± 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Zbikowska, K.; Kowalska, M.; Kowalska, H.; Rutkowska, J. Study on the Introduction of Solid Fat with a High Content of Unsaturated Fatty Acids to Gluten-Free Muffins as a Basis for Designing Food with Higher Health Value. Int. J. Mol. Sci. 2021, 22, 9220. https://doi.org/10.3390/ijms22179220
Kupiec M, Zbikowska A, Marciniak-Lukasiak K, Zbikowska K, Kowalska M, Kowalska H, Rutkowska J. Study on the Introduction of Solid Fat with a High Content of Unsaturated Fatty Acids to Gluten-Free Muffins as a Basis for Designing Food with Higher Health Value. International Journal of Molecular Sciences. 2021; 22(17):9220. https://doi.org/10.3390/ijms22179220
Chicago/Turabian StyleKupiec, Milena, Anna Zbikowska, Katarzyna Marciniak-Lukasiak, Katarzyna Zbikowska, Małgorzata Kowalska, Hanna Kowalska, and Jarosława Rutkowska. 2021. "Study on the Introduction of Solid Fat with a High Content of Unsaturated Fatty Acids to Gluten-Free Muffins as a Basis for Designing Food with Higher Health Value" International Journal of Molecular Sciences 22, no. 17: 9220. https://doi.org/10.3390/ijms22179220
APA StyleKupiec, M., Zbikowska, A., Marciniak-Lukasiak, K., Zbikowska, K., Kowalska, M., Kowalska, H., & Rutkowska, J. (2021). Study on the Introduction of Solid Fat with a High Content of Unsaturated Fatty Acids to Gluten-Free Muffins as a Basis for Designing Food with Higher Health Value. International Journal of Molecular Sciences, 22(17), 9220. https://doi.org/10.3390/ijms22179220








