Evaluation of Loco-Regional Skin Toxicity Induced by an In Situ Forming Depot after a Single Subcutaneous Injection at Different Volumes and Flow Rates in Göttingen Minipigs
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Test Item
2.2. In Vivo Administration of the Test Item
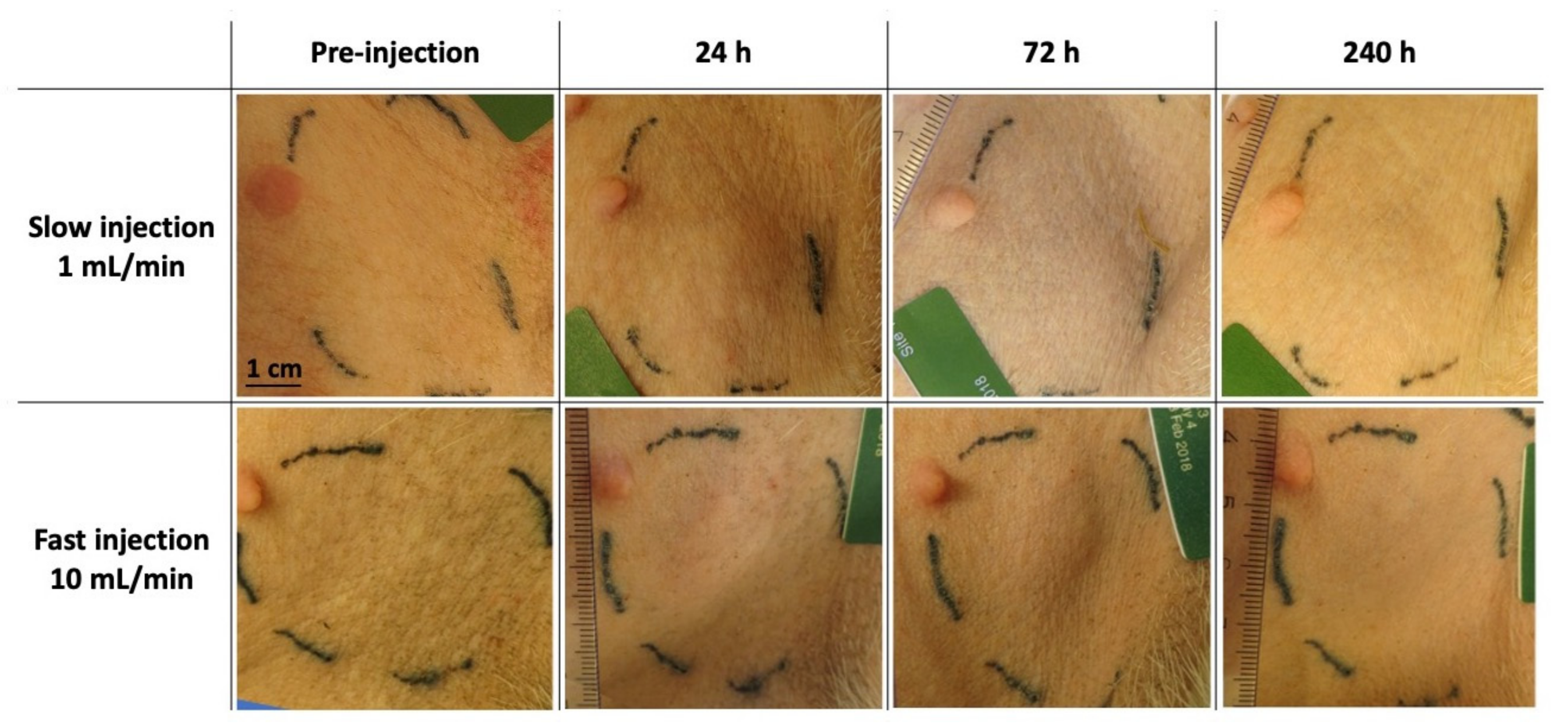
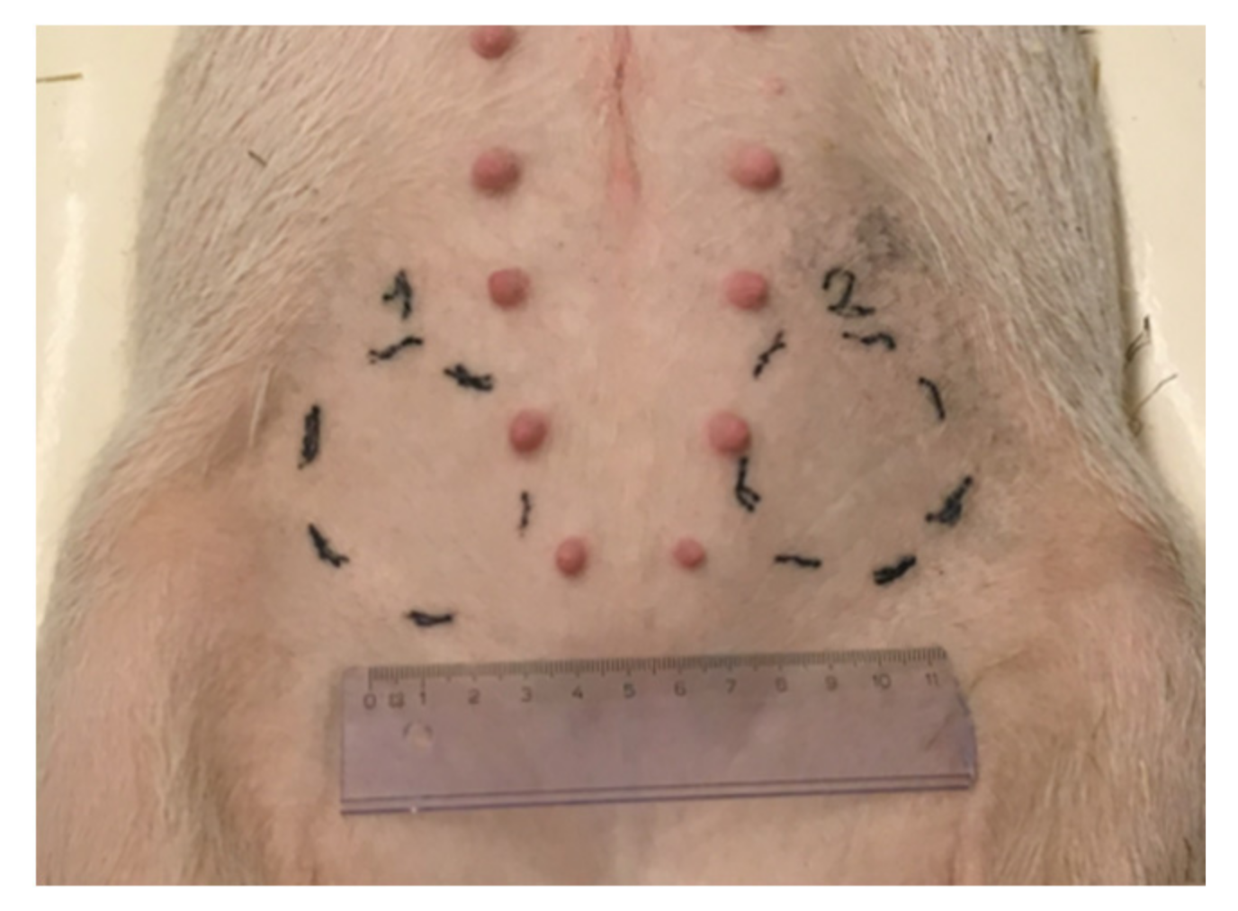
2.3. Macroscopic Observation of Loco-Regional Skin Tolerance
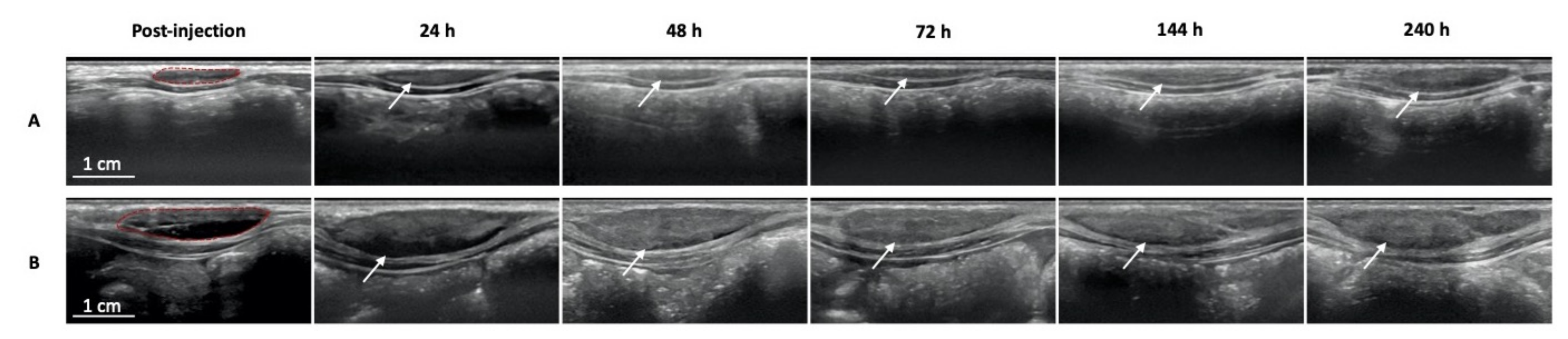
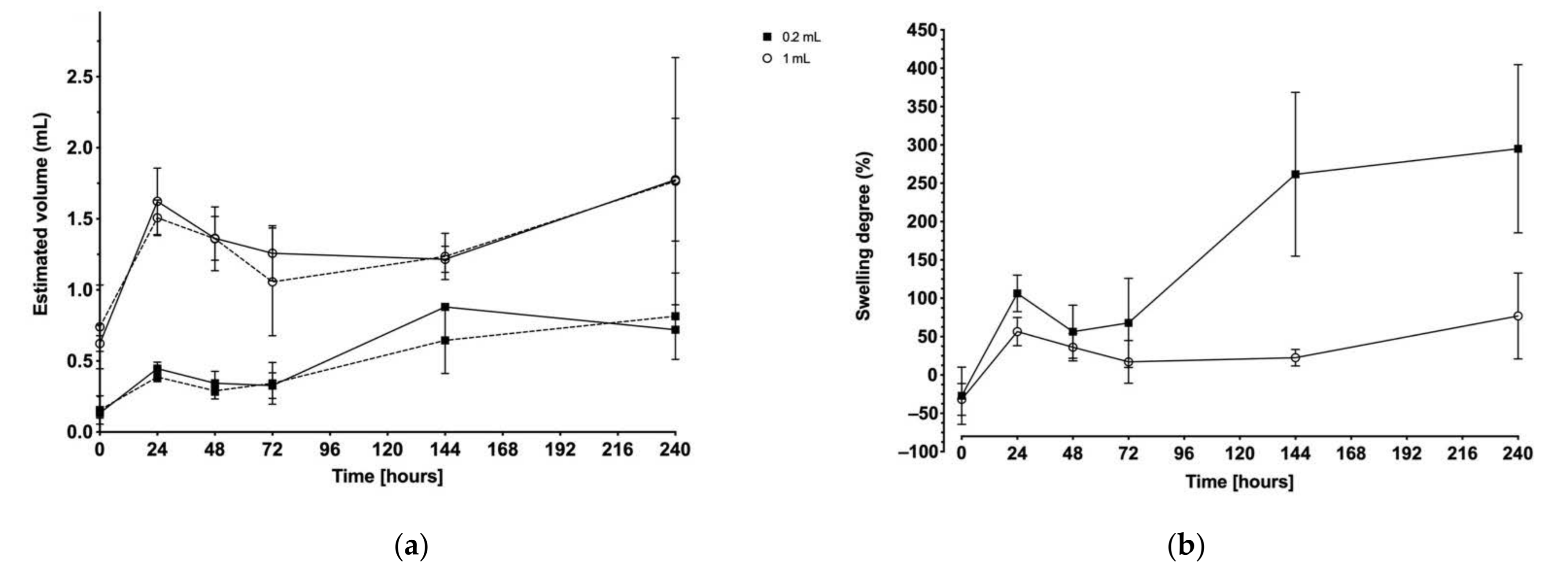
2.4. Ultrasound Imaging
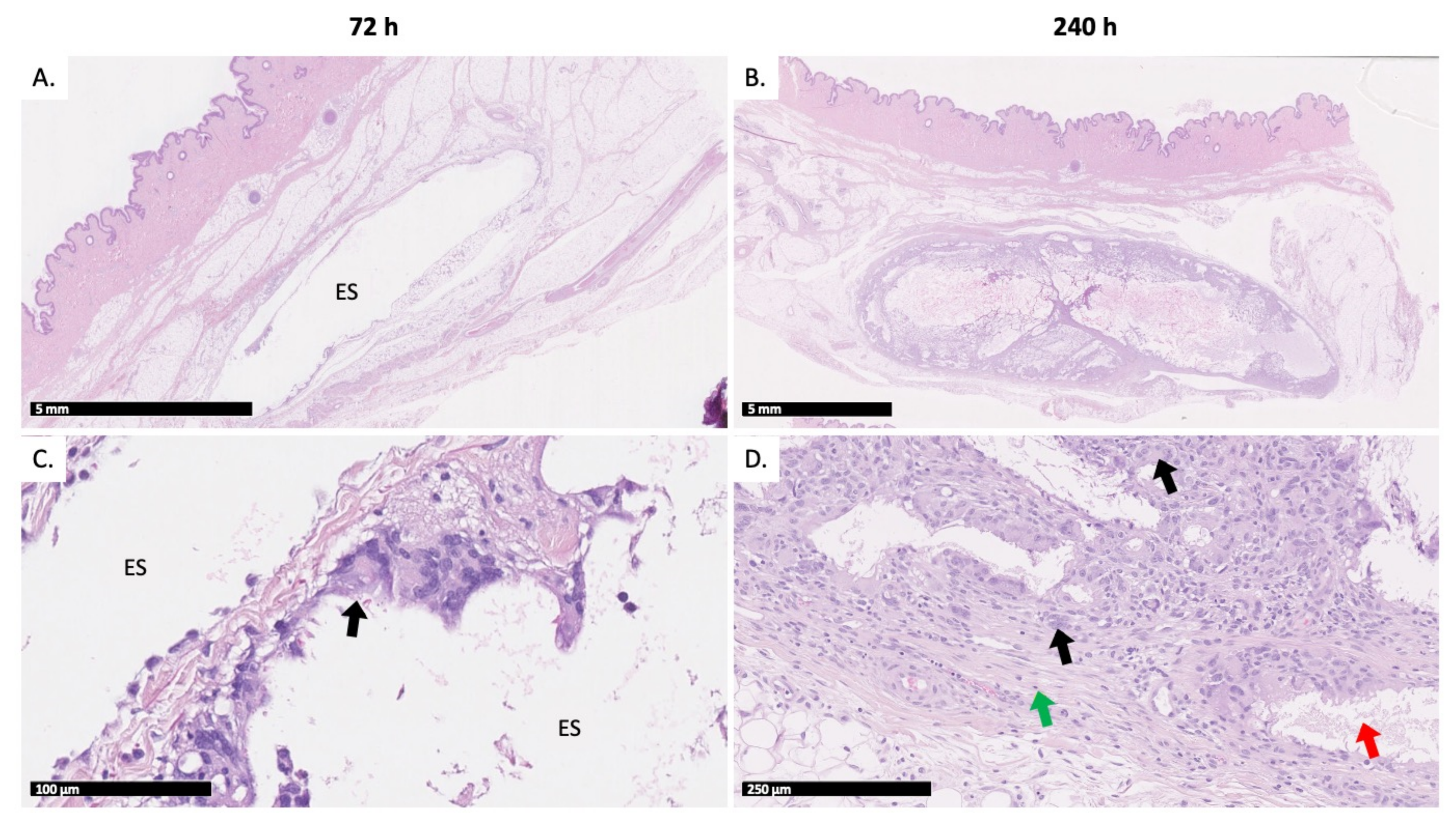
2.5. Histopathologic Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Test Item
4.3. Characterization of the Test Item
4.3.1. Dynamic Viscosity Determination
4.3.2. Assessment of Injectability
4.3.3. Bacterial Endotoxins Test
4.4. In Vivo Study Design
4.4.1. Injection Site Selection
- Group 1: 0.2 mL of test item (Animals #1 to #4);
- Group 2: 1 mL of test item (Animals #5 to #8).
- Site 1: target 1 mL/min injection flow rate;
- Site 2: target 10 mL/min injection flow rate.
4.4.2. Injection of the Test Item
4.5. Assessment of Loco-Regional Skin Tolerance
4.6. Ultrasound Imaging
4.7. Histopathologic Evaluation
4.8. Statistical Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.S. The origins and evolution of “controlled” drug delivery systems. J. Control. Release 2008, 132, 153–163. [Google Scholar] [CrossRef]
- Dash, A.K.; Cudworth, G.C., 2nd. Therapeutic applications of implantable drug delivery systems. J. Pharmacol. Toxicol. Methods 1998, 40, 1–12. [Google Scholar] [CrossRef]
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control. Release 2014, 181, 1–10. [Google Scholar] [CrossRef]
- Martín del Valle, E.M.; Galán, M.A.; Carbonell, R.G. Drug Delivery Technologies: The Way Forward in the New Decade. Ind. Eng. Chem. Res. 2009, 48, 2475–2486. [Google Scholar] [CrossRef]
- Kempe, S.; Mader, K. In situ forming implants—An attractive formulation principle for parenteral depot formulations. J. Control. Release 2012, 161, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, A.; Amsden, B. Biodegradable injectable in situ forming drug delivery systems. J. Control. Release 2002, 80, 9–28. [Google Scholar] [CrossRef]
- Potta, T.; Chun, C.; Song, S.C. Chemically crosslinkable thermosensitive polyphosphazene gels as injectable materials for biomedical applications. Biomaterials 2009, 30, 6178–6192. [Google Scholar] [CrossRef] [PubMed]
- de Jong, S.J.; De Smedt, S.C.; Demeester, J.; van Nostrum, C.F.; Kettenes-van den Bosch, J.J.; Hennink, W.E. Biodegradable hydrogels based on stereocomplex formation between lactic acid oligomers grafted to dextran. J. Control. Release 2001, 72, 47–56. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Thakur, R.R.; McMillan, H.L.; Jones, D.S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 2014, 176, 8–23. [Google Scholar] [CrossRef]
- Shah, N.H.; Railkar, A.S.; Chen, F.C.; Tarantino, R.; Kumar, S.; Murjani, M.; Palmer, D.; Infeld, M.H.; Malick, A.W. A biodegradable injectable implant for delivering micro and macromolecules using poly (lactic-co-glycolic) acid (PLGA) copolymers. J. Control. Release 1993, 27, 139–147. [Google Scholar] [CrossRef]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef]
- Gold, R.; Rieckmann, P.; Chang, P.; Abdalla, J.; Group, P.S. The long-term safety and tolerability of high-dose interferon beta-1a in relapsing-remitting multiple sclerosis: 4-year data from the PRISMS study. Eur. J. Neurol. 2005, 12, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Paquette, S.M.; Dawit, H.; Hickey, M.B.; Merisko-Liversidge, E.; Almarsson, O.; Deaver, D.R. Long-acting atypical antipsychotics: Characterization of the local tissue response. Pharm. Res. 2014, 31, 2065–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praestmark, K.A.; Jensen, C.B.; Stallknecht, B.; Madsen, N.B.; Kildegaard, J. Skin blood perfusion and cellular response to insertion of insulin pen needles with different diameters. J. Diabetes Sci. Technol. 2014, 8, 752–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, M.; Rasmussen, C.H.; Refsgaard, H.H.; Pedersen, K.M.; Kirk, R.K.; Poulsen, M.; Feidenhans’l, R. Spatial distribution of soluble insulin in pig subcutaneous tissue: Effect of needle length, injection speed and injected volume. Eur. J. Pharm. Sci. 2015, 79, 96–101. [Google Scholar] [CrossRef]
- Chan, H. Effects of injection duration on site-pain intensity and bruising associated with subcutaneous heparin. J. Adv. Nurs. 2001, 35, 882–892. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Lee, S.J. Effective method for drug injection into subcutaneous tissue. Sci. Rep. 2017, 7, 9613. [Google Scholar] [CrossRef]
- Doughty, D.V.; Clawson, C.Z.; Lambert, W.; Subramony, J.A. Understanding Subcutaneous Tissue Pressure for Engineering Injection Devices for Large-Volume Protein Delivery. J. Pharm. Sci. 2016, 105, 2105–2113. [Google Scholar] [CrossRef]
- Gaudriault, G. EP3257498B1, Biodegradable Drug Delivery Compositions; MedinCell SA: Jacou, France, 2011. [Google Scholar]
- Roberge, C.; Cros, J.M.; Serindoux, J.; Cagnon, M.E.; Samuel, R.; Vrlinic, T.; Berto, P.; Rech, A.; Richard, J.; Lopez-Noriega, A. BEPO(R): Bioresorbable diblock mPEG-PDLLA and triblock PDLLA-PEG-PDLLA based in situ forming depots with flexible drug delivery kinetics modulation. J. Control. Release 2020, 319, 416–427. [Google Scholar] [CrossRef]
- Leconet, W.; Liu, H.; Guo, M.; Le Lamer-Dechamps, S.; Molinier, C.; Kim, S.; Vrlinic, T.; Oster, M.; Liu, F.; Navarro, V.; et al. Anti-PSMA/CD3 Bispecific Antibody Delivery and Antitumor Activity Using a Polymeric Depot Formulation. Mol. Cancer Ther. 2018, 17, 1927–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, W.F.; Bhansali, S.G.; Morris, M.E. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012, 14, 559–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.C.J.; Edwards, G.A.; Martin, D.J.; Huang, H.; Crichton, M.L.; Kendall, M.A.F. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for micro-medical device translation: From mice, rats, rabbits, pigs to humans. Sci. Rep. 2017, 7, 15885. [Google Scholar] [CrossRef] [PubMed]
- Berteau, C.; Filipe-Santos, O.; Wang, T.; Rojas, H.E.; Granger, C.; Schwarzenbach, F. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med. Devices 2015, 8, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Zaybak, A.; Khorshid, L. A study on the effect of the duration of subcutaneous heparin injection on bruising and pain. J. Clin. Nurs. 2008, 17, 378–385. [Google Scholar] [CrossRef]
- Jockel, J.P.; Roebrock, P.; Shergold, O.A. Insulin depot formation in subcutaneoue tissue. J. Diabetes Sci. Technol. 2013, 7, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Watt, R.P.; Khatri, H.; Dibble, A.R.G. Injectability as a function of viscosity and dosing materials for subcutaneous administration. Int. J. Pharm. 2019, 554, 376–386. [Google Scholar] [CrossRef]
- Solorio, L.; Babin, B.M.; Patel, R.B.; Mach, J.; Azar, N.; Exner, A.A. Noninvasive characterization of in situ forming implants using diagnostic ultrasound. J. Control. Release 2010, 143, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Shao, J.; Wang, Y.; Zhang, P.; Chen, X.; Wei, Y. PLA-PEG-PLA and its electroactive tetraaniline copolymer as multi-interactive injectable hydrogels for tissue engineering. Biomacromolecules 2013, 14, 1904–1912. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, J. Long-term glycemic control and prevention of diabetes complications in vivo using oleic acid-grafted-chitosanzinc-insulin complexes incorporated in thermosensitive copolymer. J. Control. Release 2020, 323, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, D.W.; Chung, J.Y.; Shin, S.G.; Kim, S.C.; Heo, D.S.; Kim, N.K.; Bang, Y.J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Wan Kim, S.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control. Release 2001, 72, 191–202. [Google Scholar] [CrossRef]
- Schoenhammer, K.; Boisclair, J.; Schuetz, H.; Petersen, H.; Goepferich, A. Biocompatibility of an injectable in situ forming depot for peptide delivery. J. Pharm. Sci. 2010, 99, 4390–4399. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, S.; Zheng, C.; Liang, W.; Huang, Y. An in situ-forming, solid lipid/PLGA hybrid implant for long-acting antipsychotics. Soft Matter 2011, 7, 5873–5878. [Google Scholar] [CrossRef]
- Braeckman, J.; Michielsen, D. Efficacy and tolerability of 1- and 3-month leuprorelin acetate depot formulations (Eligard®/Depo-Eligard®) for advanced prostate cancer in daily practice: A Belgian prospective non-interventional study. Arch. Med. Sci. 2014, 10, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlmann, C.H.; Gross-Langenhoff, M. Efficacy and Tolerability of Leuprorelin Acetate (Eligard(R)) in Daily Practice in Germany: Pooled Data from 2 Prospective, Non-Interventional Studies with 3- or 6-Month Depot Formulations in Patients with Advanced Prostate Cancer. Urol. Int. 2018, 100, 66–71. [Google Scholar] [CrossRef]
- Royals, M.A.; Fujita, S.M.; Yewey, G.L.; Rodriguez, J.; Schultheiss, P.C.; Dunn, R.L. Biocompatibility of a biodegradable in situ forming implant system in rhesus monkeys. J. Biomed. Mater. Res. 1999, 45, 231–239. [Google Scholar] [CrossRef]
- Kranz, H.; Brazeau, G.A.; Napaporn, J.; Martin, R.L.; Millard, W.; Bodmeier, R. Myotoxicity studies of injectable biodegradable in-situ forming drug delivery systems. Int. J. Pharm. 2001, 212, 11–18. [Google Scholar] [CrossRef]
- Zheng, Y.; Tesar, D.B.; Benincosa, L.; Birnbock, H.; Boswell, C.A.; Bumbaca, D.; Cowan, K.J.; Danilenko, D.M.; Daugherty, A.L.; Fielder, P.J.; et al. Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration. MAbs 2012, 4, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.B.; Solorio, L.; Wu, H.; Krupka, T.; Exner, A.A. Effect of injection site on in situ implant formation and drug release in vivo. J. Control. Release 2010, 147, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Solorio, L.; Olear, A.M.; Zhou, H.; Beiswenger, A.C.; Exner, A.A. Effect of cargo properties on in situ forming implant behavior determined by noninvasive ultrasound imaging. Drug Deliv. Transl. Res. 2012, 2, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, P.D.; Brodbeck, K.J.; McHugh, A.J. Phase inversion dynamics of PLGA solutions related to drug delivery. J. Control. Release 1999, 58, 233–245. [Google Scholar] [CrossRef]
- Liu, H.; Venkatraman, S.S. Cosolvent effects on the drug release and depot swelling in injectable in situ depot-forming systems. J. Pharm. Sci. 2012, 101, 1783–1793. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Siepmann, F.; Siepmann, J. In-situ forming PLGA implants for intraocular dexamethasone delivery. Int. J. Pharm. 2018, 548, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Biological Responses to Materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Fournier, E.; Passirani, C.; Montero-Menei, C.N.; Benoit, J.P. Biocompatibility of implantable synthetic polymeric drug carriers: Focus on brain biocompatibility. Biomaterials 2003, 24, 3311–3331. [Google Scholar] [CrossRef]
- Nyska, A.; Schiffenbauer, Y.S.; Brami, C.T.; Maronpot, R.R.; Ramot, Y. Histopathology of biodegradable polymers: Challenges in interpretation and the use of a novel compact MRI for biocompatibility evaluation. Polym. Adv. Technol. 2014, 25, 461–467. [Google Scholar] [CrossRef]
- Rousselle, S.D.; Ramot, Y.; Nyska, A.; Jackson, N.D. Pathology of Bioabsorbable Implants in Preclinical Studies. Toxicol. Pathol. 2019, 47, 358–378. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Convention, U.S.P. Bacterial Endotoxins Test Stage 6 Harmonization. In USP 35-NF 30; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2012. [Google Scholar]
- Rose, E.H.; Vistnes, L.M.; Ksander, G.A. The panniculus carnosus in the domestic pig. Plast. Reconstr. Surg. 1977, 59, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Allmendinger, A.; Mueller, R.; Schwarb, E.; Chipperfield, M.; Huwyler, J.; Mahler, H.C.; Fischer, S. Measuring tissue back-pressure--in vivo injection forces during subcutaneous injection. Pharm. Res. 2015, 32, 2229–2240. [Google Scholar] [CrossRef] [PubMed]

| H | Viscosity | Injection Force | Endotoxin Level | |
|---|---|---|---|---|
| 1 mL/min Flow Rate | 10 mL/min Flow Rate | |||
| Test item | 625 mPa.s (5) * | 2.5 N (0.6) * | 19.0 N (0.2) * | <2.339 EU/mL |
| Target 1 mL/min | Target 10 mL/min | |||
|---|---|---|---|---|
| Flow Rate | Deviation | Flow Rate | Deviation | |
| Group 1–0.2 mL | 1.0 mL/min (0.0) * | −2% | 5.6 mL/min (1.4) * | −44% |
| Group 2–1 mL | 1.0 mL/min (0.0) * | −2% | 8.0 mL/min (1.0) * | −20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peloso, C.; Trichet, A.-P.; Descotes, J.; Richard, J.; Roberge, C.; Lopez-Noriega, A. Evaluation of Loco-Regional Skin Toxicity Induced by an In Situ Forming Depot after a Single Subcutaneous Injection at Different Volumes and Flow Rates in Göttingen Minipigs. Int. J. Mol. Sci. 2021, 22, 9250. https://doi.org/10.3390/ijms22179250
Peloso C, Trichet A-P, Descotes J, Richard J, Roberge C, Lopez-Noriega A. Evaluation of Loco-Regional Skin Toxicity Induced by an In Situ Forming Depot after a Single Subcutaneous Injection at Different Volumes and Flow Rates in Göttingen Minipigs. International Journal of Molecular Sciences. 2021; 22(17):9250. https://doi.org/10.3390/ijms22179250
Chicago/Turabian StylePeloso, Charlotte, Anne-Pascale Trichet, Jacques Descotes, Joël Richard, Christophe Roberge, and Adolfo Lopez-Noriega. 2021. "Evaluation of Loco-Regional Skin Toxicity Induced by an In Situ Forming Depot after a Single Subcutaneous Injection at Different Volumes and Flow Rates in Göttingen Minipigs" International Journal of Molecular Sciences 22, no. 17: 9250. https://doi.org/10.3390/ijms22179250
APA StylePeloso, C., Trichet, A.-P., Descotes, J., Richard, J., Roberge, C., & Lopez-Noriega, A. (2021). Evaluation of Loco-Regional Skin Toxicity Induced by an In Situ Forming Depot after a Single Subcutaneous Injection at Different Volumes and Flow Rates in Göttingen Minipigs. International Journal of Molecular Sciences, 22(17), 9250. https://doi.org/10.3390/ijms22179250






