The Amino Acid-mTORC1 Pathway Mediates APEC TW-XM-Induced Inflammation in bEnd.3 Cells
Abstract
1. Introduction
2. Results
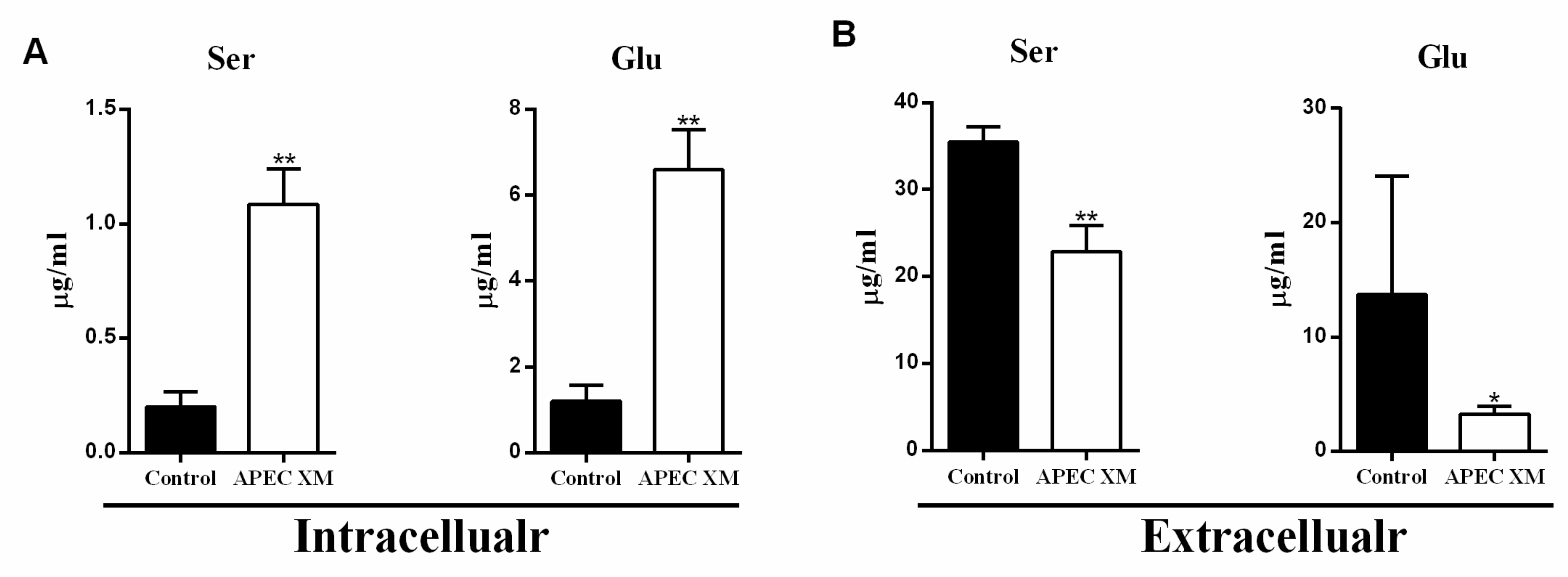
2.1. APEC XM Induced Changes of Serine and Glutamate Uptake in bEnd.3 Cells
2.2. APEC XM Infection Increases the Expression of SNAT2 and EAAT4 in bEnd.3 Cells
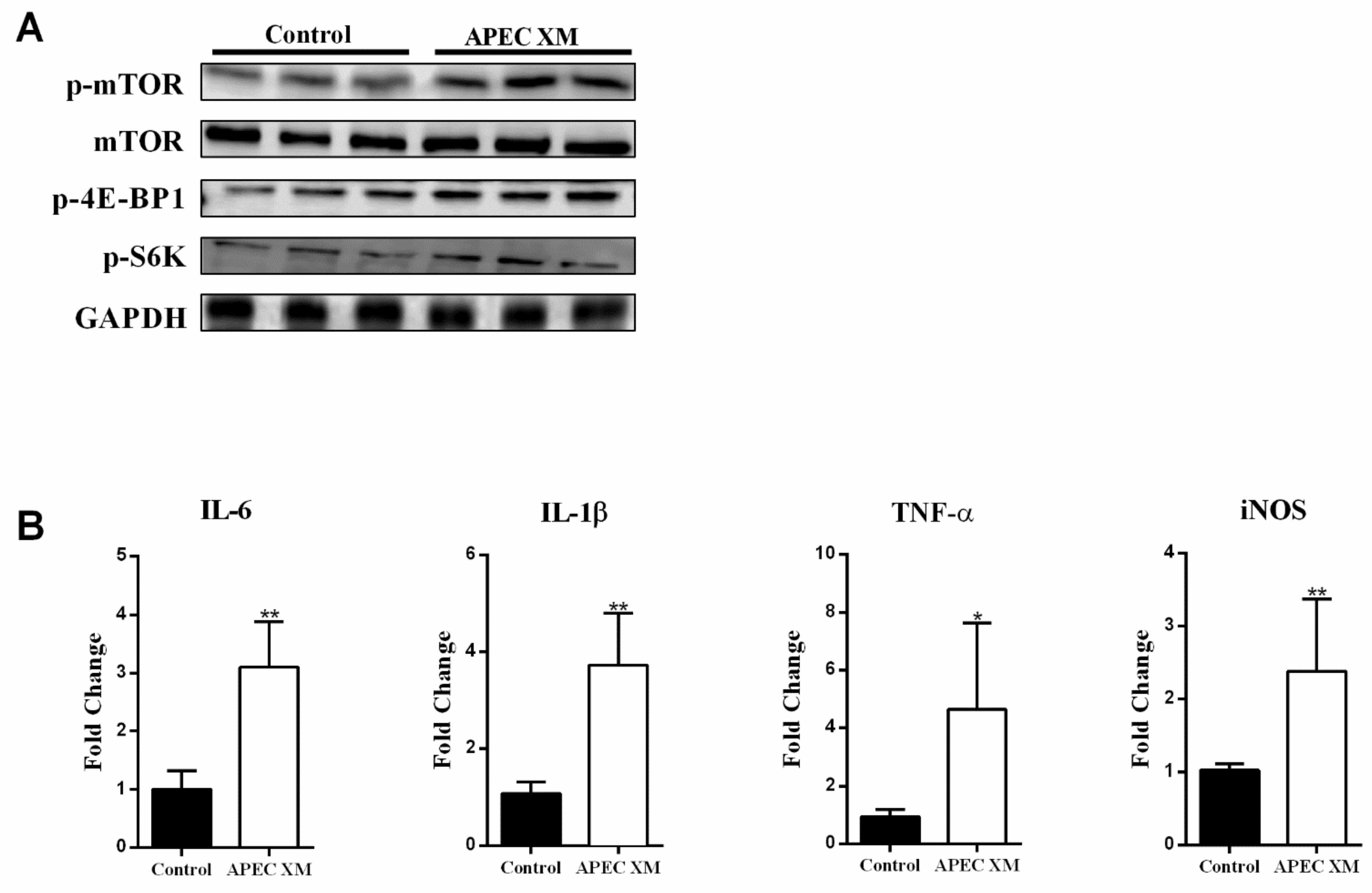
2.3. APEC XM Activates the mTORC1 Pathway and Inflammatory Responses in bEnd.3 Cells
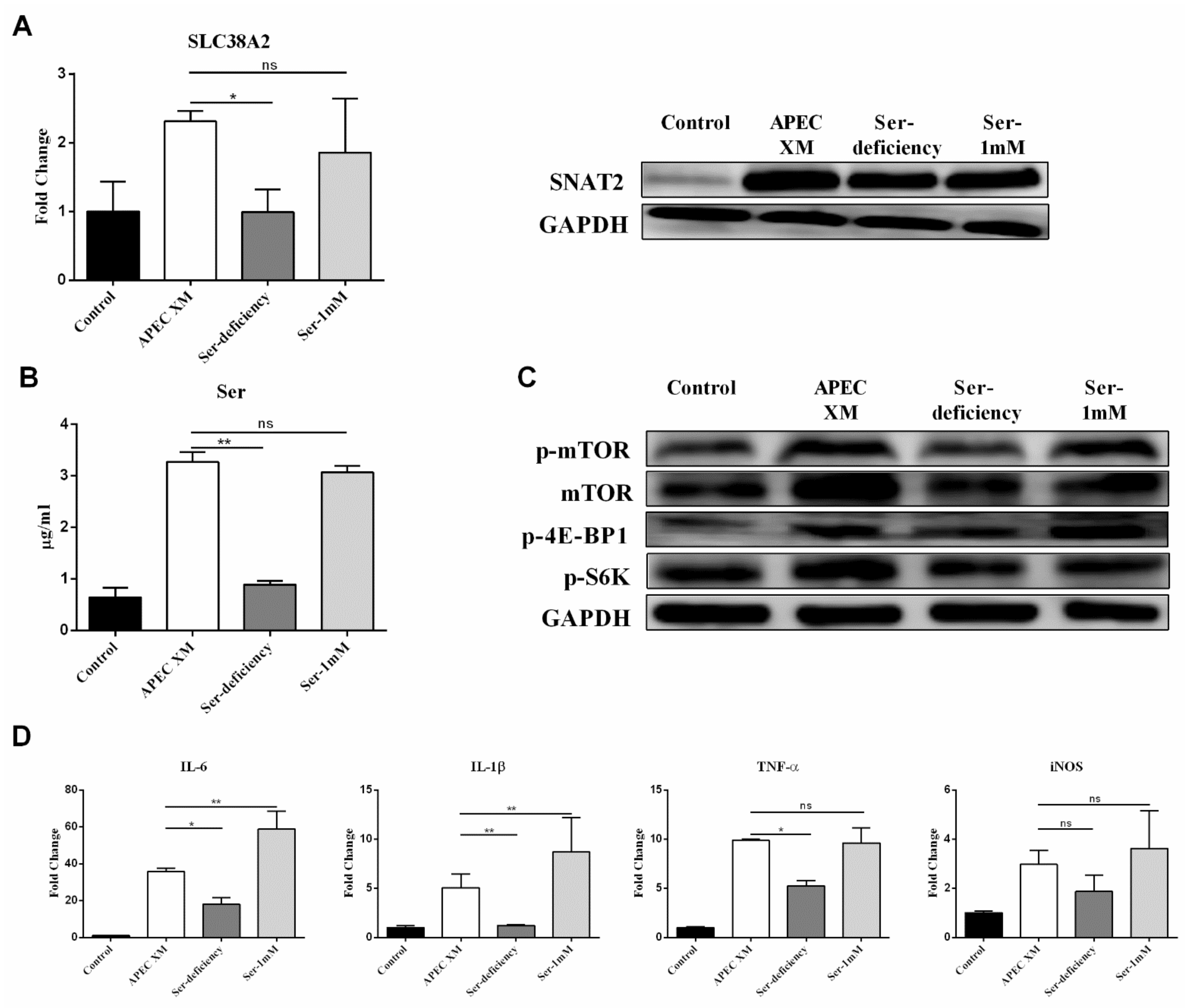
2.4. Ser Involved in APEC XM-Induced the Expression of SNAT2, Activation of mTOR Pathway and Inflammatory Responses in bEnd.3 Cells
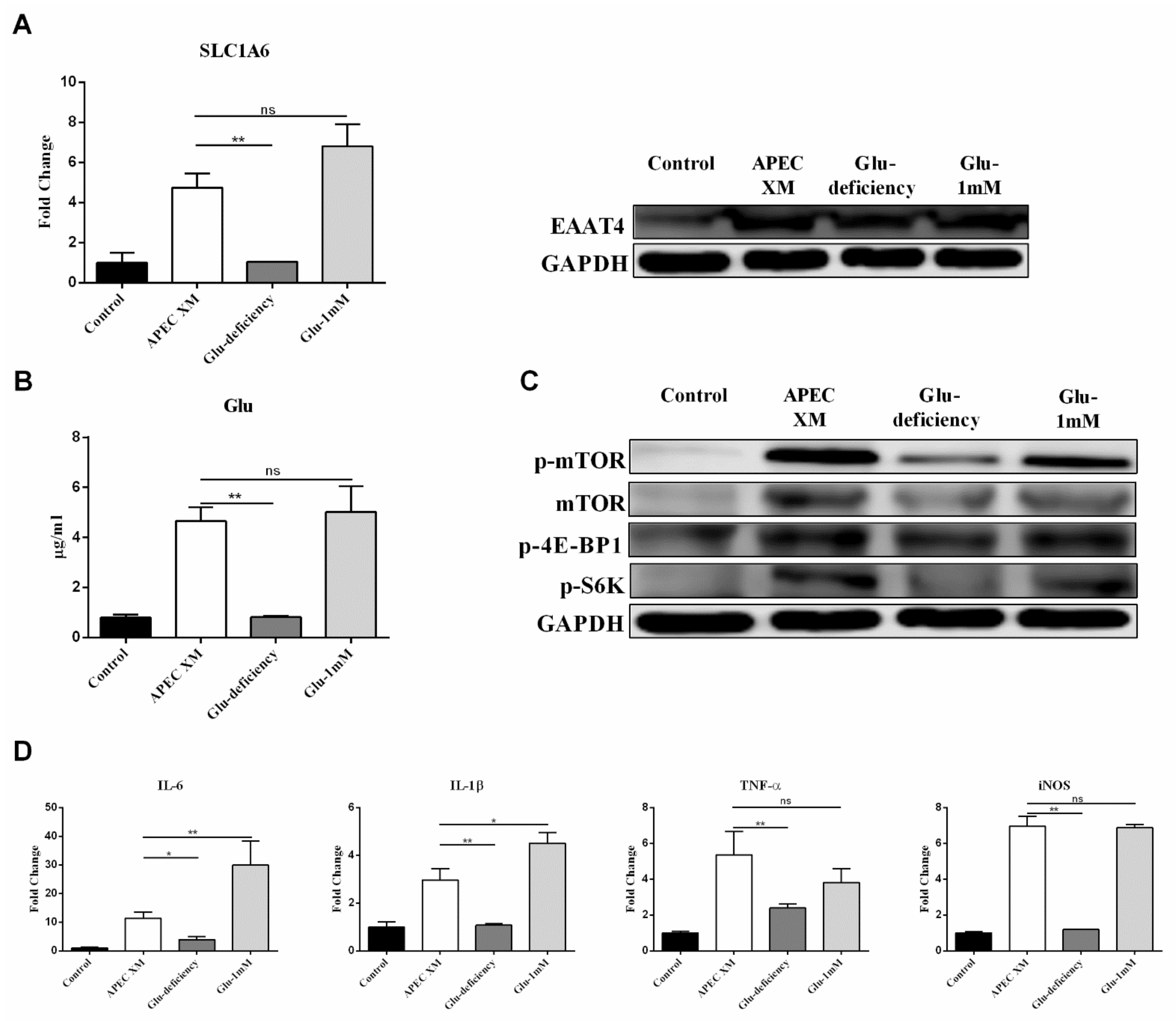
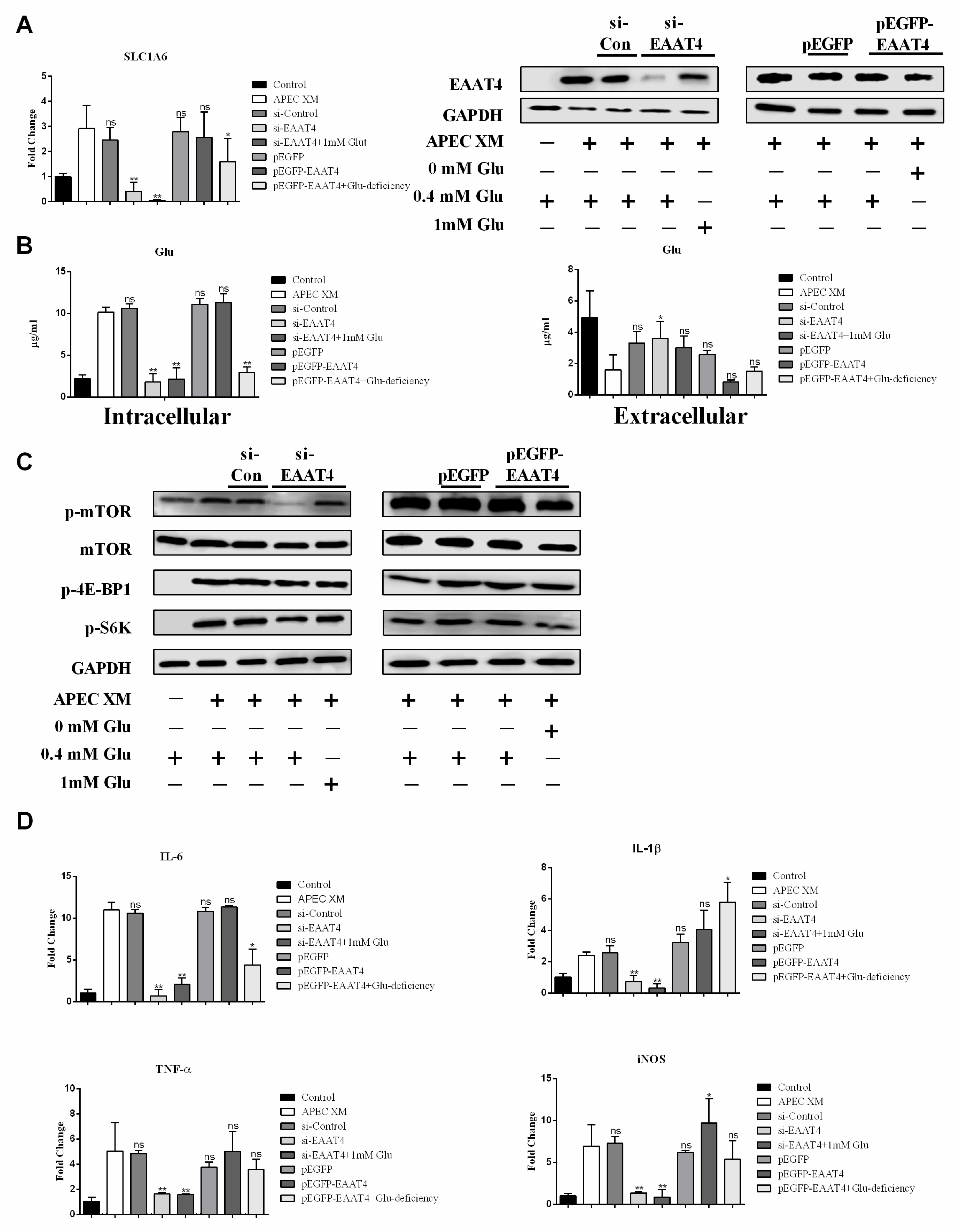
2.5. Glu Involved in APEC XM-Induced the Expression of EAAT4, Activation of mTOR Pathway and Inflammatory Responses in bEnd.3 Cells
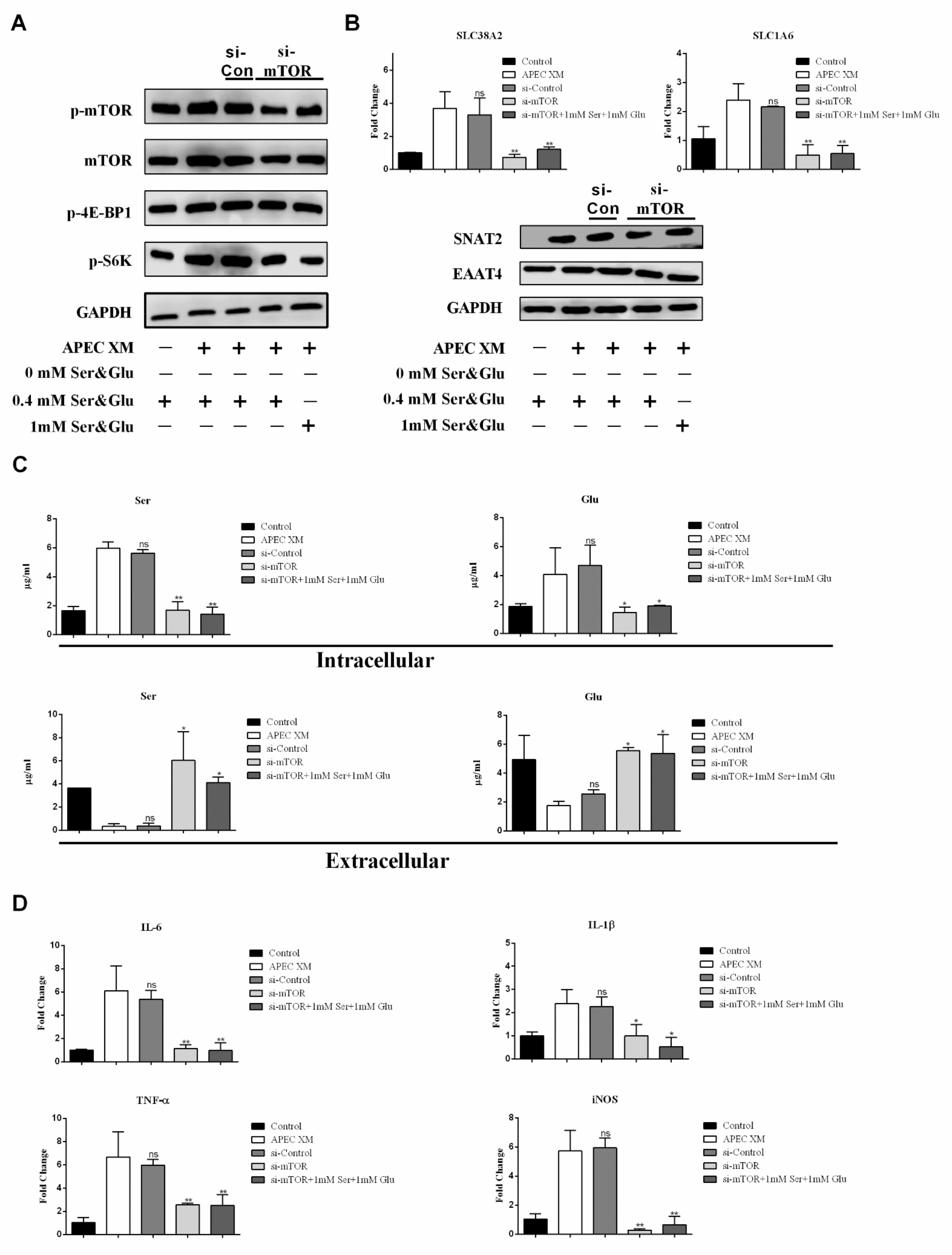
2.6. SNAT2 Promotes Ser Uptake to Activate the mTORC1 Pathway and Inflammatory Responses during APEC XM-Infection
2.7. EAAT4 Promotes Glu Transport to Activate mTORC1 and Inflammatory Responses during APEC XM Infection
2.8. APEC XM Induces Amino Acid-Driven Inflammation through The mTORC1 Pathway
3. Discussion
4. Material and Methods
4.1. Antibodies and Reagents
4.2. bEnd.3 Cells, APEC TW-XM Strain Culture and Infection
4.3. SNAT2, EAAT4 and mTORC1 siRNA Transfection of bEnd.3 Cells
4.4. Plasmids
4.5. Amino Acids Analysis
4.6. RNA Extraction and Quantitative RT-PCR
4.7. Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, C.J.; Chang, W.N.; Huang, L.T.; Huang, S.C.; Chang, Y.C.; Hung, P.L.; Lu, C.H.; Chang, C.S.; Cheng, B.C.; Lee, P.Y.; et al. Bacterial meningitis in infants: The epidemiology, clinical features, and prognostic factors. Brain Dev. 2004, 26, 168–175. [Google Scholar] [CrossRef]
- Roine, I.; Peltola, H.; Fernandez, J.; Zavala, I.; Mata, A.G.; Ayala, S.G.; Arbo, A.; Bologna, R.; Mino, G.; Goyo, J.; et al. Influence of admission findings on death and neurological outcome from childhood bacterial meningitis. Clin. Infect. Dis. 2008, 46, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Baraff, L.J.; Lee, S.I.; Schriger, D.L. Outcomes of bacterial meningitis in children: A meta-analysis. Pediatr Infect Dis. J. 1993, 12, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Grimwood, K.; Anderson, P.; Anderson, V.; Tan, L.; Nolan, T. Twelve year outcomes following bacterial meningitis: Further evidence for persisting effects. Arch. Dis. Child. 2000, 83, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Pathogenesis of bacterial meningitis: From bacteraemia to neuronal injury. Nat. Rev. Neurosci. 2003, 4, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.W.; An, C.X.; Bao, Y.L.; Zhao, X.F.; Jernigan, R.L.; Lithio, A.; Nettleton, D.; Li, L.; Wurtele, E.S.; Nolan, L.K.; et al. ArcA Controls Metabolism, Chemotaxis, and Motility Contributing to the Pathogenicity of Avian Pathogenic Escherichia coli. Infect. Immun. 2015, 83, 3545–3554. [Google Scholar] [CrossRef]
- Ewers, C.; Li, G.W.; Wilking, H.; Kiessling, S.; Alt, K.; Antao, E.M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Int. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef]
- Ewers, C.; Antao, E.M.; Diehl, I.; Philipp, H.C.; Wieler, L.H. Intestine and Environment of the Chicken as Reservoirs for Extraintestinal Pathogenic Escherichia coli Strains with Zoonotic Potential. Appl. Environ. Microbiol. 2009, 75, 184–192. [Google Scholar] [CrossRef]
- Mitchell, N.M.; Johnson, J.R.; Johnston, B.; Curtiss, R.; Mellataa, M. Zoonotic Potential of Escherichia coli Isolates from Retail Chicken Meat Products and Eggs. Appl. Environ. Microbiol. 2015, 81, 1177–1187. [Google Scholar] [CrossRef]
- Krishnan, S.; Chang, A.C.; Hodges, J.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Nicholson, B.A.; Nolan, L.K.; Prasadarao, N.V. Serotype O18 avian pathogenic and neonatal meningitis Escherichia coli strains employ similar pathogenic strategies for the onset of meningitis. Virulence 2015, 6, 777–786. [Google Scholar] [CrossRef]
- Wylezinski, L.S.; Hawiger, J. Interleukin 2 Activates Brain Microvascular Endothelial Cells Resulting in Destabilization of Adherens Junctions. J. Biol. Chem. 2016, 291, 22913–22923. [Google Scholar] [CrossRef]
- Al-Obaidi, M.M.J.; Desa, M.N.M. Mechanisms of Blood Brain Barrier Disruption by Different Types of Bacteria, and Bacterial-Host Interactions Facilitate the Bacterial Pathogen Invading the Brain. Cell. Mol. Neurobiol. 2018, 38, 1349–1368. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Winkler, F.; Koedel, U.; Kastenbauer, S.; Pfister, H.W. Differential expression of nitric oxide synthases in bacterial meningitis: Role of the inducible isoform for blood-brain barrier breakdown. J. Infect Dis. 2001, 183, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Broer, S.; Broer, A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017, 474, 1935–1963. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J.; et al. Amino Acids As Mediators of Metabolic Cross Talk between Host and Pathogen. Front. Immunol. 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.K.; Liao, Y.X.; Ding, X.Y.; Jiang, Y.; Yan, J.M.; Xia, Y.Y.; Tan, B.; Lin, Z.J.; Duan, J.L.; Jia, X.M.; et al. Slc6a13 deficiency promotes Th17 responses during intestinal bacterial infection. Mucosal Immunol. 2019, 12, 531–544. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Xiao, H.; Chen, S.; Liu, G.; Tan, B.; Li, N.; Peng, Y.; Li, T.; Zeng, B.; et al. Intestinal Microbiota-Derived GABA Mediates Interleukin-17 Expression during Enterotoxigenic Escherichia coli Infection. Front. Immunol. 2016, 7, 685. [Google Scholar] [CrossRef]
- Cabezas-Llobet, N.; Camprubi, S.; Garcia, B.; Alberch, J.; Xifro, X. Human alpha 1-antitrypsin protects neurons and glial cells against oxygen and glucose deprivation through inhibition of interleukins expression. Bba-Gen Subj. 2018, 1862, 1852–1861. [Google Scholar] [CrossRef]
- Guerra-Romero, L.; Tureen, J.H.; Fournier, M.A.; Makrides, V.; Tauber, M.G. Amino acids in cerebrospinal and brain interstitial fluid in experimental pneumococcal meningitis. Pediatr. Res. 1993, 33, 510–513. [Google Scholar] [CrossRef]
- Buondonno, I.; Sassi, F.; Carignano, G.; Dutto, F.; Ferreri, C.; Pili, F.G.; Massaia, M.; Nisoli, E.; Ruocco, C.; Porrino, P.; et al. From mitochondria to healthy aging: The role of branched-chain amino acids treatment: MATeR a randomized study. Clin. Nutr. 2019, 39, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.K.; Zou, L.X.; Li, N.Z.; Wang, Y.; Liu, G.; Peng, Y.Y.; Ding, J.N.; Cai, L.C.; Yin, Y.L.; Wu, G.Y. Dietary arginine supplementation enhances immune responses to inactivated Pasteurella multocida vaccination in mice. Br. J. Nutr. 2013, 109, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Chen, S.; Yin, J.; Duan, J.; Li, T.; Liu, G.; Feng, Z.; Tan, B.; Yin, Y.; Wu, G. Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J Nutr. 2014, 144, 988–995. [Google Scholar] [CrossRef]
- Ren, W.K.; Duan, J.L.; Yin, J.; Liu, G.; Cao, Z.; Xiong, X.; Chen, S.; Li, T.J.; Yin, Y.L.; Hou, Y.Q.; et al. Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids. 2014, 46, 2403–2413. [Google Scholar] [CrossRef]
- Pupo, E.; Avanzato, D.; Middonti, E.; Bussolino, F.; Lanzetti, L. KRAS-Driven Metabolic Rewiring Reveals Novel Actionable Targets in Cancer. Front. Oncol. 2019, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, M.; Law, D.; Dowd, G.C.; Ireton, K. Host Serine/Threonine Kinases mTOR and Protein Kinase C-alpha Promote InlB-Mediated Entry of Listeria monocytogenes. Infect. Immun. 2017, 85, e00087-17. [Google Scholar] [CrossRef]
- Li, K.; Wei, X.M.; Zhang, L.B.; Chi, H.; Yang, J.L. Raptor/mTORC1 Acts as a Modulatory Center to Regulate Anti-bacterial Immune Response in Rockfish. Front Immunol 2019, 10, 2953. [Google Scholar] [CrossRef]
- Manifava, M.; Smith, M.; Rotondo, S.; Walker, S.; Niewczas, I.; Zoncu, R.; Clark, J.; Ktistakis, N.T. Dynamics of mTORC1 activation in response to amino acids. eLife 2016, 5, e19960. [Google Scholar] [CrossRef] [PubMed]
- Van Skike, C.E.; Jahrling, J.B.; Olson, A.B.; Sayre, N.L.; Hussong, S.A.; Ungvari, Z.; Lechleiter, J.D.; Galvan, V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H693–H703. [Google Scholar] [CrossRef]
- Palmer, C.S.; Cherry, C.L.; Sada-Ovalle, I.; Singh, A.; Crowe, S.M. Glucose Metabolism in T Cells and Monocytes: New Perspectives in HIV Pathogenesis. Ebiomedicine 2016, 6, 31–41. [Google Scholar] [CrossRef]
- Sanman, L.E.; Qian, Y.; Eisle, N.A.; Ng, T.M.; Van der Linden, W.A.; Monack, D.M.; Weerapana, E.; Bogyo, M. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. eLife 2016, 5, e13663. [Google Scholar] [CrossRef]
- Shi, L.B.; Salamon, H.; Eugenin, E.A.; Pine, R.; Cooper, A.; Gennaro, M.L. Infection with Mycobacterium tuberculosis induces the Warburg effect in mouse lungs. Sci. Rep. 2015, 5, 18176. [Google Scholar] [CrossRef]
- Xia, L.; Dai, L.; Yang, Q. Transmissible gastroenteritis virus infection decreases arginine uptake by downregulating CAT-1 expression. Vet. Res. 2018, 49, 95. [Google Scholar] [CrossRef]
- Dato, S.; Hoxha, E.; Crocco, P.; Iannone, F.; Passarino, G.; Rose, G. Amino acids and amino acid sensing: Implication for aging and diseases. Biogerontology 2019, 20, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Hyde, R.; Taylor, P.M.; Hundal, H.S. Amino acid transporters: Roles in amino acid sensing and signalling in animal cells. Biochem. J. 2003, 373, 1–18. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Duan, J.; Liu, G.; Tan, B.; Yang, G.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. mTORC1 signaling and IL-17 expression: Defining pathways and possible therapeutic targets. Eur. J. Immunol. 2016, 46, 291–299. [Google Scholar] [CrossRef]
- Park, J.C.; Baik, S.H.; Han, S.H.; Cho, H.J.; Choi, H.; Kim, H.J.; Choi, H.; Lee, W.; Kim, D.K.; Mook-Jung, I. Annexin A1 restores Abeta1-42 -induced blood-brain barrier disruption through the inhibition of RhoA-ROCK signaling pathway. Aging Cell 2017, 16, 149–161. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Yuan, C.; Zhang, D.; Ma, Y.; Ding, X.Y.; Zhu, G.Q. BHV-1 induced oxidative stress contributes to mitochondrial dysfunction in MDBK cells. Vet. Res. 2016, 47, 47. [Google Scholar] [CrossRef]
- Chi, F.; Wang, L.; Zheng, X.Y.; Wu, C.H.; Jong, A.; Sheard, M.A.; Shi, W.; Huang, S.H. Meningitic Escherichia coli K1 Penetration and Neutrophil Transmigration Across the Blood-Brain Barrier are Modulated by Alpha7 Nicotinic Receptor. PLoS ONE 2011, 6, e25016. [Google Scholar] [CrossRef][Green Version]
- Liu, W.T.; Lv, Y.J.; Yang, R.C.; Fu, J.Y.; Liu, L.; Wang, H.; Cao, Q.; Tan, C.; Chen, H.C.; Wang, X.R. New insights into meningitic Escherichia coli infection of brain microvascular endothelial cells from quantitative proteomics analysis. J. Neuroinf. 2018, 15, 291. [Google Scholar] [CrossRef]
- Wessler, L.B.; de Miranda Ramos, V.; Bittencourt Pasquali, M.A.; Fonseca Moreira, J.C.; de Oliveira, J.; Scaini, G.; Streck, E.L. Administration of branched-chain amino acids increases the susceptibility to lipopolysaccharide-induced inflammation in young Wistar rats. Int. J. Dev. Neurosci. 2019, 78, 210–214. [Google Scholar] [CrossRef]
- Carosi, J.M.; Sargeant, T.J. Rapamycin and Alzheimer disease: A double-edged sword? Autophagy 2019, 15, 1460–1462. [Google Scholar] [CrossRef]
- Haas, L.T.; Strittmatter, S.M. Oligomers of Amyloid Prevent Physiological Activation of the Cellular Prion Protein-Metabotropic Glutamate Receptor 5 Complex by Glutamate in Alzheimer Disease. J. Biol. Chem. 2016, 291, 17112–17121. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharm. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Dalton, W.B. Parkin on serine: A Parkinson disease gene suppresses serine synthesis in cancer. J. Clin. Invest. 2020, 130, 2820–2822. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, M.; Choi, Y.; Shin, T. Upregulation of Cathepsins in Olfactory Bulbs Is Associated with Transient Olfactory Dysfunction in Mice with Experimental Autoimmune Encephalomyelitis. Mol. Neurobiol. 2020, 57, 3412–3423. [Google Scholar] [CrossRef]
- Gironi, M.; Dalla-Costa, G.; Frigo, M.; Rovaris, M.; Clerici, M.; Bazzini, C.; Andreoni, S.; Comi, G.; Furlan, R.; Ferrarese, C.; et al. Platelet glutamate uptake and Th1 cells inversely correlate in relapsing/remitting and in progressive multiple sclerosis. Mult. Scler. Relat. Dis. 2020, 41, 102007. [Google Scholar] [CrossRef]
- Fan, W.W.; Ye, Y.F.; Chen, Z.; Shao, Y.N.; Xie, X.L.; Zhang, W.W.; Liu, H.P.; Li, C.H. Metabolic product response profiles of Cherax quadricarinatus towards white spot syndrome virus infection. Dev. Comp. Immunol. 2016, 61, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.K.; Liu, S.P.; Chen, S.; Zhang, F.M.; Li, N.Z.; Yin, J.; Peng, Y.Y.; Wu, L.; Liu, G.; Yin, Y.L.; et al. Dietary L-glutamine supplementation increases Pasteurella multocida burden and the expression of its major virulence factors in mice. Amino Acids 2013, 45, 947–955. [Google Scholar] [CrossRef]
- Lecointre, M.; Hauchecorne, M.; Chaussivert, A.; Marret, S.; Leroux, P.; Jegou, S.; Leroux-Nicollet, I.; Gonzalez, B.J.; Henry, V.J. The efficiency of glutamate uptake differs between neonatal and adult cortical microvascular endothelial cells. J. Cereb. Blood Flow Metab. 2014, 34, 764–767. [Google Scholar] [CrossRef]
- Scott, G.S.; Bowman, S.R.; Smith, T.; Flower, R.J.; Bolton, C. Glutamate-stimulated peroxynitrite production in a brain-derived endothelial cell line is dependent on N-methyl-D-aspartate (NMDA) receptor activation. Biochem. Pharmacol. 2007, 73, 228–236. [Google Scholar] [CrossRef][Green Version]
- Williams, B.L.; Yaddanapudi, K.; Hornig, M.; Lipkin, W.I. Spatiotemporal analysis of Purkinje cell degeneration relative to parasagittal expression domains in a model of neonatal viral infection. J. Virol. 2007, 81, 2675–2687. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kawagoe, Y.; Matsuda, T.; Ebihara, I.; Koide, H. Effects of polymyxin B-immobilized fiber hemoperfusion on amino acid imbalance in septic encephalopathy. Blood Purif. 2003, 21, 282–286. [Google Scholar] [CrossRef]
- Macrez, R.; Stys, P.K.; Vivien, D.; Lipton, S.A.; Docagne, F. Mechanisms of glutamate toxicity in multiple sclerosis: Biomarker and therapeutic opportunities. Lancet Neurol 2016, 15, 1089–1102. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Misra, U.K.; Kalita, J. A study of glutamate levels, NR1, NR2A, NR2B receptors and oxidative stress in rat model of Japanese encephalitis. Physiol Behav 2017, 171, 256–267. [Google Scholar] [CrossRef]
- Broer, S. The SLC38 family of sodium-amino acid co-transporters. Pflug. Arch. 2014, 466, 155–172. [Google Scholar] [CrossRef]
- Shigeri, Y.; Shimamoto, K.; Yasuda-Kamatani, Y.; Seal, R.P.; Yumoto, N.; Nakajima, T.; Amara, S.G. Effects of threo-beta-hydroxyaspartate derivatives on excitatory amino acid transporters (EAAT4 and EAAT5). J. Neurochem. 2001, 79, 297–302. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basilio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-kappaB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Srivastava, M.; Saqib, U.; Liu, D.; Faisal, S.M.; Sugathan, S.; Bishnoi, S.; Baig, M.S. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int. Immunopharmacol. 2016, 40, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Laufenberg, L.J.; Pruznak, A.M.; Navaratnarajah, M.; Lang, C.H. Sepsis-induced changes in amino acid transporters and leucine signaling via mTOR in skeletal muscle. Amino Acids 2014, 46, 2787–2798. [Google Scholar] [CrossRef]
- Pan, H.J.; Zhong, X.P.; Lee, S. Sustained activation of mTORC1 in macrophages increases AMPK alpha-dependent autophagy to maintain cellular homeostasis. BMC Biochem. 2016, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.H.; Li, F.N.; Tan, K.R.; Liu, H.N.; Li, Y.H.; Liu, Y.Y.; Kong, X.F.; Tang, Y.L.; Wu, G.Y.; Yin, Y.L. Key mediators of intracellular amino acids signaling to mTORC1 activation. Amino Acids 2015, 47, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Yecies, J.L.; Zhang, H.H.; Howell, J.J.; Nicholatos, J.; Harputlugil, E.; Bronson, R.T.; Kwiatkowski, D.J.; Manning, B.D. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci. Signal 2012, 5, ra24. [Google Scholar] [CrossRef] [PubMed]
- Mazelin, L.; Panthu, B.; Nicot, A.S.; Belotti, E.; Tintignac, L.; Teixeira, G.; Zhang, Q.; Risson, V.; Baas, D.; Delaune, E.; et al. mTOR inactivation in myocardium from infant mice rapidly leads to dilated cardiomyopathy due to translation defects and p53/JNK-mediated apoptosis. J. Mol. Cell Cardiol. 2016, 97, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Golub, T.R.; Sabatini, D.M. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol. Cell Biol. 2002, 22, 5575–5584. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yin, J.; Wu, M.; Liu, G.; Yang, G.; Xion, Y.; Su, D.; Wu, L.; Li, T.; Chen, S.; et al. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS ONE 2014, 9, e88335. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Xu, S.; Wang, Y.; Bin, P.; Zhu, G. The Amino Acid-mTORC1 Pathway Mediates APEC TW-XM-Induced Inflammation in bEnd.3 Cells. Int. J. Mol. Sci. 2021, 22, 9245. https://doi.org/10.3390/ijms22179245
Zhang D, Xu S, Wang Y, Bin P, Zhu G. The Amino Acid-mTORC1 Pathway Mediates APEC TW-XM-Induced Inflammation in bEnd.3 Cells. International Journal of Molecular Sciences. 2021; 22(17):9245. https://doi.org/10.3390/ijms22179245
Chicago/Turabian StyleZhang, Dong, Shu Xu, Yiting Wang, Peng Bin, and Guoqiang Zhu. 2021. "The Amino Acid-mTORC1 Pathway Mediates APEC TW-XM-Induced Inflammation in bEnd.3 Cells" International Journal of Molecular Sciences 22, no. 17: 9245. https://doi.org/10.3390/ijms22179245
APA StyleZhang, D., Xu, S., Wang, Y., Bin, P., & Zhu, G. (2021). The Amino Acid-mTORC1 Pathway Mediates APEC TW-XM-Induced Inflammation in bEnd.3 Cells. International Journal of Molecular Sciences, 22(17), 9245. https://doi.org/10.3390/ijms22179245




