Site-Specific Fracture Healing: Comparison between Diaphysis and Metaphysis in the Mouse Long Bone
Abstract
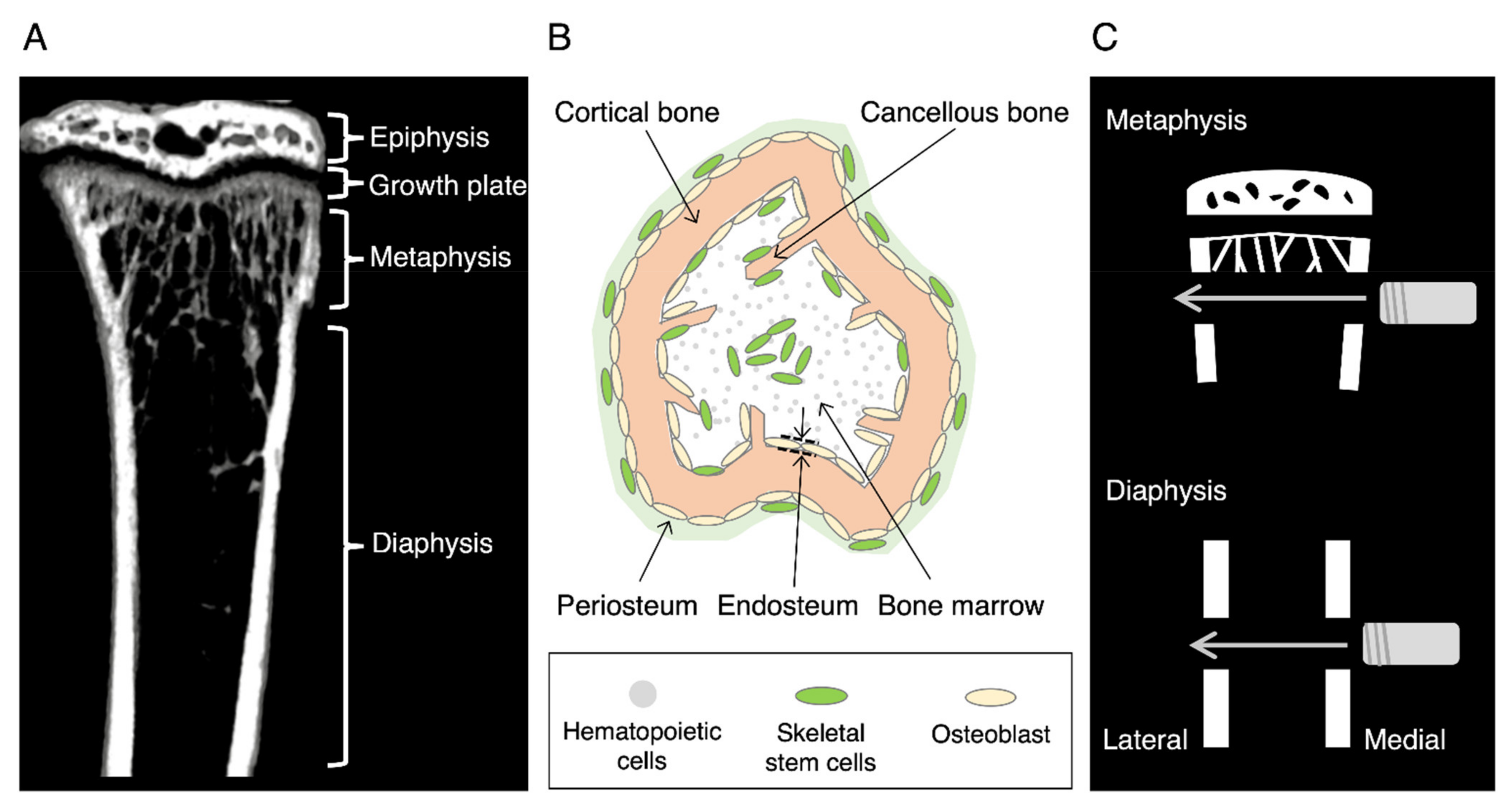
:1. Introduction
2. Animal Models of Fracture Healing
2.1. Closed Fracture Model
2.2. Open Fracture Model
3. Fracture Healing in a Drill Hole Model
3.1. Diaphysis Healing
3.1.1. Endochondral Ossification at the Periosteal Side of the Cortical Bone
3.1.2. Intramembranous Ossification at the Periosteal Side of the Cortical Bone
3.1.3. Medullary Callus
3.2. Metaphyseal Healing
3.2.1. Medullary Callus
3.2.2. Endosteal Callus
4. Estrogen in Fracture Healing
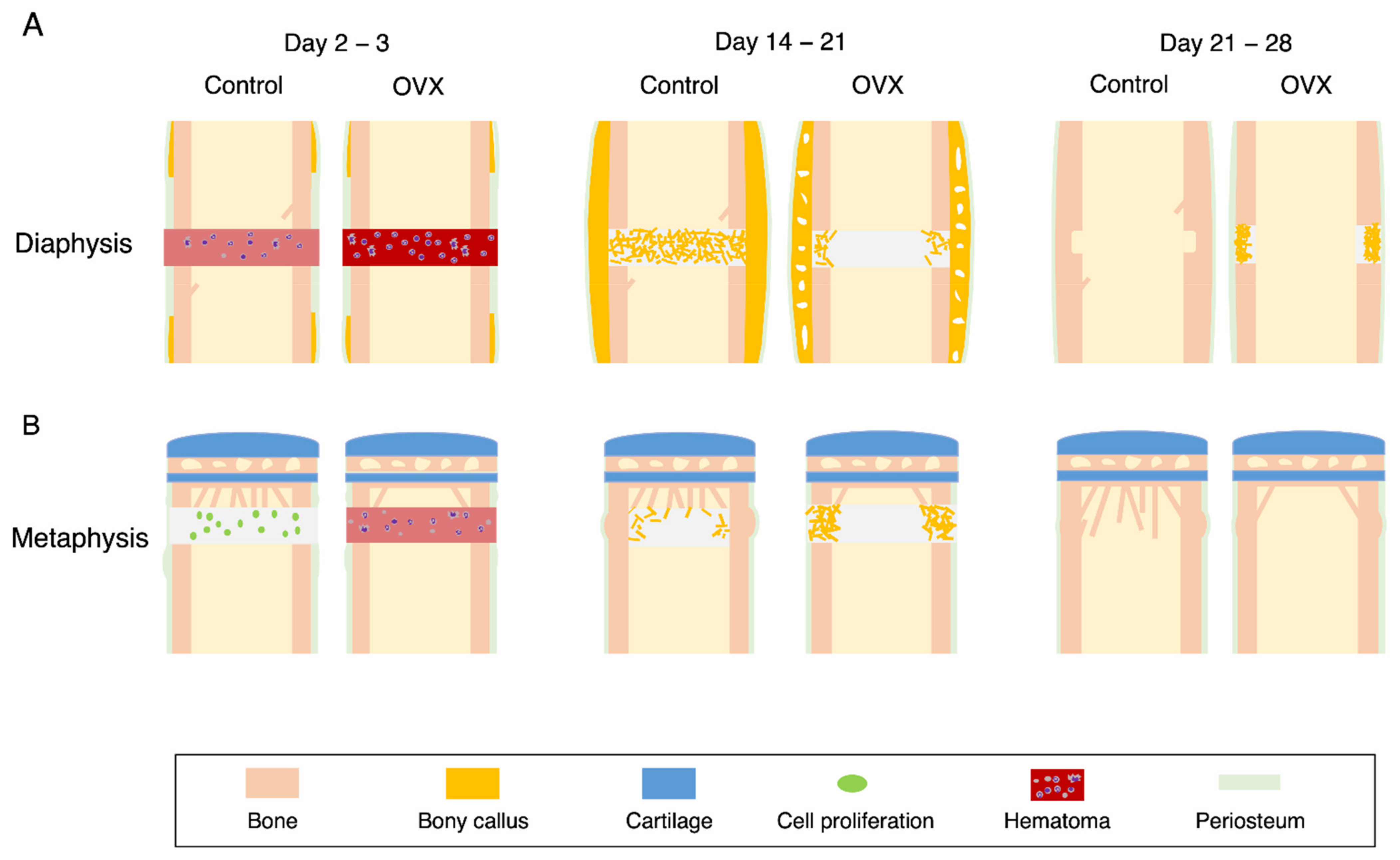
4.1. OVX Mice
4.2. Diaphyseal Healing in the OVX Mice
4.3. Metaphyseal Healing in the OVX Mice
4.4. Effects of Estrogen Administration on Fracture Healing in the OVX Animals
4.5. Phenotypes of Estrogen Receptor Alpha Knockout Mice
5. Cells Involved in Fracture Healing
5.1. Macrophages
5.2. Osteoblasts
5.3. Osteoclasts
6. Hox Code in Skeleton
6.1. Cortical Bone versus Cancellous Bone
6.2. Hox Genes in Skeleton
6.3. Hox Genes in Fracture Healing
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Hong, Z.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Kenkre, J.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. Int. J. Lab. Med. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.-R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Matsuo, K.; Irie, N. Osteoclast–osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.A.; Aitken, S.A.; Simpson, A.H.R.W. The risk of non-union per fracture: Current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017, 88, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.E.; Moore-Lotridge, S.N.; Hysong, A.A.; Posey, S.L.; Robinette, J.P.; Blum, D.M.; Benvenuti, M.A.; Cole, H.A.; Egawa, S.; Okawa, A.; et al. Bone Fracture Acute Phase Response—A Unifying Theory of Fracture Repair: Clinical and Scientific Implications. Clin. Rev. Bone Miner. Metab. 2018, 16, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Amsel, S.; Maniatis, A.; Tavassoli, M.; Crosby, W.H. The significance of intramedullary cancellous bone formation in the repair of bone marrow tissue. Anat. Rec. 1969, 164, 101–111. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Otsuka, H.; Takito, J.; Nakamura, M. Decisive differences in the bone repair processes of the metaphysis and diaphysis in young mice. Bone Reports 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Fujikawa, K.; Matsuki-Fukushima, M.; Nakamura, M. Repair processes of flat bones formed via intramembranous versus endochondral ossification. J. Oral Biosci. 2020, 62, 52–57. [Google Scholar] [CrossRef]
- Wang, D.; Gilbert, J.R.; Zhang, X.; Zhao, B.; Ker, D.F.E.; Cooper, G.M. Calvarial Versus Long Bone: Implications for Tailoring Skeletal Tissue Engineering. Tissue Eng. Part B Rev. 2020, 26, 46–63. [Google Scholar] [CrossRef]
- Driessen, J.H.M.; Hansen, L.; Eriksen, S.A.; van Onzenoort, H.A.W.; Henry, R.M.A.; van den Bergh, J.; Abrahamsen, B.; Vestergaard, P.; de Vries, F. The epidemiology of fractures in Denmark in 2011. Osteoporos. Int. 2016, 27, 2017–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, Y.; Matsubara, H.; Fang, X.; Hayashi, K.; Nomura, I.; Ugaji, S.; Hamada, T.; Tsuchiya, H. Adipose-derived stem cell sheets accelerate bone healing in rat femoral defects. PLoS ONE 2019, 14, e0214488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.T.; Han, D.C.; Zhang, P.X.; Han, N.; Kou, Y.H.; Yin, X.F.; Jiang, B.G. A special healing pattern in stable metaphyseal fractures. Acta Orthop. 2015, 86, 238–242. [Google Scholar] [CrossRef]
- Sandberg, O.; Aspenberg, P. Different effects of indomethacin on healing of shaft and metaphyseal fractures. Acta Orthop. 2015, 86, 243–247. [Google Scholar] [CrossRef]
- Sandberg, O.H.; Aspenberg, P. Glucocorticoids inhibit shaft fracture healing but not metaphyseal bone regeneration under stable mechanical conditions. Bone Jt. Res. 2015, 4, 170–175. [Google Scholar] [CrossRef]
- Inoue, S.; Fujikawa, K.; Matsuki-Fukushima, M.; Nakamura, M. Effect of ovariectomy induced osteoporosis on metaphysis and diaphysis repair process. Injury 2021, 52, 1300–1309. [Google Scholar] [CrossRef]
- Hart, N.H.; Newton, R.U.; Tan, J.; Rantalainen, T.; Chivers, P.; Siafarikas, A.; Nimphius, S. Biological basis of bone strength: Anatomy, physiology and measurement. J. Musculoskelet. Neuronal Interact. 2020, 20, 347–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.F.; Iuliano-Burns, S.; Ghasem-Zadeh, A.; Zebaze, R.; Seeman, E. Rapid growth produces transient cortical weakness: A risk factor for metaphyseal fractures during puberty. J. Bone Miner. Res. 2010, 25, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Williams, J.N.; Li, Y.; Valiya Kambrath, A.; Sankar, U. The Generation of Closed Femoral Fractures in Mice: A Model to Study Bone Healing. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Histing, T.; Garcia, P.; Holstein, J.H.; Klein, M.; Matthys, R.; Nuetzi, R.; Steck, R.; Laschke, M.W.; Wehner, T.; Bindl, R.; et al. Small animal bone healing models: Standards, tips, and pitfalls results of a consensus meeting. Bone 2011, 49, 591–599. [Google Scholar] [CrossRef]
- Gunderson, Z.J.; Campbell, Z.R.; McKinley, T.O.; Natoli, R.M.; Kacena, M.A. A comprehensive review of mouse diaphyseal femur fracture models. Injury 2020, 51, 1439–1447. [Google Scholar] [CrossRef]
- Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef]
- Sandberg, O.H.; Tätting, L.; Bernhardsson, M.E.; Aspenberg, P. Temporal role of macrophages in cancellous bone healing. Bone 2017, 101, 129–133. [Google Scholar] [CrossRef]
- Schell, H.; Duda, G.N.; Peters, A.; Tsitsilonis, S.; Johnson, K.A.; Schmidt-Bleek, K. The haematoma and its role in bone healing. J. Exp. Orthop. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Schlickewei, C.W.; Kleinertz, H.; Thiesen, D.M.; Mader, K.; Priemel, M.; Frosch, K.-H.; Keller, J. Current and Future Concepts for the Treatment of Impaired Fracture Healing. Int. J. Mol. Sci. 2019, 20, 5805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Charoenlarp, P.; Rajendran, A.K.; Iseki, S. Role of fibroblast growth factors in bone regeneration. Inflamm. Regen. 2017, 37, 10. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Olsen, B.R. Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev. Dyn. 2017, 246, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Könnecke, I.; Serra, A.; El Khassawna, T.; Schlundt, C.; Schell, H.; Hauser, A.; Ellinghaus, A.; Volk, H.D.; Radbruch, A.; Duda, G.N.; et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 2014, 64, 155–165. [Google Scholar] [CrossRef]
- Ono, T.; Okamoto, K.; Nakashima, T.; Nitta, T.; Hori, S.; Iwakura, Y.; Takayanagi, H. IL-17-producing γδT cells enhance bone regeneration. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Bougioukli, S.; Ortega, B.; Arevalo, E.; Lieberman, J.R.; McMahon, A.P. Sox9 positive periosteal cells in fracture repair of the adult mammalian long bone. Bone 2017, 103, 12–19. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Ono, N. The diverse origin of bone-forming osteoblasts. J. Bone Miner. Res. 2021, 36, 1432–1447. [Google Scholar] [CrossRef] [PubMed]
- Supakul, S.; Yao, K.; Ochi, H.; Shimada, T.; Hashimoto, K.; Sunamura, S.; Mabuchi, Y.; Tanaka, M.; Akazawa, C.; Nakamura, T.; et al. Pericytes as a source of osteogenic cells in bone fracture healing. Int. J. Mol. Sci. 2019, 20, 1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julien, A.; Kanagalingam, A.; Martínez-Sarrà, E.; Megret, J.; Luka, M.; Ménager, M.; Relaix, F.; Colnot, C. Direct contribution of skeletal muscle mesenchymal progenitors to bone repair. Nat. Commun. 2021, 12, 2860. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Xu, L.; Zwingenberger, S.; Gibon, E.; Goodman, S.B.; Li, G. Mesenchymal stem cells homing to improve bone healing. J. Orthop. Transl. 2017, 9, 19–27. [Google Scholar] [CrossRef]
- Duchamp De Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beresford, W.A. (Ed.) Chondroid Bone, Secondary Cartilage and Metaplasia; Urban & Schwarzenberg: Baltimore, MD, USA, 1981; ISBN 978-0806702612. [Google Scholar]
- Yokoi, H.; Take, Y.; Uchida, R.; Magome, T.; Shimomura, K.; Mae, T.; Okamoto, T.; Hanai, T.; Chong, Y.; Sato, S.; et al. Vibration acceleration promotes endochondral formation during fracture healing through cellular chondrogenic differentiation. PLoS ONE 2020, 15, e0229127. [Google Scholar] [CrossRef] [PubMed]
- Kates, S.L.; Ackert-Bicknell, C.L. How do bisphosphonates affect fracture healing? Injury 2016, 47, S65–S68. [Google Scholar] [CrossRef] [Green Version]
- Jarry, L.; Uhthoff, H.K. Differences in healing of metaphyseal and diaphyseal fractures. Can. J. Surg. 1971, 14, 127–135. [Google Scholar]
- Stuermer, E.K.; Sehmisch, S.; Rack, T.; Wenda, E.; Seidlova-Wuttke, D.; Tezval, M.; Wuttke, W.; Frosch, K.H.; Stuermer, K.M. Estrogen and raloxifene improve metaphyseal fracture healing in the early phase of osteoporosis. A new fracture-healing model at the tibia in rat. Langenbeck’s Arch. Surg. 2010, 395, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolios, L.; Hoerster, A.K.; Sehmisch, S.; Malcherek, M.C.; Rack, T.; Tezval, M.; Seidlova-Wuttke, D.; Wuttke, W.; Stuermer, K.M.; Stuermer, E.K. Do estrogen and alendronate improve metaphyseal fracture healing when applied as osteoporosis prophylaxis? Calcif. Tissue Int. 2010, 86, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jingushi, S.; Joyce, M.E.; Bolander, M.E. Genetic expression of extracellular matrix proteins correlates with histologic changes during fracture repair. J. Bone Miner. Res. 1992, 7, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Han, N.; Xue, F.; Zhang, P. A novel specialized staging system for cancellous fracture healing, distinct from traditional healing pattern of diaphysis corticalfracture? Int. J. Clin. Exp. Med. 2015, 8, 1301–1304. [Google Scholar]
- Tätting, L.; Sandberg, O.; Bernhardsson, M.; Ernerudh, J.; Aspenberg, P. Different composition of leucocytes in cortical and cancellous bone healing in a mouse model. Bone Jt. Res. 2018, 7, 620–628. [Google Scholar] [CrossRef]
- Hoerth, R.M.; Seidt, B.M.; Shah, M.; Schwarz, C.; Willie, B.M.; Duda, G.N.; Fratzl, P.; Wagermaier, W. Mechanical and structural properties of bone in non-critical and critical healing in rat. Acta Biomater. 2014, 10, 4009–4019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.Y.; Lieu, S.; Lu, C.; Colnot, C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone 2010, 47, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, T.; Pinho, S.; Ahmed, J.; Kunisaki, Y.; Hanoun, M.; Mendelson, A.; Ono, N.; Kronenberg, H.M.; Frenette, P.S. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 2014, 29, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89–107. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, G.; Qiao, L.; Ge, Q.; Chen, D.; Xu, Z.; Shi, D.; Dai, J.; Qin, J.; Teng, H.; et al. Age-dependent variations of cancellous bone in response to ovariectomy in C57BL/6J mice. Exp. Ther. Med. 2018, 15, 3623–3632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beil, F.T.; Barvencik, F.; Gebauer, M.; Seitz, S.; Rueger, J.M.; Ignatius, A.; Pogoda, P.; Schinke, T.; Amling, M. Effects of estrogen on fracture healing in mice. J. Trauma-Inj. Infect. Crit. Care 2010, 69, 1259–1265. [Google Scholar] [CrossRef]
- Namkung-Matthai, H.; Appleyard, R.; Jansen, J.; Hao Lin, J.; Maastricht, S.; Swain, M.; Mason, R.; Murrell, G.A..; Diwan, A..; Diamond, T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 2001, 28, 80–86. [Google Scholar] [CrossRef]
- He, Y.X.; Zhang, G.; Pan, X.H.; Liu, Z.; Zheng, L.; Chan, C.W.; Lee, K.M.; Cao, Y.P.; Li, G.; Wei, L.; et al. Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: A drill-hole defect model. Bone 2011, 48, 1388–1400. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Fischer, V.; Prystaz, K.; Liedert, A.; Ignatius, A. The inflammatory phase of fracture healing is influenced by oestrogen status in mice. Eur. J. Med. Res. 2017, 22, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Haffner-Luntzer, M.; Kemmler, J.; Heidler, V.; Prystaz, K.; Schinke, T.; Amling, M.; Kovtun, A.; Rapp, A.E.; Ignatius, A.; Liedert, A. Inhibition of midkine augments osteoporotic fracture healing. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Spiro, A.S.; Khadem, S.; Jeschke, A.; Marshall, R.P.; Pogoda, P.; Ignatius, A.; Amling, M.; Beil, F.T. The SERM raloxifene improves diaphyseal fracture healing in mice. J. Bone Miner. Metab. 2013, 31, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Kalbitz, M.; Müller-Graf, F.; Gebhard, F.; Ignatius, A.; Liedert, A.; Haffner-Luntzer, M. Influence of Menopause on Inflammatory Cytokines during Murine and Human Bone Fracture Healing. Int. J. Mol. Sci. 2018, 19, 2070. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.Y.; Zhang, Z.M.; Zheng, X.C.; Wang, L.; Huang, M.J.; Qin, S.; Chen, J.; Lai, P.L.; Yang, C.L.; Liu, J.; et al. Endogenous n-3 polyunsaturated fatty acids (PUFAs) mitigate ovariectomy-induced bone loss by attenuating bone marrow adipogenesis in FAT1 transgenic mice. Drug Des. Devel. Ther. 2013, 7, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.L.; Leung, K.S.; Cheung, W.H. Low-magnitude high-frequency vibration enhances gene expression related to callus formation, mineralization and remodeling during osteoporotic fracture healing in rats. J. Orthop. Res. 2014, 32, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.G.; Zhang, Z.M.; Zhang, Y.H.; Jiang, S.D.; Jiang, L.S.; Dai, L.Y. Changes of substance P during fracture healing in ovariectomized mice. Regul. Pept. 2010, 159, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Thormann, U.; Khawassna, T.E.; Ray, S.; Duerselen, L.; Kampschulte, M.; Lips, K.; Von Dewitz, H.; Heinemann, S.; Heiss, C.; Szalay, G.; et al. Differences of bone healing in metaphyseal defect fractures between osteoporotic and physiological bone in rats. Injury 2014, 45, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen Prevents Bone Loss via Estrogen Receptor α and Induction of Fas Ligand in Osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef]
- Martin-Millan, M.; Almeida, M.; Ambrogini, E.; Han, L.; Zhao, H.; Weinstein, R.S.; Jilka, R.L.; O’Brien, C.A.; Manolagas, S.C. The Estrogen Receptor-α in Osteoclasts Mediates the Protective Effects of Estrogens on Cancellous But Not Cortical Bone. Mol. Endocrinol. 2010, 24, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, M.; Iyer, S.; Martin-Millan, M.; Bartell, S.M.; Han, L.; Ambrogini, E.; Onal, M.; Xiong, J.; Weinstein, R.S.; Jilka, R.L.; et al. Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual. J. Clin. Investig. 2013, 123, 394–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, J.; Akhavan, N.S.; Mullins, A.P.; Arjmandi, B.H. Macrophage Polarization and Osteoporosis: A Review. Nutrients 2020, 12, 2999. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Chang, M.K.; Maylin, E.R.; Kohler, T.; Müller, R.; Wu, A.C.; Van Rooijen, N.; Sweet, M.J.; Hume, D.A.; Raggatt, L.J.; et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J. Bone Miner. Res. 2011, 26, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Wullschleger, M.E.; Alexander, K.A.; Wu, A.C.K.; Millard, S.M.; Kaur, S.; Maugham, M.L.; Gregory, L.S.; Steck, R.; Pettit, A.R. Fracture Healing via Periosteal Callus Formation Requires Macrophages for Both Initiation and Progression of Early Endochondral Ossification. Am. J. Pathol. 2014, 184, 3192–3204. [Google Scholar] [CrossRef] [PubMed]
- Vi, L.; Baht, G.S.; Whetstone, H.; Ng, A.; Wei, Q.; Poon, R.; Mylvaganam, S.; Grynpas, M.; Alman, B.A. Macrophages Promote Osteoblastic Differentiation In Vivo: Implications in Fracture Repair and Bone Homeostasis. J. Bone Miner. Res. 2015, 30, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Georgouli, T.; Blokhuis, T.J.; Pape, H.C.; Giannoudis, P.V. Pharmacological agents and impairment of fracture healing: What is the evidence? Injury 2008, 39, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Liu, X.; Tan, L.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; Kwok Yeung, K.W.; Chu, P.K.; et al. Modulation of the mechanosensing of mesenchymal stem cells by laser-induced patterning for the acceleration of tissue reconstruction through the Wnt/β-catenin signaling pathway activation. Acta Biomater. 2020, 101, 152–167. [Google Scholar] [CrossRef]
- Hu, D.P.; Ferro, F.; Yang, F.; Taylor, A.J.; Chang, W.; Miclau, T.; Marcucio, R.S.; Bahney, C.S. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 2017, 144, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative Effects of Transplanted Mesenchymal Stem Cells in Fracture Healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [Green Version]
- Siclari, V.A.; Zhu, J.; Akiyama, K.; Liu, F.; Zhang, X.; Chandra, A.; Nah, H.D.; Shi, S.; Qin, L. Mesenchymal progenitors residing close to the bone surface are functionally distinct from those in the central bone marrow. Bone 2013, 53, 575–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, S.K.; Kusumbe, A.P.; Schiller, M.; Zeuschner, D.; Bixel, M.G.; Milia, C.; Gamrekelashvili, J.; Limbourg, A.; Medvinsky, A.; Santoro, M.M.; et al. Blood flow controls bone vascular function and osteogenesis. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, B.; Ghuman, M.S.; Cama, G.; Rawlinson, S.C.F.; Grigoriadis, A.E.; Hughes, F.J. Site-specific differences in osteoblast phenotype, mechanical loading response and estrogen receptor-related gene expression. Mol. Cell. Endocrinol. 2018, 477, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhardsson, M.; Sandberg, O.; Aspenberg, P. Experimental models for cancellous bone healing in the rat. Acta Orthop. 2015, 86, 745–750. [Google Scholar] [CrossRef]
- Lanske, B.; Chandler, H.; Pierce, A.; Brown, J.; Ominsky, M.; Kostenuik, P.; Hattersley, G. Abaloparatide, a PTH receptor agonist with homology to PTHrP, enhances callus bridging and biomechanical properties in rats with femoral fracture. J. Orthop. Res. 2019, 37, 812–820. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kang, K.-C.; Kim, J.W.; Lim, S.-J.; Hahn, M.H. Current Role and Application of Teriparatide in Fracture Healing of Osteoporotic Patients: A Systematic Review. J. Bone Metab. 2017, 24, 65. [Google Scholar] [CrossRef] [Green Version]
- Yoon, B.-H.; Kim, K.-C. Does Teriparatide Improve Fracture Union? A Systematic Review. J. Bone Metab. 2020, 27, 167–174. [Google Scholar] [CrossRef]
- Eastman, K.; Gerlach, M.; Piec, I.; Greeves, J.; Fraser, W. Effectiveness of parathyroid hormone (PTH) analogues on fracture healing: A meta-analysis. Osteoporos. Int. 2021. [Google Scholar] [CrossRef]
- Ebata, S.; Takahashi, J.; Hasegawa, T.; Mukaiyama, K.; Isogai, Y.; Ohba, T.; Shibata, Y.; Ojima, T.; Yamagata, Z.; Matsuyama, Y.; et al. Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months After Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders. J. Bone Jt. Surg. 2017, 99, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Sacks, D.J.; Pelis, M.; Mason, Z.D.; Graves, D.T.; Barrero, M.; Ominsky, M.S.; Kostenuik, P.J.; Morgan, E.F.; Einhorn, T.A. Comparison of Effects of the Bisphosphonate Alendronate Versus the RANKL Inhibitor Denosumab on Murine Fracture Healing. J. Bone Miner. Res. 2009, 24, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.D.; McQueen, M.M.; Tuck, C.E.; Tobias, J.H.; Wilkinson, J.M.; Biant, L.C.; Pulford, E.C.; Aldridge, S.; Edwards, C.; Roberts, C.P.; et al. Effect of Alendronic Acid on Fracture Healing: A Multicenter Randomized Placebo-Controlled Trial. J. Bone Miner. Res. 2019, 34, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Rozental, T.D.; Makhni, E.C.; Day, C.S.; Bouxsein, M.L. Improving Evaluation and Treatment for Osteoporosis Following Distal Radial Fractures. J. Bone Jt. Surg.-Am. Vol. 2008, 90, 953–961. [Google Scholar] [CrossRef]
- Adami, S.; Libanati, C.; Boonen, S.; Cummings, S.R.; Ho, P.-R.; Wang, A.; Siris, E.; Lane, J. Denosumab Treatment in Postmenopausal Women with Osteoporosis Does Not Interfere with Fracture-Healing. J. Bone Jt. Surg. 2012, 94, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Bernhardsson, M.; Sandberg, O.; Aspenberg, P. Anti-RANKL treatment improves screw fixation in cancellous bone in rats. Injury 2015, 46, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Ishikawa, K.; Kudo, Y.; Tsuchiya, K.; Matsuoka, A.; Maruyama, H.; Emori, H.; Yamamura, R.; Hayakawa, C.; Sekimizu, M.; et al. The effect of denosumab on pedicle screw fixation: A prospective 2-year longitudinal study using finite element analysis. J. Orthop. Surg. Res. 2021, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Takito, J.; Nakamura, M. Heterogeneity and Actin Cytoskeleton in Osteoclast and Macrophage Multinucleation. Int. J. Mol. Sci. 2020, 21, 6629. [Google Scholar] [CrossRef]
- Wilks, D.C.; Winwood, K.; Gilliver, S.F.; Kwiet, A.; Chatfield, M.; Michaelis, I.; Sun, L.W.; Ferretti, J.L.; Sargeant, A.J.; Felsenberg, D.; et al. Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: A pQCT study. Bone 2009, 45, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Haidekker, M.A.; Andresen, R.; Werner, H.J. Relationship Between Structural Parameters, Bone Mineral Density and Fracture Load in Lumbar Vertebrae, Based on High-Resolution Computed Tomography, Quantitative Computed Tomography and Compression Tests. Osteoporos. Int. 1999, 9, 433–440. [Google Scholar] [CrossRef]
- Strube, A.; Suominen, M.I.; Rissanen, J.P.; Mumberg, D.; Klar, U.; Halleen, J.M.; Käkönen, S.-M. The anti-tumor agent sagopilone shows antiresorptive effects both in vitro and in vivo. Osteoporos. Int. 2011, 22, 2887–2893. [Google Scholar] [CrossRef]
- Parfitt, A.M. Misconceptions (2): Turnover is always higher in cancellous than in cortical bone. Bone 2002, 30, 807–809. [Google Scholar] [CrossRef]
- Weatherholt, A.M.; Fuchs, R.K.; Warden, S.J. Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model. Bone 2013, 52, 372–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, F.; Ornitz, D.M. Development of the Endochondral Skeleton. Cold Spring Harb. Perspect. Biol. 2013, 5, a008334. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.B. A gene complex controlling segmentation in Drosophila. Nature 1978, 276, 565–570. [Google Scholar] [CrossRef]
- McGinnis, W.; Krumlauf, R. Homeobox genes and axial patterning. Cell 1992, 68, 283–302. [Google Scholar] [CrossRef]
- Wellik, D.M. Hox10 and Hox11 Genes Are Required to Globally Pattern the Mammalian Skeleton. Science 2003, 301, 363–367. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, D.C.; Rakshit, S.; Yallowitz, A.R.; Loken, L.; Jeannotte, L.; Capecchi, M.R.; Wellik, D.M. Hox patterning of the vertebrate rib cage. Development 2007, 134, 2981–2989. [Google Scholar] [CrossRef] [Green Version]
- Rux, D.R.; Song, J.Y.; Swinehart, I.T.; Pineault, K.M.; Schlientz, A.J.; Trulik, K.G.; Goldstein, S.A.; Kozloff, K.M.; Lucas, D.; Wellik, D.M. Regionally Restricted Hox Function in Adult Bone Marrow Multipotent Mesenchymal Stem/Stromal Cells. Dev. Cell 2016, 39, 653–666. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Pineault, K.M.; Dones, J.M.; Raines, R.T.; Wellik, D.M. Hox genes maintain critical roles in the adult skeleton. Proc. Natl. Acad. Sci. USA 2020, 117, 7296–7304. [Google Scholar] [CrossRef] [PubMed]
- Gersch, R.P.; Lombardo, F.; McGovern, S.C.; Hadjiargyrou, M. Reactivation of Hox gene expression during bone regeneration. J. Orthop. Res. 2005, 23, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Bais, M.; McLean, J.; Sebastiani, P.; Young, M.; Wigner, N.; Smith, T.; Kotton, D.N.; Einhorn, T.A.; Gerstenfeld, L.C. Transcriptional Analysis of Fracture Healing and the Induction of Embryonic Stem Cell–Related Genes. PLoS ONE 2009, 4, e5393. [Google Scholar] [CrossRef] [Green Version]
- Rux, D.R.; Song, J.Y.; Pineault, K.M.; Mandair, G.S.; Swinehart, I.T.; Schlientz, A.J.; Garthus, K.N.; Goldstein, S.A.; Kozloff, K.M.; Wellik, D.M. Hox11 Function Is Required for Region-Specific Fracture Repair. J. Bone Miner. Res. 2017, 32, 1750–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineault, K.M.; Song, J.Y.; Kozloff, K.M.; Lucas, D.; Wellik, D.M. Hox11 expressing regional skeletal stem cells are progenitors for osteoblasts, chondrocytes and adipocytes throughout life. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Leucht, P.; Kim, J.-B.; Amasha, R.; James, A.W.; Girod, S.; Helms, J.A. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development 2008, 135, 2845–2854. [Google Scholar] [CrossRef] [Green Version]
- Bradaschia-Correa, V.; Leclerc, K.; Josephson, A.M.; Lee, S.; Palma, L.; Litwa, H.P.; Neibart, S.S.; Huo, J.C.; Leucht, P. Author Correction: Hox gene expression determines cell fate of adult periosteal stem/progenitor cells. Sci. Rep. 2020, 10, 3220. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, R.A.; Fuhler, G.M.; Janmaat, V.T.; da Fernandes, C.J.; da Silva Feltran, G.; Oliveira, F.A.; Matos, A.A.; Oliveira, R.C.; Ferreira, M.R.; Zambuzzi, W.F.; et al. HOXA cluster gene expression during osteoblast differentiation involves epigenetic control. Bone 2019, 125, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, T.C.; Wildman, B.J.; Beloti, M.M.; Kemper, A.G.; Ferraz, E.P.; Roy, B.; Rehan, M.; Afreen, L.H.; Kim, E.; Lengner, C.J.; et al. The microRNA-23a cluster regulates the developmental HoxA cluster function during osteoblast differentiation. J. Biol. Chem. 2018, 293, 17646–17660. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.Q.; Tare, R.; Lee, S.H.; Mandeville, M.; Weiner, B.; Montecino, M.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. HOXA10 Controls Osteoblastogenesis by Directly Activating Bone Regulatory and Phenotypic Genes. Mol. Cell. Biol. 2007, 27, 3337–3352. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Sitwala, K.; Bronstein, J.; Sanders, D.; Dandekar, M.; Collins, C.; Robertson, G.; MacDonald, J.; Cezard, T.; Bilenky, M.; et al. Identification and characterization of Hoxa9 binding sites in hematopoietic cells. Blood 2012, 119, 388–398. [Google Scholar] [CrossRef]
- Bei, L.; Lu, Y.; Bellis, S.L.; Zhou, W.; Horvath, E.; Eklund, E.A. Identification of a HoxA10 Activation Domain Necessary for Transcription of the Gene Encoding β3 Integrin during Myeloid Differentiation. J. Biol. Chem. 2007, 282, 16846–16859. [Google Scholar] [CrossRef] [Green Version]
- Shah, C.A.; Wang, H.; Bei, L.; Platanias, L.C.; Eklund, E.A. HoxA10 Regulates Transcription of the Gene Encoding Transforming Growth Factor β2 (TGFβ2) in Myeloid Cells. J. Biol. Chem. 2011, 286, 3161–3176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, C.A.; Bei, L.; Wang, H.; Platanias, L.C.; Eklund, E.A. HoxA10 Protein Regulates Transcription of Gene Encoding Fibroblast Growth Factor 2 (FGF2) in Myeloid Cells. J. Biol. Chem. 2012, 287, 18230–18248. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, R.A.; Pettengell, R.; Pandha, H.S.; Morgan, R. The role of HOX genes in normal hematopoiesis and acute leukemia. Leukemia 2013, 27, 1000–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudreau, N.J.; Varner, J.A. The Homeobox Transcription Factor Hox D3 Promotes Integrin α5β1 Expression and Function during Angiogenesis. J. Biol. Chem. 2004, 279, 4862–4868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leow, J.M.; Clement, N.D.; Tawonsawatruk, T.; Simpson, C.J.; Simpson, A.H.R.W. The radiographic union scale in tibial (RUST) fractures: Reliability of the outcome measure at an independent centre. Bone Jt. Res. 2016, 5, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Osterhoff, G.; Sprague, S.; Garibaldi, A.; Bhandari, M.; Slobogean, G.P. The Radiographic Union Score for Hip (RUSH) Identifies Radiographic Nonunion of Femoral Neck Fractures. Clin. Orthop. Relat. Res. 2016, 474, 1396–1404. [Google Scholar] [CrossRef] [Green Version]

| Diaphysis | Metaphysis | |||
|---|---|---|---|---|
| Estrogen injection | − | + | − | + |
| Inflammation stage | ||||
| Neutrophils | ↑ [20,60] | ND | ↑ [20] | ND |
| Inflammatory cytokines | ↑ [60,61] | ND | ND | ND |
| Callus formation stage | ||||
| Cartilaginous callus | → [57,65] | ↑ [57] | NA | NA |
| → [62]* | ||||
| Periosteal callus (bony) | ↓ [57,59,65] | ↑ [57,62]* | NA | NA |
| Medullary callus | ↓ [59] | ↑ [20] | ↓ [20,47,48] | → [20] |
| ↑ [47,48]* | ||||
| Osteogenic markers | ↓ [59] | ND | ↓ [20] | ND |
| Remodeling stage | ||||
| Bone mineral density | ↓ [59] | ↑ [20] | ↓ [20] | ↑ [20] |
| Bone strength | ↓ [57,65] | ↑ [57] | ↓ [48] | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, S.; Takito, J.; Nakamura, M. Site-Specific Fracture Healing: Comparison between Diaphysis and Metaphysis in the Mouse Long Bone. Int. J. Mol. Sci. 2021, 22, 9299. https://doi.org/10.3390/ijms22179299
Inoue S, Takito J, Nakamura M. Site-Specific Fracture Healing: Comparison between Diaphysis and Metaphysis in the Mouse Long Bone. International Journal of Molecular Sciences. 2021; 22(17):9299. https://doi.org/10.3390/ijms22179299
Chicago/Turabian StyleInoue, Satoshi, Jiro Takito, and Masanori Nakamura. 2021. "Site-Specific Fracture Healing: Comparison between Diaphysis and Metaphysis in the Mouse Long Bone" International Journal of Molecular Sciences 22, no. 17: 9299. https://doi.org/10.3390/ijms22179299
APA StyleInoue, S., Takito, J., & Nakamura, M. (2021). Site-Specific Fracture Healing: Comparison between Diaphysis and Metaphysis in the Mouse Long Bone. International Journal of Molecular Sciences, 22(17), 9299. https://doi.org/10.3390/ijms22179299







