Endothelial Dysfunction Accelerates Impairment of Mitochondrial Function in Ageing Kidneys via Inflammasome Activation
Abstract
:1. Introduction
2. Results
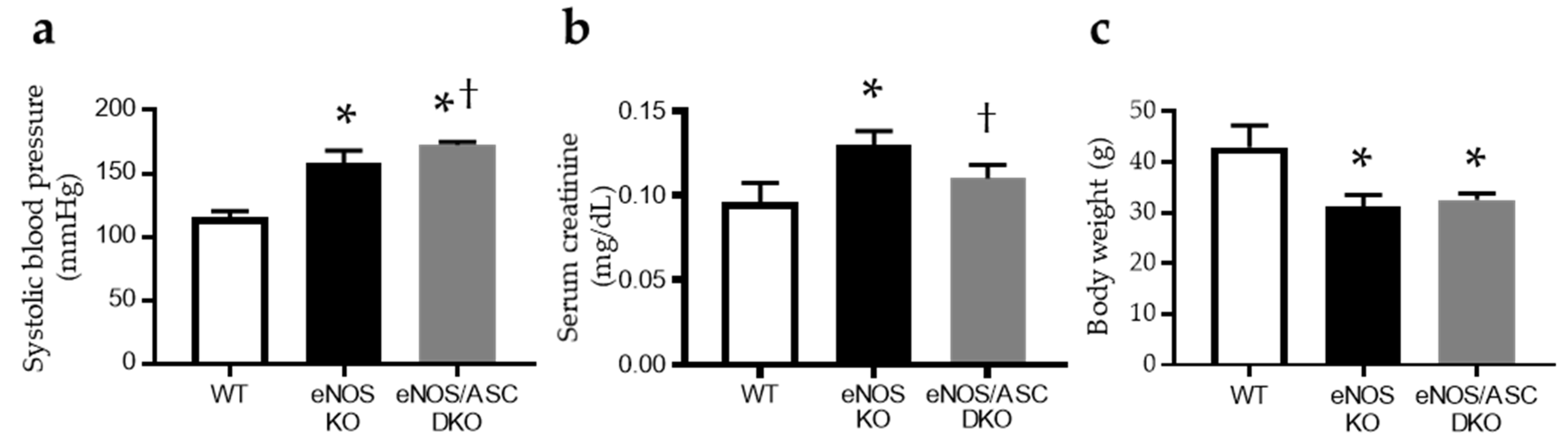
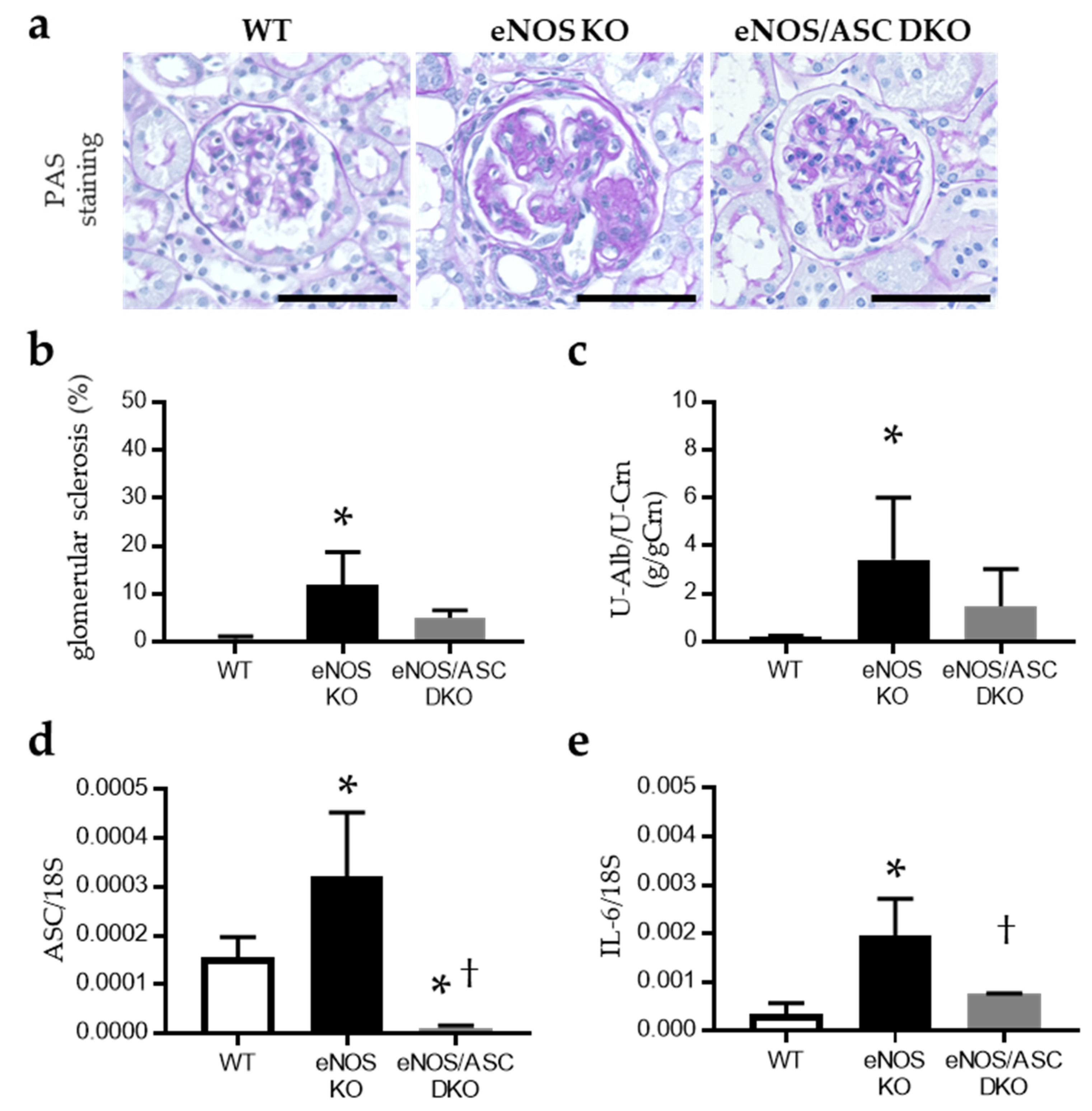
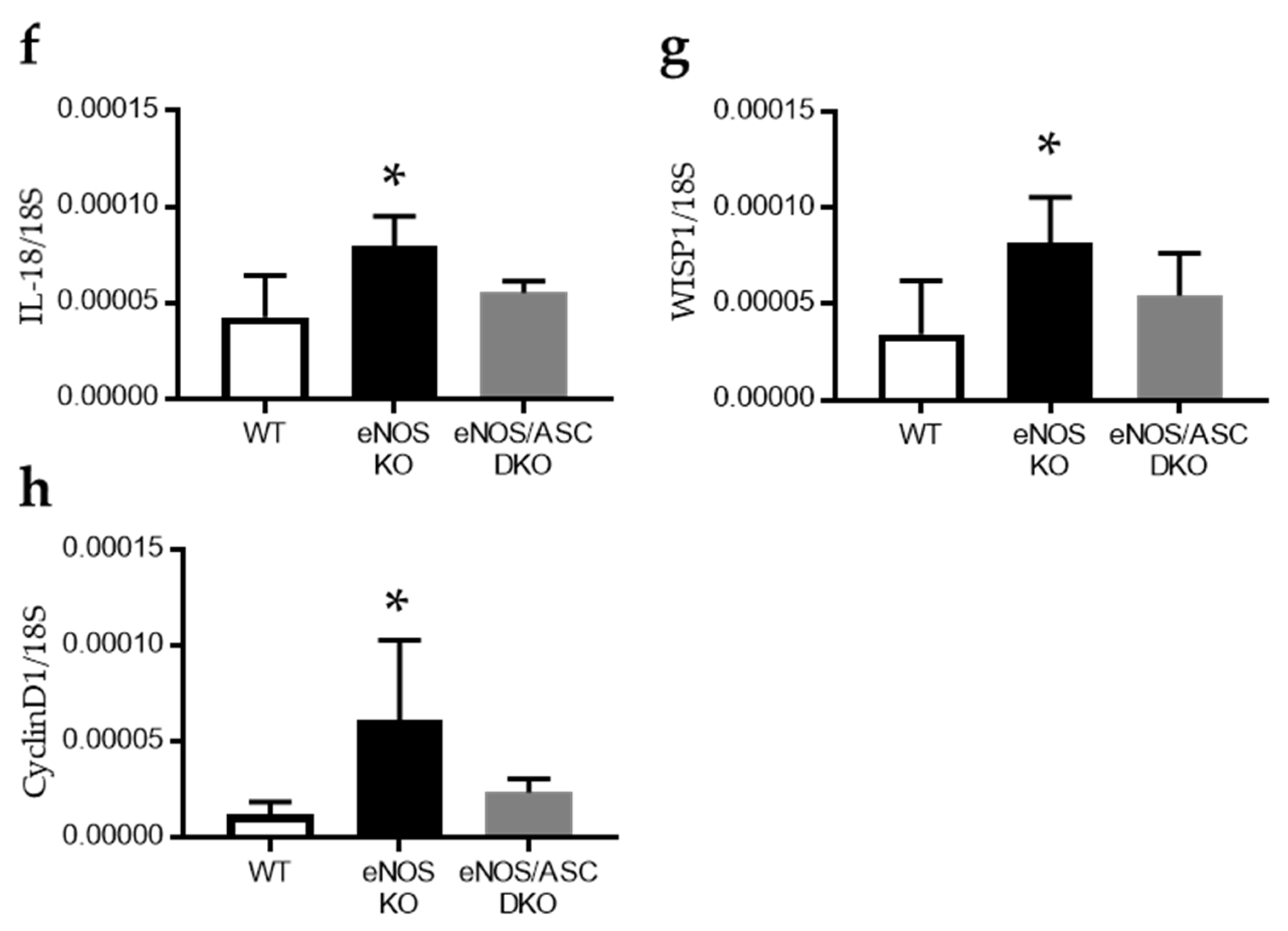
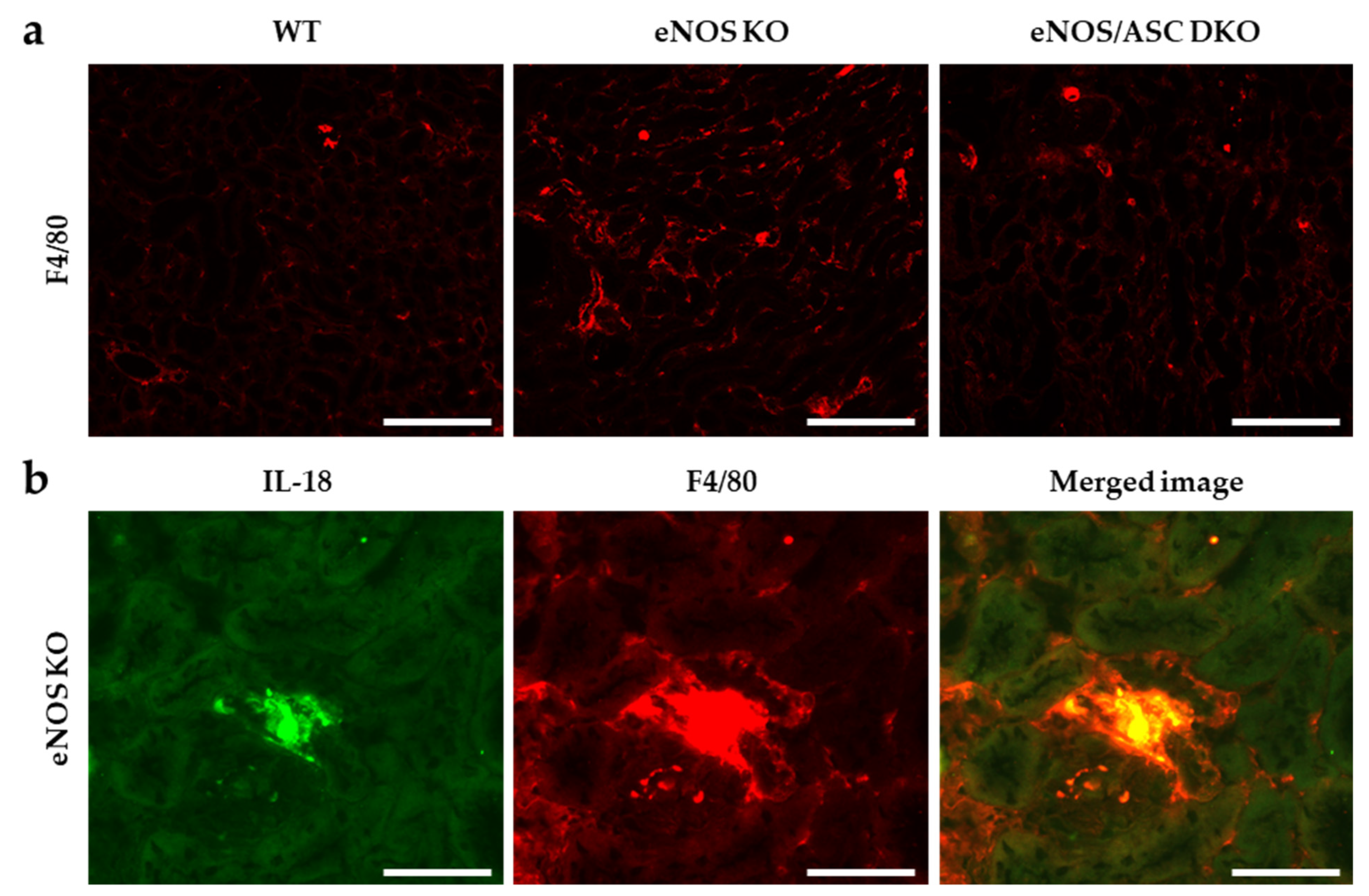
2.1. Inflammatory Response Worse in Aged eNOS KO Mice, Causing Glomerular Damage
2.2. Lacking eNOS-NO Pathway Hastens Mitochondrial Damage and Cellular Senescence
2.3. Aged-eNOS KO Mice Exhibit Impaired Fatty Acid Metabolism Associated with Mitochondrial Dysfunction in the Proximal Tubule
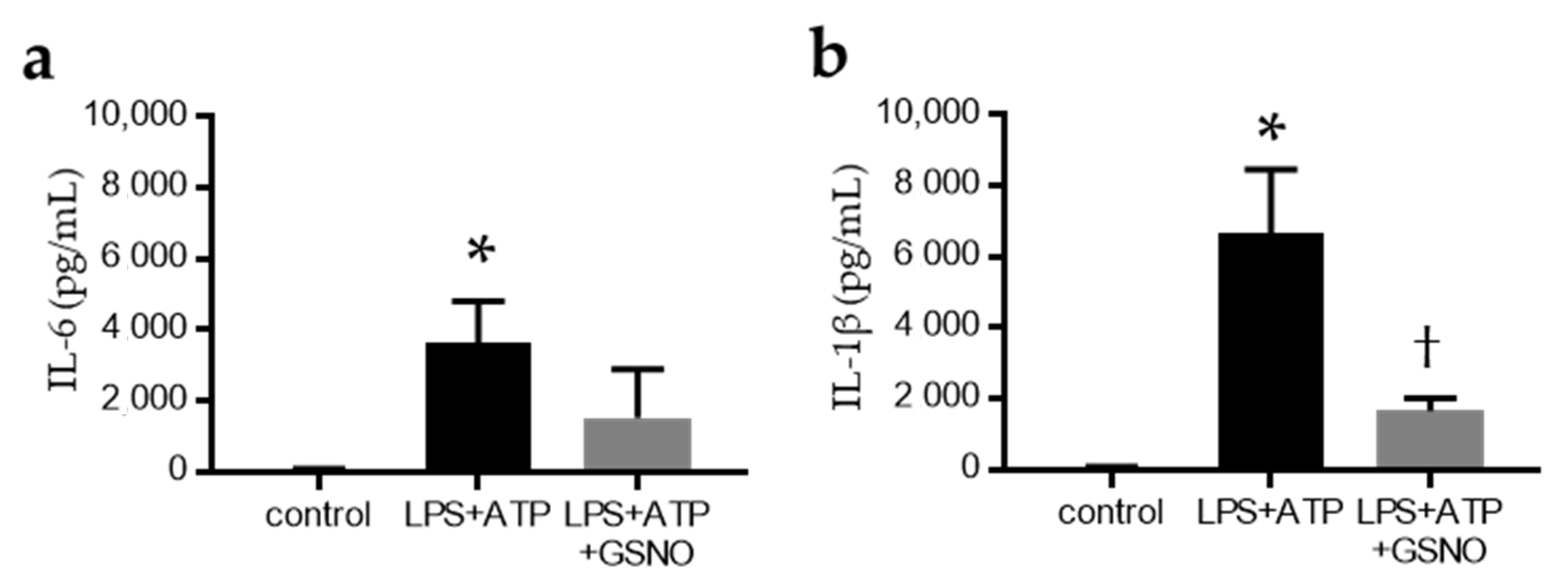
2.4. NO Suppresses the Activation of Inflammasomes in Macrophages
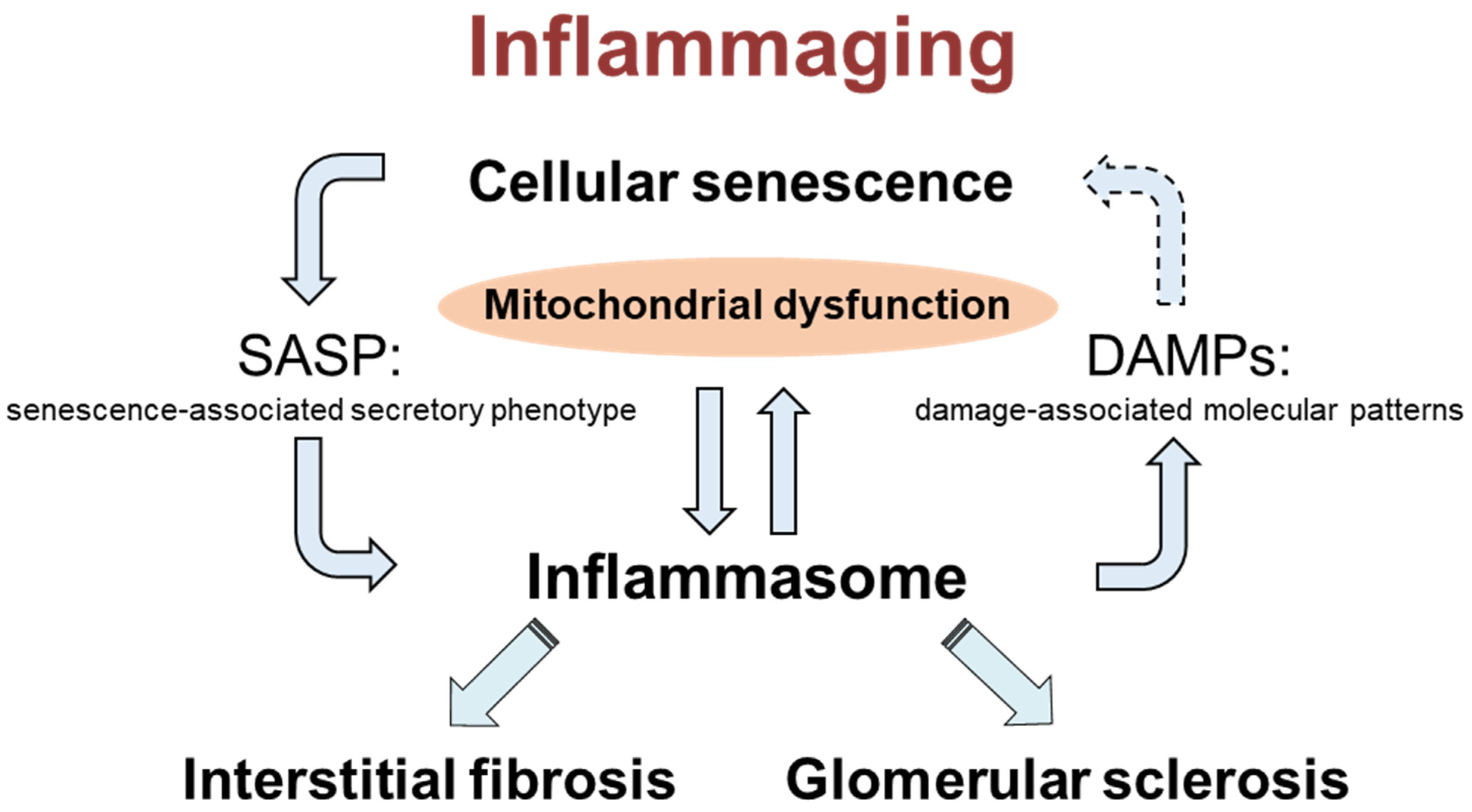
3. Discussion
4. Materials and Methods
4.1. Animal
4.2. Physiological and Biochemical Measurements
4.3. Histologic Analysis and Immunohistochemistry
4.4. Isolation of Glomeruli
4.5. Real-Time Reverse Transcription PCR
4.6. Mitochondrial Enzyme Activity
4.7. Senescence-Associated β-Galactosidase Activity
4.8. Cell Culture
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satoh, M.; Kidokoro, K.; Ozeki, M.; Nagasu, H.; Nishi, Y.; Ihoriya, C.; Fujimoto, S.; Sasaki, T.; Kashihara, N. Angiostatin production increases in response to decreased nitric oxide in aging rat kidney. Lab. Investig. 2013, 93, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Kahveci, A.S.; Barnatan, T.T.; Kahveci, A.; Adrian, A.E.; Arroyo, J.; Eirin, A.; Harris, P.C.; Lerman, A.; Lerman, L.O.; Torres, V.E.; et al. Oxidative Stress and Mitochondrial Abnormalities Contribute to Decreased Endothelial Nitric Oxide Synthase Expression and Renal Disease Progression in Early Experimental Polycystic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 1994. [Google Scholar] [CrossRef] [Green Version]
- Pourbagher-Shahri, A.M.; Farkhondeh, T.; Talebi, M.; Kopustinskiene, D.M.; Samarghandian, S.; Bernatoniene, J. An Overview of NO Signaling Pathways in Aging. Molecules 2021, 26, 4533. [Google Scholar] [CrossRef] [PubMed]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Berkowitz, D.E.; White, R.; Li, D.; Minhas, K.M.; Cernetich, A.; Kim, S.; Burke, S.; Shoukas, A.A.; Nyhan, D.; Champion, H.C.; et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003, 108, 2000–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef]
- Wever, R.; Boer, P.; Hijmering, M.; Stroes, E.; Verhaar, M.; Kastelein, J.; Versluis, K.; Lagerwerf, F.; van Rijn, H.; Koomans, H.; et al. Nitric oxide production is reduced in patients with chronic renal failure. Arter. Thromb. Vasc. Biol. 1999, 19, 1168–1172. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Lateef, A.M.; Engels, K.; Samsell, L.; Baylis, C. Basal and stimulated nitric oxide in control of kidney function in the aging rat. Am. J. Physiol. 1997, 272, R1747–R1753. [Google Scholar] [CrossRef]
- Komada, T.; Usui, F.; Shirasuna, K.; Kawashima, A.; Kimura, H.; Karasawa, T.; Nishimura, S.; Sagara, J.; Noda, T.; Taniguchi, S.; et al. ASC in renal collecting duct epithelial cells contributes to inflammation and injury after unilateral ureteral obstruction. Am. J. Pathol. 2014, 184, 1287–1298. [Google Scholar] [CrossRef]
- Kadoya, H.; Satoh, M.; Sasaki, T.; Taniguchi, S.; Takahashi, M.; Kashihara, N. Excess aldosterone is a critical danger signal for inflammasome activation in the development of renal fibrosis in mice. FASEB J. 2015, 29, 3899–3910. [Google Scholar] [CrossRef]
- Sogawa, Y.; Nagasu, H.; Iwase, S.; Ihoriyas, C.; Itano, S.; Uchida, A.; Kidokoro, K.; Taniguchi, S.; Takahashi, M.; Satoh, M.; et al. Infiltration of M1, but not M2, macrophages is impaired after unilateral ureter obstruction in Nrf2-deficient mice. Sci. Rep. 2017, 7, 8801. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Xu, Y.J.; Zheng, L.; Hu, Y.W.; Wang, Q. Pyroptosis and its relationship to atherosclerosis. Clin. Chim. Acta 2018, 476, 28–37. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Gu, X.; Chen, Y.L.; Liu, J.B.; Hou, C.X.; Lin, S.Y.; Hao, N.N.; Liang, Y.; Chen, W.; Meng, H.Y. Tolllike receptor 4 activates the NLRP3 inflammasome pathway and periodontal inflammaging by inhibiting Bmi1 expression. Int. J. Mol. Med. 2021, 47, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Ma, Y.; Bai, X.Y.; Chen, X. The Expression Changes of Inflammasomes in the Aging Rat Kidneys. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugarski, M.; Ghazi, S.; Polesel, M.; Martins, J.R.; Hall, A.M. Changes in NAD and Lipid Metabolism Drive Acidosis-Induced Acute Kidney Injury. J. Am. Soc. Nephrol. 2021, 32, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Ozawa, S.; Mori, K.; Asanuma, K.; Yanagita, M.; Uchida, S.; Nakagawa, T. ENOS deficiency causes podocyte injury with mitochondrial abnormality. Free Radic. Biol. Med. 2015, 87, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Keenan, H.A.; Li, Q.; Ishikado, A.; Kannt, A.; Sadowski, T.; Yorek, M.A.; Wu, I.H.; Lockhart, S.; Coppey, L.J.; et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat. Med. 2017, 23, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Lim, S.W.; Luo, K.; Cui, S.; Quan, Y.; Shin, Y.J.; Lee, K.E.; Kim, H.L.; Ko, E.J.; Chung, B.H.; et al. Coenzyme Q10 alleviates tacrolimus-induced mitochondrial dysfunction in kidney. FASEB J. 2019, 33, 12288–12298. [Google Scholar] [CrossRef] [Green Version]
- Satoh, M.; Kashihara, N.; Fujimoto, S.; Horike, H.; Tokura, T.; Namikoshi, T.; Sasaki, T.; Makino, H. A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J. Pharm. Exp. Ther. 2003, 305, 1183–1190. [Google Scholar] [CrossRef] [Green Version]
- Satoh, M.; Fujimoto, S.; Horike, H.; Ozeki, M.; Nagasu, H.; Tomita, N.; Sasaki, T.; Kashihara, N. Mitochondrial damage-induced impairment of angiogenesis in the aging rat kidney. Lab. Investig. 2011, 91, 190–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misawa, T.; Takahama, M.; Kozaki, T.; Lee, H.; Zou, J.; Saitoh, T.; Akira, S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013, 14, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Bruder-Nascimento, T.; Ferreira, N.S.; Zanotto, C.Z.; Ramalho, F.; Pequeno, I.O.; Olivon, V.C.; Neves, K.B.; Alves-Lopes, R.; Campos, E.; Silva, C.A.; et al. NLRP3 Inflammasome Mediates Aldosterone-Induced Vascular Damage. Circulation 2016, 134, 1866–1880. [Google Scholar] [CrossRef] [PubMed]
- Abais, J.M.; Xia, M.; Li, G.; Chen, Y.; Conley, S.M.; Gehr, T.W.B.; Boini, K.M.; Li, P.L. Nod-like receptor protein 3 (NLRP3) inflammasome activation and podocyte injury via thioredoxin-interacting protein (TXNIP) during hyperhomocysteinemia. J. Biol. Chem. 2014, 289, 27159–27168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Lin, S.; Qiu, J.; Sun, W.; Dong, M.; Xiang, Y.; Wang, L.; Du, P. NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem. Biophys. Res. Commun. 2020, 521, 791–798. [Google Scholar] [CrossRef]
- Shahzad, K.; Bock, F.; Al-Dabet, M.M.; Gadi, I.; Kohli, S.; Nazir, S.; Ghosh, S.; Ranjan, S.; Wang, H.; Madhusudhan, T.; et al. Caspase-1, but Not Caspase-3, Promotes Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2270–2275. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Guo, H.; Xu, C.; Wang, B.; Zhang, M.; Ding, F. Mitochondrial reactive oxygen species-mediated NLRP3 inflammasome activation contributes to aldosterone-induced renal tubular cells injury. Oncotarget 2016, 7, 17479–17491. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Ajay, A.K.; Chang, J.H.; Mou, S.; Zhao, H.; Kishi, S.; Li, J.; Brooks, C.R.; Xiao, S.; Woo, H.M.; et al. KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab. 2021, 33, 1042–1061.e7. [Google Scholar] [CrossRef]
- Komada, T.; Chung, H.; Lau, A.; Platnich, J.M.; Beck, P.L.; Benediktsson, H.; Duff, H.J.; Jenne, C.N.; Muruve, D.A. Macrophage Uptake of Necrotic Cell DNA Activates the AIM2 Inflammasome to Regulate a Proinflammatory Phenotype in CKD. J. Am. Soc. Nephrol. 2018, 29, 1165–1181. [Google Scholar] [CrossRef]
- Yu, J.; Nagasu, H.; Murakami, T.; Hoang, H.; Broderick, L.; Hoffman, H.M.; Horng, T. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc. Natl. Acad. Sci. USA 2014, 111, 15514–15519. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Patra, S.; Bhol, C.S.; Panigrahi, D.P.; Praharaj, P.P.; Singh, A.; Patil, S.; Dhiman, R.; et al. Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics. Int. J. Biochem. Cell Biol. 2021, 136, 106013. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Chen, S.; Chen, M.; Ma, Y.; Wang, Y.; Huang, B.; He, Z.; Zeng, Y.; Hu, Y.; Sun, S.; et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013, 23, 201–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, B.B.; Rathinam, V.A.; Martens, G.W.; Martinot, A.J.; Kornfeld, H.; Fitzgerald, K.A.; Sassetti, C.M. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat. Immunol. 2013, 14, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, T.; Mori, K.; Mukoyama, M.; Kasahara, M.; Yokoi, H.; Saito, Y.; Ogawa, Y.; Imamaki, H.; Kawanishi, T.; Ishii, A.; et al. Exacerbation of diabetic nephropathy by hyperlipidaemia is mediated by Toll-like receptor 4 in mice. Diabetologia 2012, 55, 2256–2266. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, T.; Sato, W.; Kosugi, T.; Zhang, L.; Campbell-Thompson, M.; Yoshimura, A.; Croker, B.P.; Johnson, R.J.; Nakagawa, T. Endothelial injury due to eNOS deficiency accelerates the progression of chronic renal disease in the mouse. Am. J. Physiol. Ren. Physiol. 2009, 296, F317–F327. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, M.; Takahashi, M.; Hata, T.; Kashima, Y.; Usui, F.; Morimoto, H.; Izawa, A.; Takahashi, Y.; Masumoto, J.; Koyama, J.; et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011, 123, 594–604. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, M.; Asker, N.; Gerhardt, H.; Lundkvist, A.; Johansson, B.R.; Saito, Y.; Betsholtz, C. A new method for large scale isolation of kidney glomeruli from mice. Am. J. Pathol. 2002, 161, 799–805. [Google Scholar] [CrossRef] [Green Version]


| Gene | Accession Number | Primer and TaqMan Probe Sequences (5′–3′) |
|---|---|---|
| ASC | NM_023258 | Forward primer: CACCAGCCAAGACAAGATGA |
| Reverse primer: CTCCAGGTCCATCACCAAGT | ||
| TaqMan probe: FAM-CCCTCCTCCAGGCCTTGAAGGA-TAMRA | ||
| IL-6 | NM_03168 | Forward primer: CTTCACAAGTCCGGAGAGGA |
| Reverse primer: TCCACGATTTCCCAGAGAAC | ||
| TaqMan probe: FAM-CAGAGGATACCACTCCCAACAGACCTG-TAMRA | ||
| IL-18 | NM_008360 | Forward primer: AGACAGCCTGTGTTCGAGGA |
| Reverse primer: AGAGGGTCACAGCCAGTCC | ||
| TaqMan probe: FAM-CAAAGTGCCAGTGAACCCCAGACCA-TAMRA | ||
| WISP1 | NM_018865 | Forward primer: ACACATCAAGGCAGGGAAGA |
| Reverse primer: CGCAGTACTTGGGTCGGTAG | ||
| TaqMan probe: FAM-CAGCCAGAGGAGGCCACGAACTT-TAMRA | ||
| cyclinD1 | NM_007631 | Forward primer: CCTGCTGGAGAAGGTTTAGG |
| Reverse primer: ATTGGGTTGGGAAAGTCAAG | ||
| TaqMan probe: FAM-ATTGGTCTTTCATTGGGCAACGG-TAMRA | ||
| 18S rRNA | NR_003278 | Forward primer: CCTGCGGCTTAATTTGACTC |
| Reverse primer: GACAAATCGCTCCACCAACT | ||
| TaqMan probe: FAM-TCTTTCTCGATTCCGTGGGTGGTG-TAMRA | ||
| FAM, 6-carboxyfluorescein; TAMRA, N,N,N′,N′-tetramethyl-6-carboxyrhodamine derivative. | ||
| Gene, genes used for qPCR; Accession number, locus of mRNA; Primer and TaqMan probe sequences, sequences of primer and probe. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, Y.; Umeno, R.; Nagasu, H.; Kondo, M.; Tokuyama, A.; Kadoya, H.; Kidokoro, K.; Taniguchi, S.; Takahashi, M.; Sasaki, T.; et al. Endothelial Dysfunction Accelerates Impairment of Mitochondrial Function in Ageing Kidneys via Inflammasome Activation. Int. J. Mol. Sci. 2021, 22, 9269. https://doi.org/10.3390/ijms22179269
Wada Y, Umeno R, Nagasu H, Kondo M, Tokuyama A, Kadoya H, Kidokoro K, Taniguchi S, Takahashi M, Sasaki T, et al. Endothelial Dysfunction Accelerates Impairment of Mitochondrial Function in Ageing Kidneys via Inflammasome Activation. International Journal of Molecular Sciences. 2021; 22(17):9269. https://doi.org/10.3390/ijms22179269
Chicago/Turabian StyleWada, Yoshihisa, Reina Umeno, Hajime Nagasu, Megumi Kondo, Atsuyuki Tokuyama, Hiroyuki Kadoya, Kengo Kidokoro, Shun’ichiro Taniguchi, Masafumi Takahashi, Tamaki Sasaki, and et al. 2021. "Endothelial Dysfunction Accelerates Impairment of Mitochondrial Function in Ageing Kidneys via Inflammasome Activation" International Journal of Molecular Sciences 22, no. 17: 9269. https://doi.org/10.3390/ijms22179269
APA StyleWada, Y., Umeno, R., Nagasu, H., Kondo, M., Tokuyama, A., Kadoya, H., Kidokoro, K., Taniguchi, S., Takahashi, M., Sasaki, T., & Kashihara, N. (2021). Endothelial Dysfunction Accelerates Impairment of Mitochondrial Function in Ageing Kidneys via Inflammasome Activation. International Journal of Molecular Sciences, 22(17), 9269. https://doi.org/10.3390/ijms22179269






