The Aneugenicity of Ketone Bodies in Colon Epithelial Cells Is Mediated by Microtubule Hyperacetylation and Is Blocked by Resveratrol
Abstract
1. Introduction
2. Results
2.1. Ketone Bodies Enhance Microtubule Acetylation Both in Cancerous and Non-Cancerous Cells
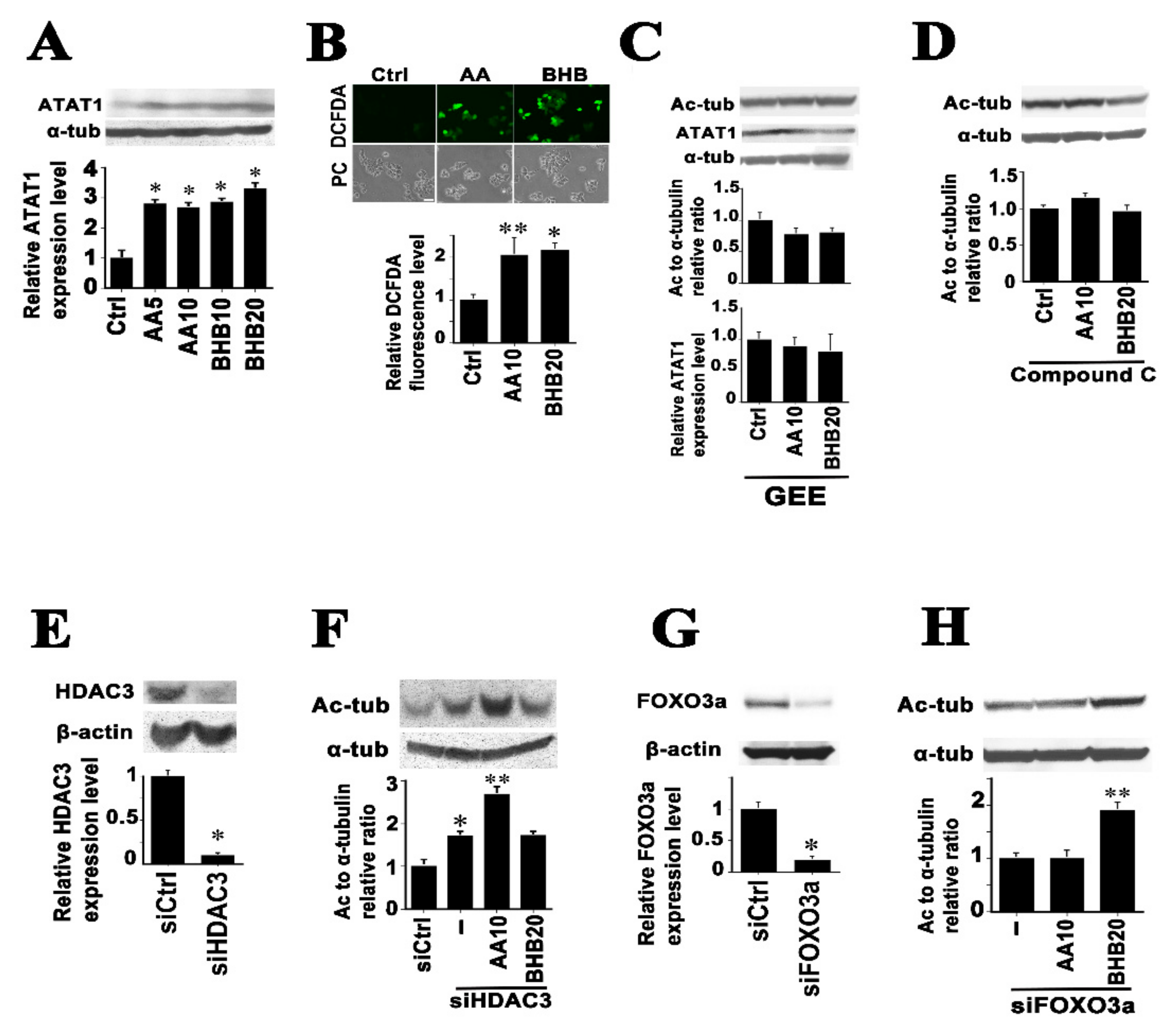
2.2. Mechanisms of Microtubule Hyperacetylation Induced by AA and BHB
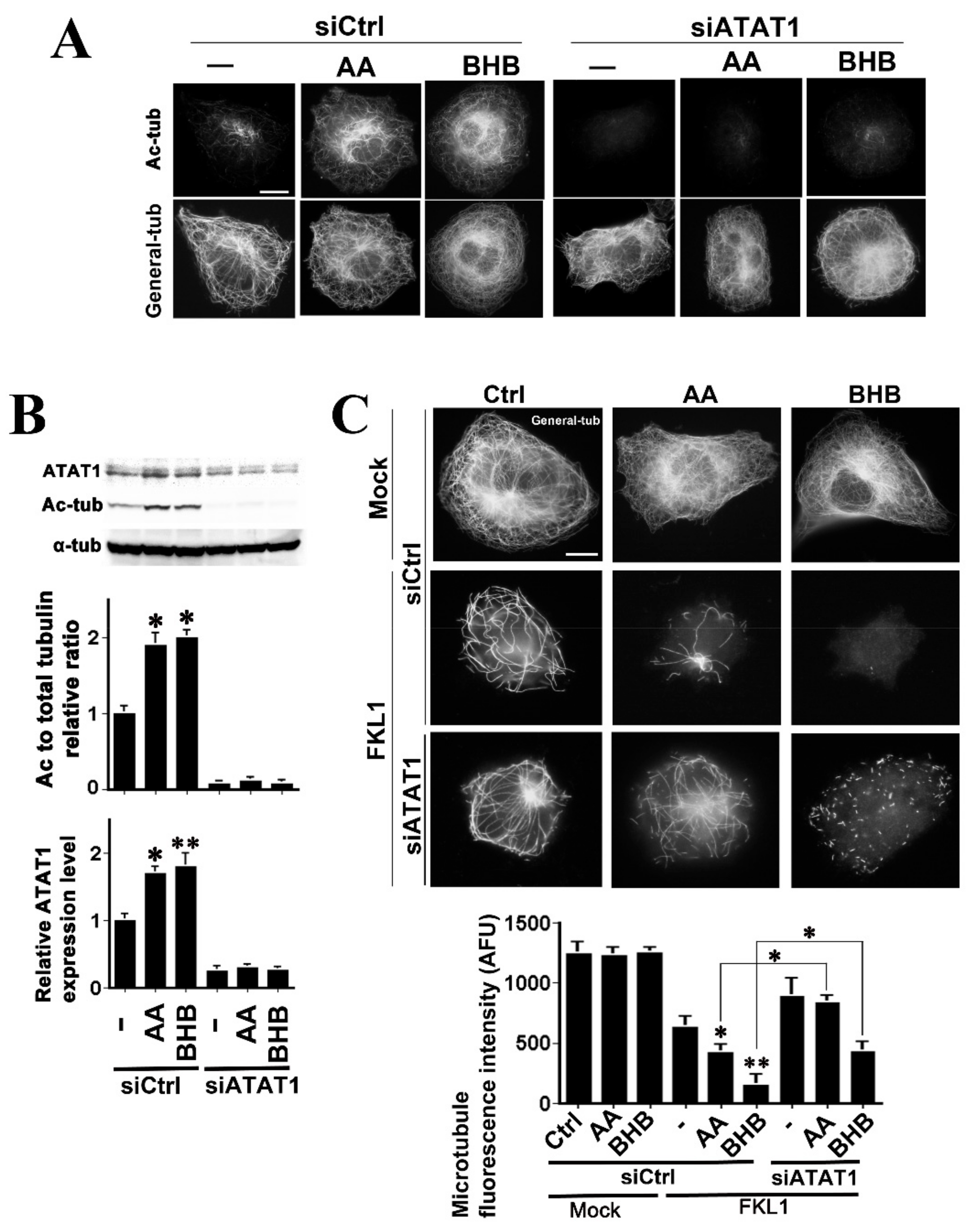
2.3. Both AA and BHB Enhance Microtubule Severing by KL1
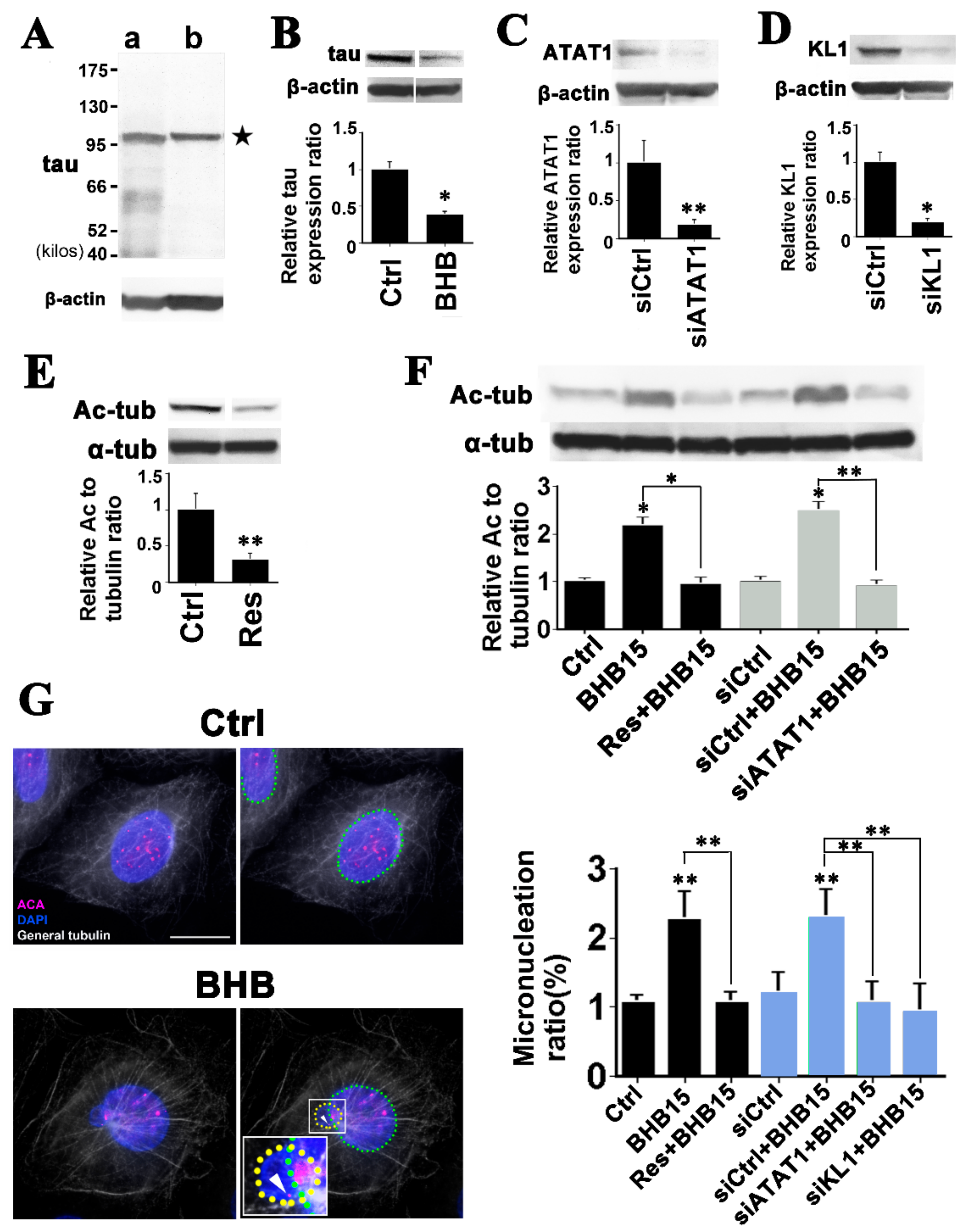
2.4. Microtubule Hyperacetylation-Mediated Aneuploidization Is Induced by Ketone Bodies in Cells of Colon Epithelial Origin
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Expression Constructs
4.3. Cell Culture, Transfection and Drug Treatments
4.3.1. Fibroblasts
4.3.2. Human Colon Cancer Cell Line
4.3.3. Human Colon Epithelial Cells (HCECs)
4.4. Western Blotting and Immunoblotting of Colon Cancer Tumor/Normal Tissue Lysate Test Strip Arrays
4.5. Semiquantitative RT-PCR
4.6. Immunofluorescence Techniques
4.7. ROS Detection
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renehan, A.; Smith, U.; Kirkman, M.S. Linking diabetes and cancer: A consensus on complexity. Lancet 2010, 375, 2201–2202. [Google Scholar] [CrossRef]
- Suh, S.; Kim, K.-W. Diabetes and cancer: Cancer should be screened in routine diabetes assessment. Diabetes Metab. J. 2019, 43, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Yuhara, H.; Steinmaus, C.; Cohen, S.E.; Corley, D.A.; Tei, Y.; Buffler, P.A. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am. J. Gastroenterol. 2011, 106, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Thevenet, J.; De Marchi, U.; Domingo, J.S.; Christinat, N.; Bultot, L.; Lefebvre, G.; Sakamoto, K.; Descombes, P.; Masoodi, M.; Wiederkehr, A. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. FASEB J. 2016, 30, 1913–1926. [Google Scholar] [CrossRef]
- Cheng, C.-W.; Biton, M.; Haber, A.L.; Gunduz, N.; Eng, G.; Gaynor, L.T.; Tripathi, S.; Calibasi-Kocal, G.; Rickelt, S.; Butty, V.; et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell 2019, 178, 1115–1131.e15. [Google Scholar] [CrossRef] [PubMed]
- Sada, N.; Lee, S.; Katsu, T.; Otsuki, T.; Inoue, T. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 2015, 347, 1362–1367. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.J.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Tajima, T.; Yoshifuji, A.; Matsui, A.; Itoh, T.; Uchiyama, K.; Kanda, T.; Tokuyama, H.; Wakino, S.; Itoh, H. β-hydroxybutyrate attenuates renal ischemia-reperfusion injury through its anti-pyroptotic effects. Kidney Int. 2019, 95, 1120–1137. [Google Scholar] [CrossRef]
- Sauer, L.A.; Dauchy, R.T. Stimulation of tumor growth in adult rats in vivo during acute streptozotocin-induced diabetes. Cancer Res. 1987, 47, 1756–1761. [Google Scholar] [PubMed]
- Goodstein, M.L.; Richtsmeier, W.J.; Sauer, L.A. The effect of an acute fast on human head and neck carcinoma xenograft: Growth effects on an ‘isolated tumor vascular pedicle’ in the nude rat. Arch. Otolaryngol. Head Neck Surg. 1993, 119, 897–902. [Google Scholar] [CrossRef]
- Huang, C.-K.; Chang, P.-H.; Kuo, W.-H.; Chen, C.-L.; Jeng, Y.-M.; Chang, K.-J.; Shew, J.-Y.; Hu, C.-M.; Lee, W.-H. Adipocytes promote malignant growth of breast tumours with monocarboxylate transporter 2 expression via β-hydroxybutyrate. Nat. Commun. 2017, 8, 14706. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Tsirigos, A.; Whitaker-Menezes, D.; Pavlides, S.; Pestell, R.G.; Chiavarina, B.; Frank, P.; Flomenberg, N.; Howell, A.; Martinez-Outschoorn, U.; et al. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epi-thelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010, 9, 3506–3514. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-B.; Fan, J.; Lin, R.; Elf, S.; Ji, Q.; Zhao, L.; Jin, L.; Seo, J.H.; Shan, C.; Arbiser, J.L.; et al. Metabolic rewiring by oncogenic BRAF V600E links ketogenesis pathway to BRAF-MEK1 signaling. Mol. Cell 2015, 59, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhan, L.; Zhou, Q.-Y.; Zhang, L.-L.; Chen, X.-M.; Hu, X.-M.; Yuan, X.-C. SIRT2 regulates microtubule stabilization in diabetic cardiomyopathy. Eur. J. Pharmacol. 2015, 764, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Nigra, A.D.; Monesterolo, N.E.; Rivelli, J.F.; Amaiden, M.R.; Campetelli, A.N.; Casale, C.H.; Santander, V.S. Alterations of hemorheological parameters and tubulin content in erythrocytes from diabetic subjects. Int. J. Biochem. Cell Biol. 2016, 74, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Boggs, A.E.; Vitolo, M.; Whipple, R.A.; Charpentier, M.S.; Goloubeva, O.G.; Ioffe, O.B.; Tuttle, K.C.; Slovic, J.; Lu, Y.; Mills, G.B.; et al. A-tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res. 2015, 75, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; You, E.; Ko, P.; Jeong, J.; Keum, S.; Rhee, S. Genetic disruption of tubulin acetyltransferase, αTAT1, inhibits proliferation and invasion of colon cancer cells through decreases in Wnt1/β-catenin signaling. Biochem. Biophys. Res. Commun. 2017, 482, 8–14. [Google Scholar] [CrossRef]
- Sudo, H. Microtubule hyperacetylation enhances KL1-dependent micronucleation under a tau deficiency in mammary epithelial cells. Int. J. Mol. Sci. 2018, 19, 2488. [Google Scholar] [CrossRef]
- Sudo, H.; Nakajima, K. The mitotic tensegrity guardian tau protects mammary epithelia from katanin-like1-induced aneuploidy. Oncotarget 2016, 7, 53712–53734. [Google Scholar] [CrossRef]
- Kanikarla, P.; Jain, S.K. Hyperketonemia (acetoacetate) upregulates NADPH oxidase 4 and elevates oxidative stress, ICAM-1, and monocyte adhesivity in endothelial cells. Cell. Physiol. Biochem. 2015, 35, 364–373. [Google Scholar] [CrossRef]
- Gadau, S. Nitrosative-induced posttranslational α-tubulin changes on high-glucose-exposed Schwannoma cell line. Neuro Endocrinol. Lett. 2013, 34, 372–382. [Google Scholar]
- Qiang, L.; Sun, X.; Austin, T.O.; Muralidharan, H.; Jean, D.C.; Liu, M.; Yu, W.; Baas, P.W. Tau does not stabilize axonal microtubules but rather enables them to have long labile domains. Curr. Biol. 2018, 28, 2181–2189.e4. [Google Scholar] [CrossRef]
- Valenstein, M.L.; Roll-Mecak, A. Graded control of microtubule severing by tubulin glutamylation. Cell 2016, 164, 911–921. [Google Scholar] [CrossRef]
- Van Dijk, J.; Bompard, G.; Cau, J.; Kunishima, S.; Rabeharivelo, G.; Mateos-Langerak, J.; Cazevieille, C.; Cavelier, P.; Boizet-Bonhoure, B.; Delsert, C.; et al. Microtubule polyglutamylation and acetylation drive microtubule dynamics critical for platelet formation. BMC Biol. 2018, 16, 116. [Google Scholar] [CrossRef]
- Nekooki-Machida, Y.; Nakakura, T.; Nishijima, Y.; Tanaka, H.; Arisawa, K.; Kiuchi, Y.; Miyashita, T.; Hagiwara, H. Dynamic localization of α-tubulin acetyltransferase ATAT1 through the cell cycle in human fibroblastic KD cells. Med. Mol. Morphol. 2018, 51, 217–226. [Google Scholar] [CrossRef]
- Mackeh, R.; Lorin, S.; Ratier, A.; Mejdoubi-Charef, N.; Baillet, A.; Bruneel, A.; Hamaï, A.; Codogno, P.; Poüs, C.; Perdiz, D. Reactive Oxygen Species, AMP-activated protein kinase, and the transcription cofactor p300 regulate α-tubulin acetyltransferase-1 (αTAT-1/MEC-17)-dependent microtubule hyperacetylation during cell stress. J. Biol. Chem. 2014, 289, 11816–11828. [Google Scholar] [CrossRef] [PubMed]
- Wickström, S.; Masoumi, K.C.; Khochbin, S.; Fässler, R.; Massoumi, R. CYLD negatively regulates cell-cycle progression by inactivating HDAC6 and increasing the levels of acetylated tubulin. EMBO J. 2010, 29, 131–144. [Google Scholar] [CrossRef]
- Krishnan, J.; Danzer, C.; Simka, T.; Ukropec, J.; Walter, K.M.; Kumpf, S.; Mirtschink, P.; Ukropcova, B.; Gasperikova, D.; Pedrazzini, T.; et al. Dietary obesity-associated Hif1 activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev. 2012, 26, 259–270. [Google Scholar] [CrossRef]
- Bacon, T.; Seiler, C.; Wolny, M.; Hughes, R.; Watson, P.; Schwabe, J.; Grigg, R.; Peckham, M. Histone deacetylase 3 indirectly modulates tubulin acetylation. Biochem. J. 2015, 472, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Qin, L.; Li, B.; Liao, Z.; Liang, J.; Xiao, X.; Xiao, X.; Mo, Y.; Huang, G.; Zhang, Z.; et al. Inactivation of HMGCL promotes proliferation and metastasis of nasopharyngeal carcinoma by suppressing oxidative stress. Sci. Rep. 2017, 7, 11954. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.S.; Hah, Y.-S.; Nilufar, R.; Han, J.; Bong, J.-H.; Kang, S.S.; Cho, G.J.; Choi, W.S. Acetoacetate protects neuronal cells from oxidative glutamate toxicity. J. Neurosci. Res. 2006, 83, 702–709. [Google Scholar] [CrossRef]
- Sudo, H.; Hashimoto, Y.; Niikura, T.; Shao, Z.; Yasukawa, T.; Ito, Y.; Yamada, M.; Hata, M.; Hiraki, T.; Kawasumi, M.; et al. Secreted aβ does not mediate neurotoxicity by antibody-stimulated amyloid precursor protein. Biochem. Biophys. Res. Commun. 2001, 282, 548–556. [Google Scholar] [CrossRef]
- Emerling, B.M.; Weinberg, F.; Snyder, C.; Burgess, Z.; Mutlu, G.M.; Viollet, B.; Budinger, G.S.; Chandel, N.S. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic. Biol. Med. 2009, 46, 1386–1391. [Google Scholar] [CrossRef]
- Nakano, A.; Kato, H.; Watanabe, T.; Min, K.-D.; Yamazaki, S.; Asano, Y.; Seguchi, O.; Higo, S.; Shintani, Y.; Asanuma, H.; et al. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat. Cell Biol. 2010, 12, 583–590. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. β-hydroxybutyrate: Much more than a metabolite. Diabetes Res. Clin. Pract. 2014, 106, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Gao, M.; Han, L.; Qiu, D.; Wang, H.; Xiong, B.; Sun, S.-C.; Liu, H.; Gu, L. HDAC3 promotes meiotic apparatus assembly in mouse oocytes via modulating tubulin acetylation. Development 2017, 144, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, X.; Liu, X.; Gao, M.; He, Y.; Xiong, B.; Liu, H. HDAC3 inhibition disrupts the assembly of meiotic apparatus during porcine oocyte maturation. J. Cell. Physiol. 2018, 234, 10178–10183. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Gao, M.; Liu, H.; Gu, L. Loss of HDAC3 contributes to meiotic defects in aged oocytes. Aging Cell 2019, 18, e13036. [Google Scholar] [CrossRef]
- Sudo, H.; Baas, P.W. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J. Neurosci. 2010, 30, 7215–7226. [Google Scholar] [CrossRef]
- Yu, W.; Qiang, L.; Solowska, J.M.; Karabay, A.; Korulu, S.; Baas, P.W. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol. Biol. Cell 2008, 19, 1485–1498. [Google Scholar] [CrossRef]
- Hirako, S.; Tsuda, H.; Ito, F.; Okazaki, Y.; Hirayama, T.; Nagasawa, H.; Nakano, T.; Imai, K.; Kotani, T.; Kikkawa, F.; et al. Role of catalytic iron and oxidative stress in nitrofen-induced congenital diaphragmatic hernia and its amelioration by Saireito (TJ-114). J. Clin. Biochem. Nutr. 2017, 61, 176–182. [Google Scholar] [CrossRef]
- Wijesekara, N.; Gonçalves, R.A.; Ahrens, R.; De Felice, F.G.; Fraser, P.E. Tau ablation in mice leads to pancreatic β cell dysfunction and glucose intolerance. FASEB J. 2018, 32, 3166–3173. [Google Scholar] [CrossRef]
- Cheung, K.; Senese, S.; Kuang, J.; Bui, N.; Ongpipattanakul, C.; Gholkar, A.; Cohn, W.; Capri, J.; Whitelegge, J.P.; Torres, J.Z. Proteomic analysis of the mammalian katanin family of microtubule-severing enzymes defines katanin p80 subunit B-like 1 (KATNBL1) as a regulator of mammalian katanin microtubule-severing. Mol. Cell. Proteom. 2016, 15, 1658–1669. [Google Scholar] [CrossRef]
- McNally, F.J.; Roll-Mecak, A. Microtubule-severing enzymes: From cellular functions to molecular mechanism. J. Cell Biol. 2018, 217, 4057–4069. [Google Scholar] [CrossRef]
- Kırımtay, K.; Selçuk, E.; Kelle, D.; Erman, B.; Karabay, A. p53 regulates katanin-p60 promoter in HCT 116 cells. Gene 2020, 727, 144241. [Google Scholar] [CrossRef] [PubMed]
- Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Genetic instability in colorectal cancers. Nature 1997, 386, 623–627. [Google Scholar] [CrossRef]
- Huda, N.; Erdene-Ochir, E.; Pan, C.-H. Assay for phosphorylation and microtubule binding along with localization of tau protein in colorectal cancer cells. J. Vis. Exp. 2017, 10, e55932. [Google Scholar] [CrossRef]
- Farhana, L.; Nangia-Makker, P.; Arbit, E.; Shango, K.; Sarkar, S.; Mahmud, H.; Hadden, T.; Yu, Y.; Majumdar, A.P.N. Bile acid: A potential inducer of colon cancer stem cells. Stem Cell Res. Ther. 2016, 7, 181. [Google Scholar] [CrossRef]
- Sudo, H.; Baas, P.W. Strategies for diminishing katanin-based loss of microtubules in tauopathic neurodegenerative diseases. Hum. Mol. Genet. 2010, 20, 763–778. [Google Scholar] [CrossRef]
- Rossi, G.; Redaelli, V.; Contiero, P.; Fabiano, S.; Tagliabue, G.; Perego, P.; Benussi, L.; Bruni, A.C.; Filippini, G.; Farinotti, M.; et al. Tau mutations serve as a novel risk factor for cancer. Cancer Res. 2018, 78, 3731–3739. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Guo, W.; Zhu, X.; Ahuja, N.; Fu, T. MAPT promoter CpG island hypermethylation is associated with poor prognosis in patients with stage II colorectal cancer. Cancer Manag. Res. 2019, 11, 7337–7343. [Google Scholar] [CrossRef]
- Fischer, I.; Baas, P.W. Resurrecting the mysteries of big tau. Trends Neurosci. 2020, 43, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Whiteside, C.M.; Maarouf, C.L.; Walker, D.G.; Beach, T.G.; Sue, L.I.; Garcia, A.; Dunckley, T.; Meechoovet, B.; Reiman, E.M.; et al. The presence of select tau species in human peripheral tissues and their relation to alzheimer’s disease. J. Alzheimer’s Dis. 2016, 51, 345–356. [Google Scholar] [CrossRef]
- Baur, J.; Sinclair, D. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.O.A.; Yamamoto, T.; Maehara, K.; Nogami, J.; Ohkawa, Y.; Miura, H.; Poonperm, R.; Hiratani, I.; Nakayama, H.; Nakao, M.; et al. The Eleanor ncRNAs activate the topological domain of the ESR1 locus to balance against apoptosis. Nat. Commun. 2019, 10, 3778. [Google Scholar] [CrossRef]
- Suzuki, K.; Koike, T. Resveratrol abolishes resistance to axonal degeneration in slow Wallerian degeneration (WldS) mice: Activation of SIRT2, an NAD-dependent tubulin deacetylase. Biochem. Biophys. Res. Commun. 2007, 359, 665–671. [Google Scholar] [CrossRef]
- Misawa, T.; Saitoh, T.; Kozaki, T.; Park, S.; Takahama, M.; Akira, S. Resveratrol inhibits the acetylated α-tubulin-mediated assembly of the NLRP3-inflammasome. Int. Immunol. 2015, 27, 425–434. [Google Scholar] [CrossRef]
- Sayd, S.; Thirant, C.; El-Habr, E.A.; Lipecka, J.; Dubois, L.G.; Bogeas, A.; Tahiri-Jouti, N.; Chneiweiss, H.; Junier, M.-P. Sirtuin-2 activity is required for glioma stem cell proliferation arrest but not necrosis induced by resveratrol. Stem Cell Rev. Rep. 2014, 10, 103–113. [Google Scholar] [CrossRef]
- Crasta, K.; Ganem, N.; Dagher, R.; Lantermann, A.B.; Ivanova, E.V.; Pan, Y.; Nezi, L.; Protopopov, A.; Chowdhury, D.; Pellman, D. DNA breaks and chromosome pulverization from errors in mitosis. Nature 2012, 482, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; Battistoni, A.; Antoccia, A.; Degrassi, F.; Tanzarella, C. Changes in microtubule organization after exposure to a benzimidazole derivative in Chinese hamster cells. Mutagenesis 2000, 15, 507–515. [Google Scholar] [CrossRef][Green Version]
- Taggart, A.K.P.; Kero, J.; Gan, X.; Cai, T.-Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.-J.; et al. (d)-β-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.; Mellinger, J.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A Is a G-protein–coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Misawa, T.; Saitoh, T. Macrophages control innate inflammation. Diabetes Obes. Metab. 2013, 15, 10–18. [Google Scholar] [CrossRef]
- Liu, Y.; Dentin, R.; Chen, D.; Hedrick, S.; Ravnskjaer, K.; Schenk, S.; Milne, J.; Meyers, D.J.; Cole, P.; Yates, J., III; et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 2008, 456, 269–273. [Google Scholar] [CrossRef]
- Belcheva, A.; Irrazabal, T.; Robertson, S.J.; Streutker, C.; Maughan, H.; Rubino, S.; Moriyama, E.H.; Copeland, J.K.; Surendra, A.; Kumar, S.; et al. Gut microbial metabolism drives transformation of Msh2-deficient colon epithelial cells. Cell 2014, 158, 288–299. [Google Scholar] [CrossRef]
- Cherbuy, C.; Andrieux, C.; Honvo-Houeto, E.; Thomas, M.; Ide, C.; Druesne, N.; Chaumontet, C.; Darcy-Vrillon, B.; Duée, P.-H. Expression of mitochondrial HMGCoA synthase and glutaminase in the colonic mucosa is modulated by bacterial species. Eur. J. Biochem. 2003, 271, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Laghi, L.; Negri, F.; Gaiani, F.; Cavalleri, T.; Grizzi, F.; De Angelis, G.L.; Malesci, a. prognostic and predictive cross-roads of microsatellite instability and immune response to colon cancer. Int. J. Mol. Sci. 2020, 21, 9680. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Bi, J.; Wei, Q.; Jiang, L.; Guan, Q.; Zhang, M.; Song, X.; Chen, T.; Fan, J.; Li, X.; et al. Pan-cancer analysis of NLRP3 inflammasome with potential implications in prognosis and immunotherapy in human cancer. Briefings Bioinform. 2020, 22, bbaa345. [Google Scholar] [CrossRef]
- Steiner, P. Brain fuel utilization in the developing brain. Ann. Nutr. Metab. 2019, 75, 8–18. [Google Scholar] [CrossRef]
- Karabay, A.; Yu, W.; Solowska, J.M.; Baird, D.H.; Baas, P.W. Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules. J. Neurosci. 2004, 24, 5778–5788. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Yu, W.; Liu, M.; Solowska, J.M.; Baas, P.W. Basic fibroblast growth factor elicits formation of interstitial axonal branches via enhanced severing of microtubules. Mol. Biol. Cell 2010, 21, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O.I.; Baas, P.W. Microtubules and growth cones: Motors drive the turn. Trends Neurosci. 2016, 39, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Banks, G.; Lassi, G.; Hoerder-Suabedissen, A.; Tinarelli, F.; Simon, M.M.; Wilcox, A.; Lau, P.; Lawson, T.N.; Johnson, S.; Rutman, A.; et al. A missense mutation in Katnal1 underlies behavioural, neurological and ciliary anomalies. Mol. Psychiatry 2018, 23, 713–722. [Google Scholar] [CrossRef]
- Cooper, M.A.; Menta, B.W.; Perez-Sanchez, C.; Jack, M.M.; Khan, Z.W.; Ryals, J.M.; Winter, M.; Wright, D.E. A ketogenic diet reduces metabolic syndrome-induced allodynia and promotes peripheral nerve growth in mice. Exp. Neurol. 2018, 306, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, Y.; Shirai, T.; Kobayashi, T.; Hino, O.; Tsujii, Y.; Inoue, H.; Kazami, M.; Tadokoro, T.; Suzuki, T.; Kobayashi, K.-I.; et al. The tuberous sclerosis complex model Eker (TSC2+/−) rat exhibits hyperglycemia and hyperketonemia due to decreased glycolysis in the liver. Arch. Biochem. Biophys. 2016, 590, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Fine, E.J.; Miller, A.; Quadros, E.V.; Sequeira, J.M.; Feinman, R.D. Acetoacetate reduces growth and ATP concentration in cancer cell lines which over-express uncoupling protein 2. Cancer Cell Int. 2009, 9, 14. [Google Scholar] [CrossRef]
- Thaler, S.; Choragiewicz, T.J.; Rejdak, R.; Fiedorowicz, M.; Turski, W.A.; Tulidowicz-Bielak, M.; Zrenner, E.; Schuettauf, F.; Zarnowski, T. Neuroprotection by acetoacetate and β-hydroxybutyrate against NMDA-induced RGC damage in rat—Possible involvement of kynurenic acid. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1729–1735. [Google Scholar] [CrossRef]
- Ho, M.; Chen, T.; Liu, J.; Dowling, P.; Hideshima, T.; Zhang, L.; Morelli, E.; Camci-Unal, G.; Wu, X.; Tai, Y.-T.; et al. Targeting histone deacetylase 3 (HDAC3) in the bone marrow microenvironment inhibits multiple myeloma proliferation by modulating exosomes and IL-6 trans-signaling. Leukemia 2020, 34, 196–209. [Google Scholar] [CrossRef]
- Sonbuchner, T.M.; Rath, U.; Sharp, D.J. KL1 is a novel microtubule severing enzyme that regulates mitotic spindle architecture. Cell Cycle 2010, 9, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ganguly, A.; Yin, S.; Cabral, F. Megakaryocyte lineage-specific class VI β-tubulin suppresses microtubule dynamics, fragments microtubules, and blocks cell division. Cytoskeleton 2011, 68, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Berent-Spillson, A.; Russell, J.W. Metabotropic glutamate receptor 3 protects neurons from glucose-induced oxidative injury by increasing intracellular glutathione concentration. J. Neurochem. 2007, 101, 342–354. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudo, H.; Kubo, A. The Aneugenicity of Ketone Bodies in Colon Epithelial Cells Is Mediated by Microtubule Hyperacetylation and Is Blocked by Resveratrol. Int. J. Mol. Sci. 2021, 22, 9397. https://doi.org/10.3390/ijms22179397
Sudo H, Kubo A. The Aneugenicity of Ketone Bodies in Colon Epithelial Cells Is Mediated by Microtubule Hyperacetylation and Is Blocked by Resveratrol. International Journal of Molecular Sciences. 2021; 22(17):9397. https://doi.org/10.3390/ijms22179397
Chicago/Turabian StyleSudo, Haruka, and Akira Kubo. 2021. "The Aneugenicity of Ketone Bodies in Colon Epithelial Cells Is Mediated by Microtubule Hyperacetylation and Is Blocked by Resveratrol" International Journal of Molecular Sciences 22, no. 17: 9397. https://doi.org/10.3390/ijms22179397
APA StyleSudo, H., & Kubo, A. (2021). The Aneugenicity of Ketone Bodies in Colon Epithelial Cells Is Mediated by Microtubule Hyperacetylation and Is Blocked by Resveratrol. International Journal of Molecular Sciences, 22(17), 9397. https://doi.org/10.3390/ijms22179397





