Usage of Natural Volatile Organic Compounds as Biological Modulators of Disease
Abstract
:1. Introduction
2. Biological Effects of Natural Volatile Compounds
2.1. (+)-3-Carene
2.2. Camphene
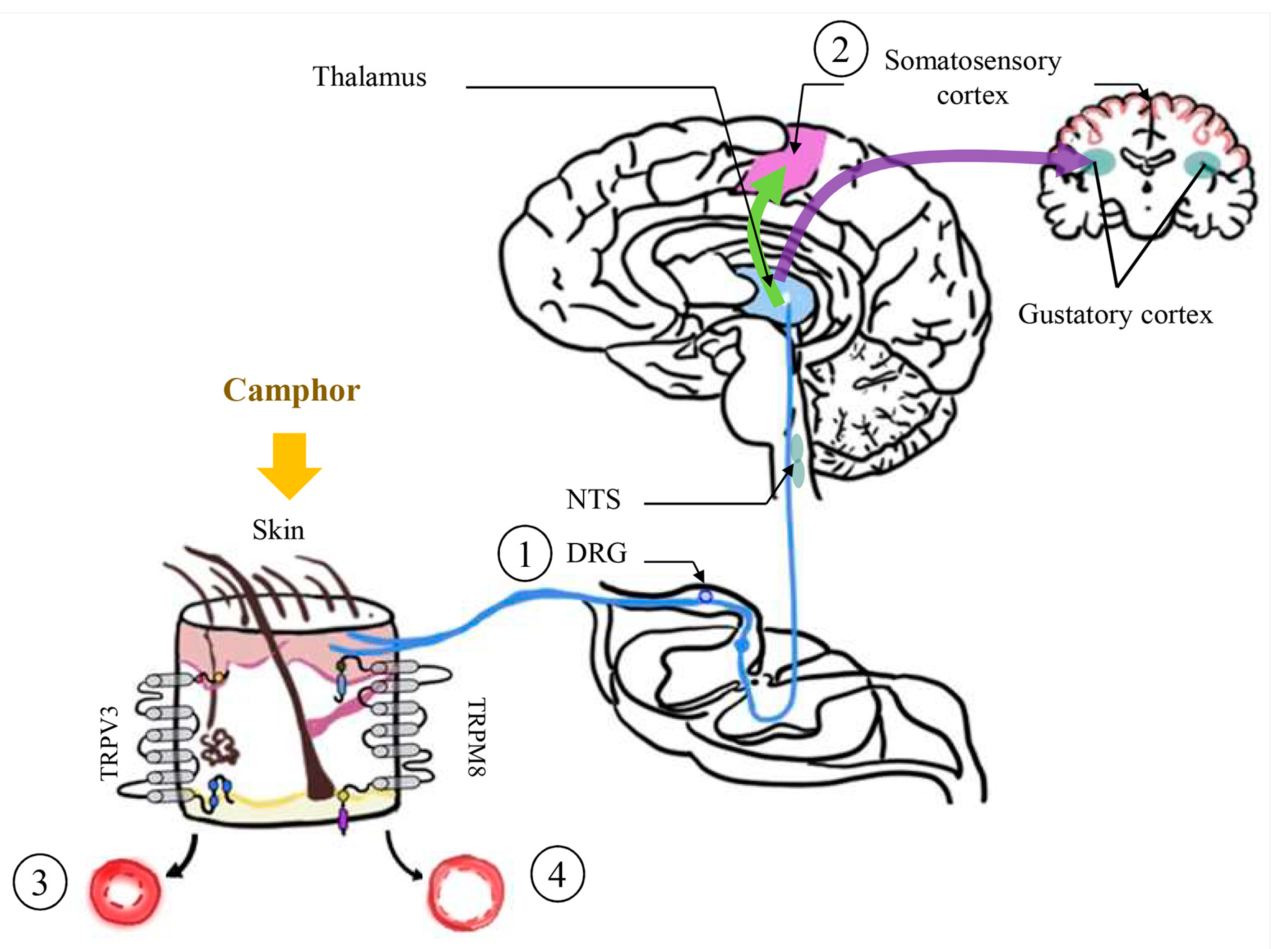
2.3. Camphor
2.4. 1,8-Cineol
2.5. p-Cymene
2.6. Limonene
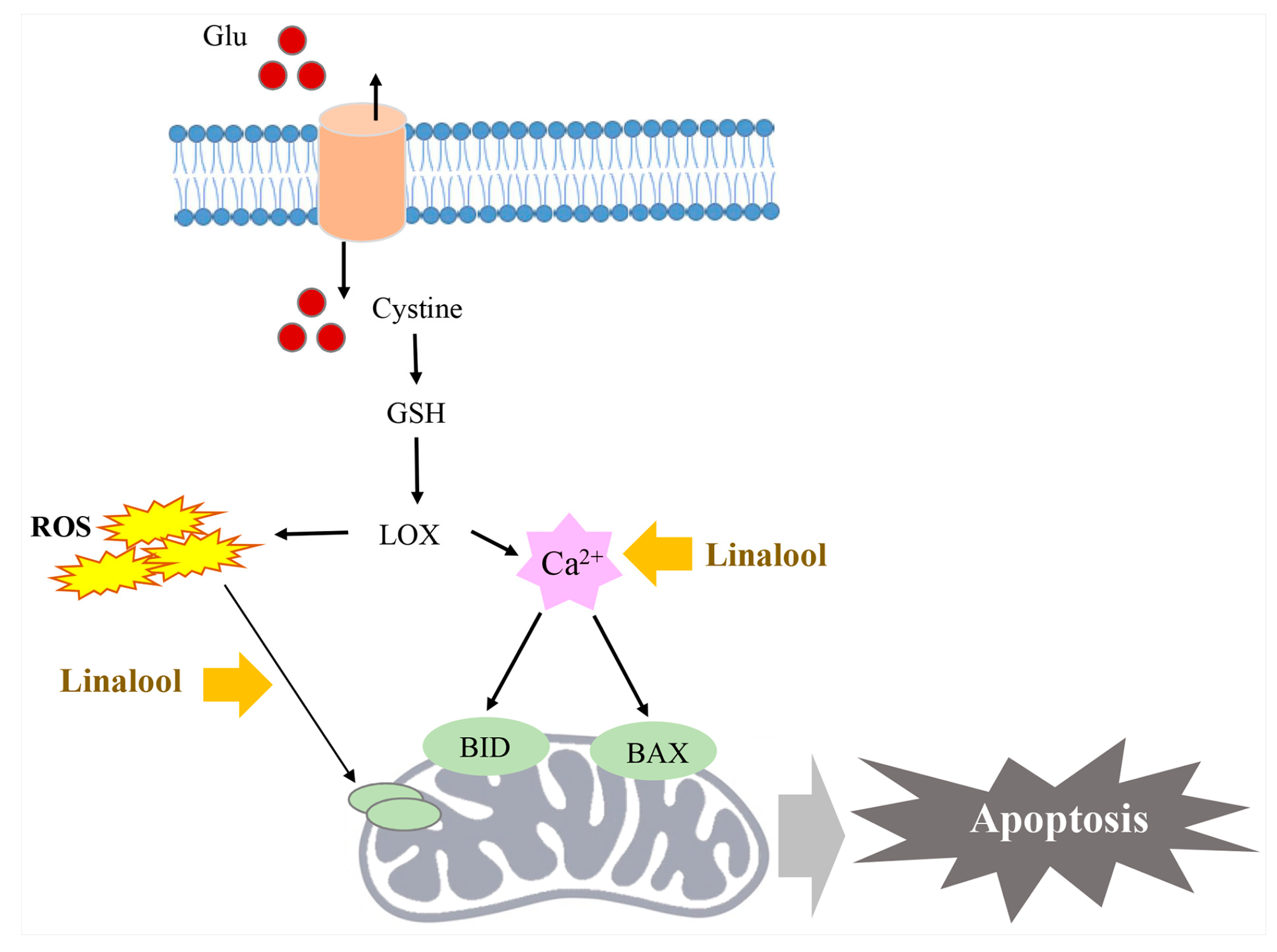
2.7. Linalool
2.8. Myrcene
2.9. α-Phellandrene
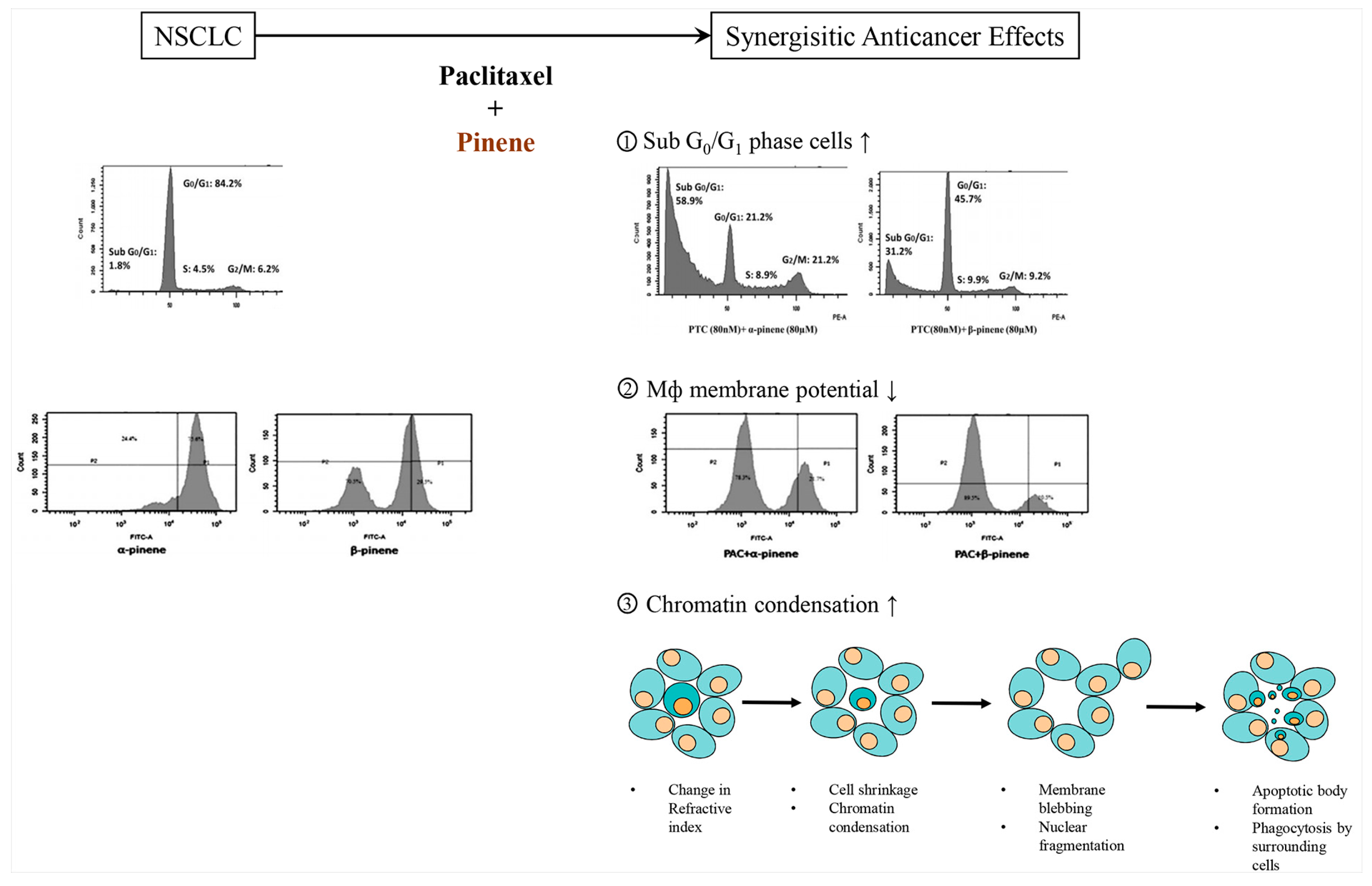
2.10. Pinene
2.10.1. α-Pinene
2.10.2. β-Pinene
2.11. α-Terpinen
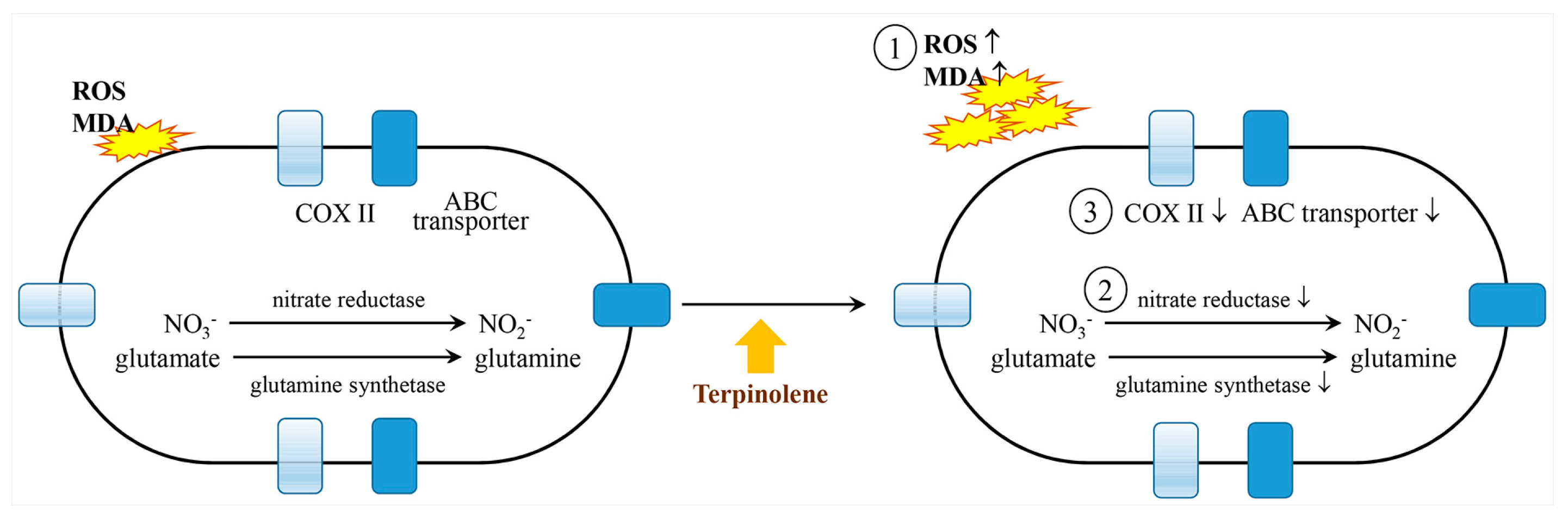
2.12. Terpinolene
3. NVOC Safety
4. Perspectives
Funding
Conflicts of Interest
References
- Health Canada. Available online: http://www.hc-sc.gc.ca/ewh-semt/pubs/air/office_building-immeubles_bureaux/organic-organiques-eng.php (accessed on 26 August 2021).
- European Solvents Downstream Users Coordination Group. How Are Solvents Regulated? Why Do Products Contain VOC Solvents? And Why Do We (Still) Use Them? Focus: Solvents and VOCs. Available online: https://www.esig.org/wp-content/uploads/2020/04/VOC_IAQ_final_202002.pdf (accessed on 26 August 2021).
- The National Institute of Standards and Technology Home Page. Available online: http://cpcb.nic.in/displaypdf.php?id=aG9tZS9haXItcG9sbHV0aW9uL05vLTE0LTE5ODEucGRm (accessed on 29 July 2021).
- Definitions of VOC and ROG; California Air Resources Board: Sacramento, CA, USA, November 2004.
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant. Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Furstenberg-Hagg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [Green Version]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Llusia, J.; Penuelas, J.; Gimeno, B. Seasonal and species-specific response of VOC emissions by Mediterranean woody plant to elevated ozone concentrations. Atmos. Environ. 2002, 36, 3931–3938. [Google Scholar] [CrossRef]
- Niederbacher, B.; Winkler, J.B.; Schnitzler, J.P. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Botany. 2015, 66, 5403–5416. [Google Scholar] [CrossRef]
- Penuelas, J.; Llusia, J. The complexity of factors driving volatile organic compound emissions by plants. Biol. Plant. 2001, 44, 481–487. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant. Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Hansen, U.; Seufert, G. Temperature and light dependence of beta-caryophyllene emission rates. J. Geophys. Res. Atmos. 2003, 108, 4801. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Loreto, F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of Kudzu leaves. Oecologia 1993, 95, 328–333. [Google Scholar] [CrossRef]
- Staudt, M.; Seufert, G. Light-dependent emission of monoterpenes by holm oak (Quercus ilex L). Naturwissenschaften 1995, 82, 89–92. [Google Scholar] [CrossRef]
- Ebel, R.C.; Mattheis, J.P.; Buchanan, D.A. Drought stress of apple-trees alters leaf emissions of volatile compounds. Physiol. Plant. 1995, 93, 709–712. [Google Scholar] [CrossRef]
- Vallat, A.; Gu, H.; Dorn, S. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry 2005, 66, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Delfine, S. Emission of isoprene from salt-stressed Eucalyptus globulus leaves. Plant. Physiol. 2000, 123, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Heiden, A.C.; Hoffmann, T.; Kahl, J.; Kley, D.; Klockow, D.; Langebartels, C.; Mehlhorn, H.; Sandermann, H., Jr.; Schraunder, M.; Schuh, G.; et al. Emission of volatile organic compounds from ozone-exposed plants. Ecol. Appl. 1999, 9, 1160–1167. [Google Scholar] [CrossRef]
- Vuorinen, T.; Nerg, A.M.; Holopainen, J.K. Ozone exposure triggers the emission of herbivore-induced plant volatiles, but dose not disturb tritrophic singalling. Environ. Pollut. 2004, 131, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Yang, H.; Yoon, M.; Gadhe, C.G.; Pae, A.N.; Cho, S.; Lee, C.J. 3-Carene, a phytoncide from pine tree has a sleep-enhancing effect by targeting the GABAA-benzodiazepine receptors. Exp. Neurobiol. 2019, 28, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Vallianou, I.; Hadzopoulou-Cladaras, M. Camphene, a plant derived monoterpene, exerts its hypolipidemic action by affecting SREBP-1 and MTP expression. PLoS ONE 2016, 11, e0147117. [Google Scholar] [CrossRef] [Green Version]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.A.; Matsuo, A.L.; Travassos, L.R.; et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Lenis-Rojas, O.A.; Robalo, M.P.; Tomaz, A.I.; Carvalho, A.; Fernandes, A.R.; Marques, F.; Folgueira, M.; Yanez, J.; Vazquez-Garcia, D.; Torres, M.L.; et al. RuII(p-cymene) compounds as effective and selective anticancer candidates with no toxicity in vivo. Inorgan. Chem. 2018, 57, 13150–13166. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med. Sci. Monit. 2019, 25, 6631–6638. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Turkez, H.; Tasdemir, S. Anticancer and antioxidant properties of terpinolene in rat brain cells. Arh. Hig. Rada. Toksikol. 2013, 64, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.Y.; Song, J.; Yun, H.S.; Lee, I.-S.; Koo, B.-S.; et al. Neuroprotective effects of limonene (+) against Aβ42-induced neurotoxicity in a Drosophila model of Alzheimer’s disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Sabogal-Guaqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dogla, A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019, 118, 10295. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.U.S.; Hellman, B.; Nyberg, F.; Amir, N.; Jayaraj, R.L.; Petroianu, G.; Adem, A. Myrcene attenuates renal inflammation and oxidative stress in the adrenalectomized rat model. Molecules 2020, 25, 4492. [Google Scholar] [CrossRef]
- Du, Y.; Luan, J.; Jiang, R.P.; Liu, J.; Ma, Y. Myrcene exerts anti-asthmatic activity in neonatal rats via modulating the matrix remodeling. Immunopathol. Pharmacol. 2020, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Agarwal, K.; Singh, M.; Saxena, A.; Yadav, P.; Maurya, A.K.; Yadav, A.; Tandon, S.; Chanda, D.; Bawankule, D.U. Essential oil from waste leaves of Curcuma longa L. alleviates skin inflammation. Inflammopharmacology 2018, 26, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Grando, T.H.; Souza, C.F.; Gressler, L.T.; Stefani, L.M.; de Silva, A.S.; Monteiro, S.G. In vitro and in vivo action of terpinen-4-ol, γ-terpinene, and α-terpinene against Trypanosoma evansi. Exp. Parasiol. 2016, 162, 43–48. [Google Scholar] [CrossRef]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov (accessed on 29 July 2021).
- Falk, A.; Lof, A.; Hagberg, M.; Hjelm, E.W.; Wang, Z. Human exposure to 3-carene by inhalation: Toxicokinetics, effects on pulmonary function and occurrence of irritative and CNS symptoms. Toxicol. Appl. Pharm. 1991, 110, 198–205. [Google Scholar] [CrossRef]
- Schlumpf, M.; Durrer, S.; Faass, O.; Ehnes, C.; Fuetsch, M.; Gaille, C.; Henseler, M.; Hofkamp, L.; Maerkel, K.; Reolon, S.; et al. Developmental toxicity of UV filters and environmental exposure: A review. Int. J. Androl. 2008, 31, 144–151. [Google Scholar] [CrossRef]
- Dorsam, B.; Wu, C.F.; Efferth, T.; Kaina, B.; Fahrer, J. The eucalyptus oil ingredient 1,8-cineol induces oxidative DNA damage. Arch. Toxicol. 2015, 89, 797–805. [Google Scholar] [CrossRef]
- Ravichandran, C.; Badgujar, P.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food. Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef]
- Audrain, H.; Kenward, C.; Lovell, C.R.; Green, C.; Ormerod, A.D.; Sansom, J.; Chowdhury, M.M.U.; Cooper, S.M.; Johnston, G.A.; Wilkinson, M.; et al. Allergy to oxidized limonene and linalool is frequent in the U.K. Br. J. Dermatol. 2014, 171, 292–297. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.C.; Schmidt, E. Tee tree oil: Contact allergy and chemical composition. Contact Dermatitis 2016, 75, 129–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/443156 (accessed on 29 July 2021).
- Shu, H.; Chen, H.; Wang, X.; Hu, Y.; Yun, Y.; Zhong, Q.; Chen, W.; Chen, W. Antimicrobial activity and proposed action mechanism of 3-carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules 2019, 24, 3246. [Google Scholar] [CrossRef] [Green Version]
- Langsi, J.D.; Nukenine, E.N.; Oumarou, K.M.; Moktar, H.; Fokunang, C.N.; Mbata, G.N. Evaluation of the insecticidal activities of α-pinene and 3-carene on Sitophilus zeamais motschulsky (Coleoptera: Curculionidae). Insects 2020, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Illikoud, N.; Jaffres, E.; Zagorec, M. Bronchothrix thermosphacta. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ray, B.; Bhunia, A. Fundamental Food Microbiology; CRC Press: Boca Raton, FL, USA, 2004; Volume 64, p. 33. [Google Scholar]
- Nukenine, E.N. Stored product pests. Jul. –Kuhn-Arch. 2010, 425, 26–41. [Google Scholar]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/6616 (accessed on 29 July 2021).
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected endemic mycoses. Lancet Infect. Dis. 2017, 17, e367–e377. [Google Scholar] [CrossRef]
- Silva, K.S.F.E.; Neto, B.R.D.S.; Zambuzzi-Carvalho, P.F.; Oliveira, C.M.D.; Pires, L.B.; Kato, L.; Bailao, A.M.; Parente-Rocha, J.A.; Hernandez, O.; Ochoa, J.G.M.; et al. Response of Paracoccidioides lutzii to the antifungal camphene thiosemicarbazide determined by proteomic analysis. Future. Microbiol. 2018, 13, 1473–1496. [Google Scholar] [CrossRef]
- Benelli, G.; Govindarajan, M.; Rajeswary, M.; Vaseeharan, B.; Alyahya, S.A.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Maggi, F. Insecticidal activity of camphene, zerumbone and α-humulene from Cheilocostus speciosus rhizome essential oil against the Old-World bollworm, Helicoverpa armigera. Ectoxicol. Environ. Saf. 2018, 148, 781–786. [Google Scholar] [CrossRef]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-coA reductase activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, M.; Kakkar, P. Plant derived antioxidnats−geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol In Vitro 2009, 23, 295–301. [Google Scholar] [CrossRef]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/230921 (accessed on 29 July 2021).
- Impact of Mosquito-Borne Diseases Worldwide in 2015. Available online: https://www.statista.com/statistics/515118/mosquito-borne-diseases-global-impact (accessed on 29 July 2021).
- Pohlit, A.M.; Lopes, N.P.; Gama, R.A.; Tadei, W.P.; de Andrade Neto, V.F. Patent literature on mosquito repellent inventions which contain plant essential oils−a review. Planta Med. 2011, 77, 598–617. [Google Scholar] [CrossRef] [Green Version]
- Lischewski, A.; Harmsen, D.; Wilms, K.; Baier, G.; Gunzer, U.; Klinker, H.; Wilhelm, M.; Schwinn, A.; Hacker, J. Molecular epidemiology of Candida albicans isolates from AIDS and cancer patients using a novel standardized CARE-2 DNA fingerprinting technique. Mycoses. 1999, 42, 371–383. [Google Scholar] [CrossRef]
- Xu, S.X.; McCormick, J.K. Staphylococcal superantigens in colonization and disease. Front. Cell. Infect. Microbiol. 2012, 2, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diggle, S.; Whitely, M. Microbe profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat. Microbiology. 2020, 166, 30–33. [Google Scholar] [CrossRef]
- Poole, K. Efflux-mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 2004, 10, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Kotaka, T.; Kimur, S.; Kashiwayanagi, M.; Iwamoto, J. Camphor induces cold and warm sensations with increases in skin and muscle blood flow in human. Biol. Pharm Bull. 2014, 37, 1913–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selescu, T.; Ciobanu, A.C.; Dobre, C.; Reid, G.; Babes, A. Camphor activates and sensitizes transient receptor potential melastatin 8 (TRPM8) to cooling and icilin. Chem. Senses. 2013, 38, 563–575. [Google Scholar] [CrossRef]
- Steinhoff, M.; Biro, T. A TR(I)P to pruritus research: Role of TRPV3 in inflammation and itch. J. Invet. Dermatol. 2009, 129, 531–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/2758 (accessed on 29 July 2021).
- Boland, D.J.; Brophy, J.J.; House, A.P.N. Eucalyptus Leaf Oils: Use, Chemistry, Distillation and Marketing; Inkata Press: Melbourne, Australia, 1991; p. 6. [Google Scholar]
- Li, Y.; Lai, Y.; Wang, Y.; Liu, N.; Zhang, F.; Xu, P. 1,8-cineol protect against influenza-virus-induced pneumonia in mice. Inflammation 2016, 39, 1582–1593. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, A.; Starkel, P.; Galle, M.; Crespo, R. 1,8-cineole promotes G0/G1 cell cycle arrest and oxidative stress-induced senescence n HepG2 cells and sensitizes cells to anti-senescence drugs. Life Sci. 2020, 243, 117271. [Google Scholar] [CrossRef]
- Juergens, L.J.; Worth, H.; Juergens, U.R. New perspectives for mucolytic, anti-inflammatory and adjunctive therapy with 1,8-cineole in COPD and asthma: Review on the new therapeutic approach. Adv. Ther. 2020, 37, 1737–1753. [Google Scholar] [CrossRef] [Green Version]
- Juergens, U.R.; Engelen, T.; Racke, K.; Stober, M.; Gillissen, A.; Vetter, H. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm. Pharmacol. Ther. 2004, 17, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, J.; Fang, C.; Tang, F. 1,8-cineol attenuates LPS-induced acute pulmonary inflammation in mice. Inflammation 2014, 37, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.F.W.; Muller, J.; Zeuner, M.-T.; Hauser, S.; Seidel, T.; Klenke, C.; Grunwald, L.-M.; Schomann, T.; Widera, D.; Sudhoff, H.; et al. 1,8-cineol inhibits nuclear translocation of NF-κB p65 and NF-κB-dependent transcriptional activity. Biochim. Biophy. Acta 2013, 1833, 2866–2878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/7463 (accessed on 29 July 2021).
- Yang, T.-S.; Chao, L.K.-P.; Liu, T.-T. Antimicrobial activity of the essential oil of Glossogyne tenuifolia against selected pathogens. J. Sci. Food Agric. 2014, 94, 2965–2971. [Google Scholar] [CrossRef]
- de Oliveira, T.M.; de Carvalho, R.B.F.; da Costa, I.H.F.; de Oliveira, G.A.L.; de Souza, A.A.; de Lima, S.G.; de Freitas, R.M. Evaluation of p-cymene, a natural antioxidant. Pharm. Biol. 2015, 53, 423–428. [Google Scholar] [CrossRef]
- Xie, G.; Chen, N.; Soromou, L.W.; Liu, F.; Xiong, Y.; Wu, Q.; Li, H.; Feng, H.; Liu, G. p-cymene protects mice against lipopolysaccharide-induced acute lung injury by inhibiting inflammatory cell activation. Molecules 2012, 17, 8159–8173. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Chi, G.; Jiang, L.; Soromou, L.W.; Chen, N.; Huo, M.; Guo, W.; Deng, X.; Feng, H. p-cymene modulates in vitro and in vivo cytokine production by inhibiting MAPK and NF-κB activation. Inflammation 2013, 36, 529–537. [Google Scholar] [CrossRef]
- Dougnon, G.; Ito, M. Role of ascaridole and p-cymene in the sleeping-promoting effects of Dysphania ambrosioides essential oil via the GABAergic system in a ddY mouse inhalation model. J. Nat. Prod. 2021, 84, 91–100. [Google Scholar] [CrossRef]
- Santos, W.B.R.; Melo, M.A.O.; Alves, R.S.; Brito, R.G.D.; Rabelo, T.K.; Prado, L.D.S.; Silva, V.K.D.S.; Bezerra, D.P.; Menezes-Filho, J.E.R.D.; Souza, D.S.; et al. p-cymene attenuates cancer pain via inhibitory pathways and modulation of calcium currents. Phytomed. 2019, 61, 152836. [Google Scholar] [CrossRef] [PubMed]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/22311 (accessed on 29 July 2021).
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial susceptibility and antibacterial mechanism of limonene against Listeria monocytogenes. Molecules 2020, 25, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, M.C.; Vieira, A.J.; Beserra, F.P.; Pellizzon, C.H.; Nobrega, R.H.; Rozza, A.L. Gastroprotective effect of limonene in rats: Influence on oxidative stress, inflammation and gene expression. phytomedicine. 2019, 53, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Yang, Z.-Y.; Fan, G.; Ren, J.-N.; Yin, K.-J.; Pan, S.-Y. Antidepressant-like effect of Citrus sinensis (L.) osbeck essential oil and its main component limonene on mice. J. Agric. Food Chem. 2019, 67, 13817–13828. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Kiumarsi, A.; Moghadam, A.R.; Alizadeh, J.; Marzban, H.; Ghavami, S. Perillyl alcohol (monoterpene alcohol), limonene. Enzymes 2014, 36, 7–32. [Google Scholar]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/6549 (accessed on 29 July 2021).
- Ponte, H.A.S.; Lima, M.I.D.O.; Lima, E.D.O.; Pereira, F.D.O. Linalool modulates dermatophyte susceptibility to azole drugs. Med. Mycol. 2019, 0, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Norwood, K.; Kennedy, P.J.; Leslie, J.C. Effects of linalool on extinction of mouse operant behavior. Behav. Pharmacol. 2020, 31, 73–80. [Google Scholar] [CrossRef]
- Yingngam, B.; Brantner, A.H. Factorial design of essential oil extraction from Fagraea fragrans Roxb. Flowers and evaluation of its biological activities for perfumery and cosmetic applications. Int. J. Cosmet. Sci. 2015, 37, 272–281. [Google Scholar] [CrossRef]
- Kim, M.-G.; Kim, S.-M.; Min, J.-H.; Kwon, O.-K.; Park, M.-H.; Park, J.-W.; Ahn, H.I.; Hwang, J.-Y.; Oh, S.-R.; Lee, J.-W.; et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharm. 2019, 74, 105706. [Google Scholar] [CrossRef]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/31253 (accessed on 29 July 2021).
- Fabbri, J.; Maggiore, M.A.; Pensel, P.E.; Albani, C.M.; Denegri, G.M.; Elissondo, M.C. Could beta-myrcene be an alternative to albendazole for the treatment of experimental cystic echinococcosis? Acta Trop. 2018, 187, 5–12. [Google Scholar] [CrossRef]
- Jansen, C.; Shimoda, L.M.N.; Kawakami, J.K.; Ang, L.; Bacani, A.J.; Baker, J.D.; Badowski, C.; Speck, M.; Stokes, A.J.; Small-Howard, A.L.; et al. Myrcene and terpene regulation of TRPV1. Channels 2019, 13, 344–366. [Google Scholar] [CrossRef] [Green Version]
- Hwang, E.; Ngo, H.T.T.; Park, B.; Seo, S.-A.; Yang, J.E.; Yi, T.-H. Myrcene, an aromatic volatile compound, ameliorates human skin extrinsic aging via regulation of MMPs production. Am. J. Chin. Med. 2017, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/443160 (accessed on 29 July 2021).
- Pavela, R.; Maggi, F.; Cianfaglione, K.; Canale, A.; Benelli, G. Promising insecticidal efficacy of the essential oils from the halophyte Echinophora spinosa (Apiaceae) growing in Corsica Island, France. Environ. Sci. Pollut. Res. Int. 2020, 27, 14454–14464. [Google Scholar] [CrossRef] [PubMed]
- Canales-Martinez, M.; Rivera-Yanez, C.R.; Salas-Oropeza, J.; Lopez, H.R.; Jimenez-Estrada, M.; Rosas-Lopez, R.; Duran, D.A.; Flores, C.; Hernandez, L.B.; Rodriguez-Monroy, M.A. Antimicrobial activity of Bursera morelensis ramirez essential oil. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 74–82. [Google Scholar]
- De Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef]
- Winnacker, M. Pinenes: Abundant and renewable building blocks for a variety of sustainable polymers. Angew. Chem. Int. Ed. 2018, 57, 14362–14371. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juca, D.M.; da Silva, M.T.B.; Palheta Junior, R.C.; de Lima, F.J.; Okoba, W.; Lahlou, S.; de Oliveira, R.B.; dos Santos, A.A.; Magalhaes, P.J.C. The essential oil of Eucalyptus tereticornis and its constituents α- and β-pinene, show accelerative properties on rat gastrointestinal transit. Planta Med. 2011, 77, 57–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Guo, S.; Liu, X.; Gao, X. Synergistic antitumor effect of alpha-pinene and beta-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC). Drug Res. 2015, 65, 214–218. [Google Scholar]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Kovac, J.; Simunovic, K.; Wu, Z.; Klancnik, A.; Bucar, F.; Zhang, Q.; Mozina, S.S. Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLoS ONE 2015, 10, e0122871. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Scutti, J.A.B.; Masaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiacease) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Woo, J.; Pae, A.N.; Um, M.Y.; Cho, N.C.; Park, K.D.; Yoon, M.; Kim, J.; Lee, C.J.; Cho, S. alpha-Pinene, a major constituent of pine tree oils, enhances non-rapid eye movement sleep in mice through GAGAA-benzodiazepine receptors. Mol. Pharmacol. 2016, 90, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.-Y.; Chung, C.-k.; Seo, J.-H.; Rah, S.-Y.; Kim, H.-M.; Jeong, H.-J. The therapeutic efficacy of α-pinene in an experimental mouse model of allergic rhinitis. Int. Immunopharmacol. 2014, 23, 273–282. [Google Scholar] [CrossRef]
- Mahajan, P.; Singh, H.P.; Kaur, S.; Batish, D.R.; Kohli, R.K. β-Pinene moderates Cr(VI) phytotoxicity by quenching reactive oxygen species and altering antioxidant machinery in maize. Environ. Sci. Pollut. Res. 2019, 26, 456–463. [Google Scholar] [CrossRef] [PubMed]
- de Macedo Andrade, A.C.; Rosalen, P.L.; Freires, I.A.; Scotti, L.; Scotti, M.T.; Aquino, S.G.; de Castro, R.D. Antifungal activity, mode of action, docking prediction and anti-biofilm effects of (+)-β-piene enantiomers against Cadida spp. Curr. Trend. Med. Chem. 2018, 18, 10. [Google Scholar]
- Moreira, I.J.A.; Menezes, P.P.; Serafini, M.R.; Araujo, A.A.S.; Quintans-Junior, L.J.; Bonjardim, L.R.; Filho, V.J.S.; Junior, D.B.P.; Santos, S.L.; Junior, W.L.; et al. Characterization and antihypertensive effect of the complex of (-)-β-pinene in β-cyclodextrin. Curr. Pharm. Biotechnol. 2016, 17, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Schnitzler, P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran. J. Microbiol. 2014, 6, 149–155. [Google Scholar]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/7462 (accessed on 29 July 2021).
- Morais, L.P.D.; Silva, A.A.; Silva, R.E.R.D.; Navarro, D.M.A.F.; Coutinho, H.D.M.; Menezes, I.R.A.D.; Kerntopf, M.R.; Cunha, F.A.B.D.; Leal-Cardoso, J.H.; Barbosa, R. Myorelaxant action of the Cysphania ambrosioides (L.) mosyakin & clemants essential oil and its major constituent α-terpinene in isolated rat trachea. Food Chem. 2020, 325, 126923. [Google Scholar]
- de Morais Oliveira-Tintino, C.D.; Tintino, S.R.; Limaverde, P.W.; Figueredo, F.G.; Campina, F.F.; da Cunha, F.A.B.; da Costa, R.H.S.; Pereira, P.S.; Lima, L.F.; de Matos, Y.M.L.S.; et al. Inhibition of the essential oil from Chenophodium ambrosioides L. and α-terpinene on the NorA efflux-pump of Staphylococcus aureus. Food Chem. 2018, 262, 72–77. [Google Scholar]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/11463 (accessed on 29 July 2021).
- Scherf, J.R.; dos Santos, C.R.B.; de Freitas, T.S.; Rocha, J.E.; Macedo, N.S.; Lima, J.N.M.; Coutinho, H.D.M.; da Cunha, F.A.B. Effect of terpinolene against the resistant Staphylococcus aureus stain, carrier of the efflux pump QacC and β-lactamase gene, and its toxicity in the Drosophila melanogaster model. Microb. Pathog. 2020, 149, 104528. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ito, M. The sedative effect of inhaled terpinolene in mice and its structure-activity relationship. J. Nat. Med. 2013, 67, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Sengupta, A.; Gupta, K.; Gupta, B. Molecular phylogenetic study and expression analysis of ATP-binding cassette transporter gene family in Oryza sativa in response to salt stress. Comput. Biol. Chem. 2015, 54, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rayon, E.; Guilhermino, L.; Irazola, M.; Ivanina, A.V.; Sokolova, I.M.; Izagirre, U.; Marigomez, I. The influence of short-term experimental fasting on biomarker responsiveness in oil WAF exposed mussels. Aquat. Toxicol. 2019, 206, 164–175. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, Y.; Huang, W.; He, L.; Lin, Z.; Zhou, J.; He, Q. Toxic effects of terpinolene on Microcystis qeruginosa: Physiological, metabolism, gene transcription, and growth effect. Sci. Total. Environ. 2020, 719, 137376. [Google Scholar] [CrossRef] [PubMed]
- Siegel, E.; Wason, S. Camphor toxicity. Pediatr. Clon. North. Am. 1986, 33, 375–379. [Google Scholar] [CrossRef]
- Lam, H.R.; Ladefoged, O.; Ostergaard, G.; Lund, S.P.; Simonsen, L. Four weeks’ inhalation exposure of rats to p-cymene affects regional and synaptosomal neurochemistry. Pharmacol. Toxicol. 1996, 79, 225–230. [Google Scholar] [CrossRef]
- Mog, S.R.; Zang, Y.J. Safety assessment of food additives: Case example with myrcene, a synthetic flavoring agent. Toxicol. Pathol. 2019, 47, 1035–1037. [Google Scholar] [CrossRef]
- Johard, U.; Larsson, K.; Lof, A.; Eklund, A. Controlled short-time terpene exposure induces an increase of the macrophages and the mast cells in bronchoalveolar lavage fluid. Am. J. Ind. Med. 1993, 23, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Pubchem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/14896 (accessed on 29 July 2021).
- Araujo, I.B.; Souza, C.A.M.; De-Carbalho, R.R.; Kuriyama, S.N.; Rodrigues, R.P.; Vollmer, R.S.; Alves, E.N.; Paumgartten, F.J.R. Study of the embryofoetotoxicity of α-terpinene in the rat. Food Chem. Toxicol. 1996, 34, 477–482. [Google Scholar] [CrossRef]
- Hall, D.E.; Robert, J.A.; Keeling, C.I.; Domanski, D.; Quesada, A.L.; Jancsik, S.; Kuzyk, M.A.; Hamberger, B.; Borchers, C.H.; Bohlmann, J. An integrated genomic, proteomic and biochemical analysis of (+)-3-carene biosynthesis in Sitka spruce (Picea sitchensis) genotypes that are resistant or susceptible to white pine weevil. Plant. 2011, 65, 936–948. [Google Scholar] [CrossRef]
- Boruga, O.; Jianu, C.; Misca, C.; Golet, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life. 2014, 7, 56–60. [Google Scholar] [PubMed]
- Ibrahim, E.A.; Wang, M.; Radwan, M.M.; Wanas, A.S.; Majumdar, C.G.; Avula, B.; Wang, W.-H.; Khan, I.; Chandra, S.; Lata, H.; et al. Analysis of terpenes in Cannabis sativa L. using GC/MS: Method development, validation, and application. Planta Med. 2019, 85, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Benali, T.; Habbadi, K.; Khabbach, A.; Marmouzi, I.; Zengin, G.; Bouyahya, A.; Chamkhi, I.; Chtibi, H.; Aanniz, T.; Achbbani, E.H.; et al. GC-MS analysis, antioxidant and antimicrobial activities of Achillea odorata subsp. Pectinata and Ruta montana essential oils and their potential use as food preservatives. Foods. 2020, 9, 668. [Google Scholar]
- Rabinovich, D. The father of toxicology? Chem. Int. 2009, 30, 3. [Google Scholar]
- Bostrom, J.; Brown, D.G.; Young, R.J.; Keseru, G.M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 2018, 17, 709–727. [Google Scholar] [CrossRef]
- NTP TR-557: National Toxicology Program Technical Report-557. Toxicology and carcinogenesis studies of beta-myrcene (CAS No. 123–35–3) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program. Tech. Rep. Ser. 2010, 557, 1–163. [Google Scholar]
- Food Additive Regulations; Synthetic Flavoring Agents and Adjuvants. Available online: https://www.federalregister.gov/documents/2018/10/09/2018-21807/food-additive-regulations-synthetic-flavoring-agents-and-adjuvants (accessed on 26 August 2021).
 : (+)-3-carene;
: (+)-3-carene;  : GABAA-benzodiazepine receptor.
: GABAA-benzodiazepine receptor.
 : (+)-3-carene;
: (+)-3-carene;  : GABAA-benzodiazepine receptor.
: GABAA-benzodiazepine receptor.

 : to inhibit the follow action.
: to inhibit the follow action.
 : to inhibit the follow action.
: to inhibit the follow action.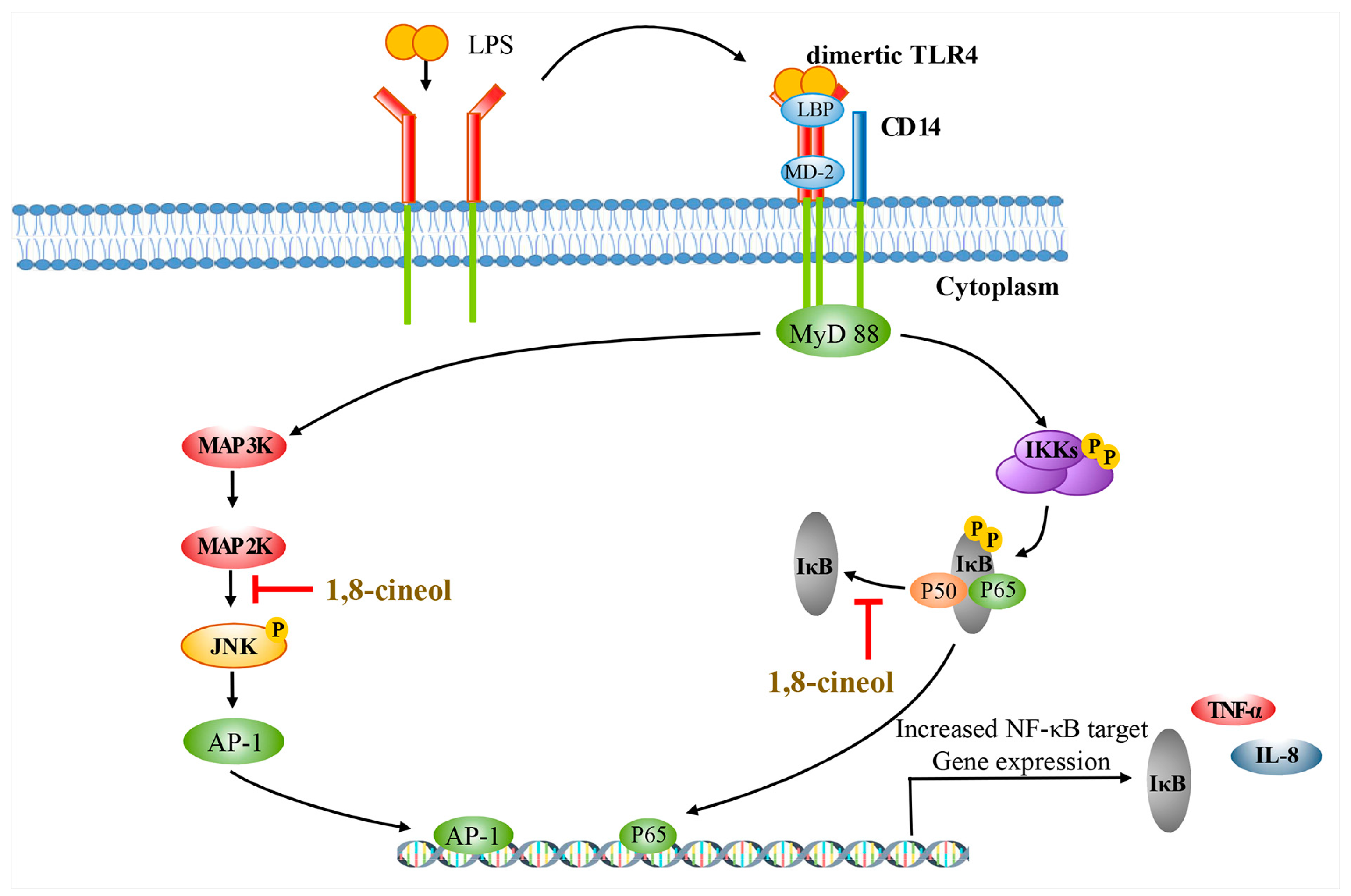

 : to inhibit the follow action.
: to inhibit the follow action.
 : to inhibit the follow action.
: to inhibit the follow action.
 : to inhibit the follow action.
: to inhibit the follow action.
 : to inhibit the follow action.
: to inhibit the follow action.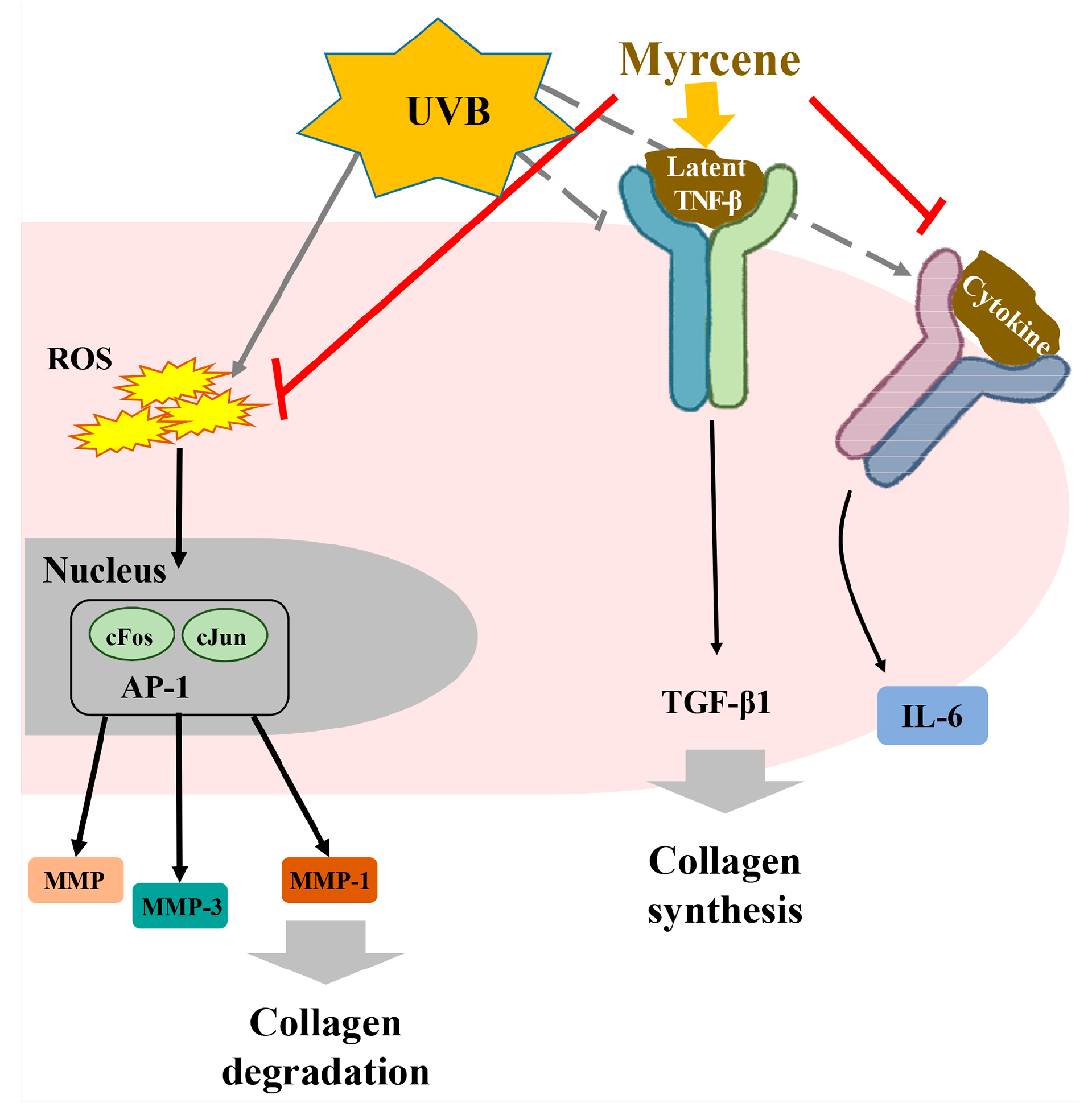
 : to inhibit the follow action.
: to inhibit the follow action.
 : to inhibit the follow action.
: to inhibit the follow action.

| Natural Volatile Organic Compound | Chemical Structure | Molecular Weight (g/mol) | Toxicological Effect | References |
|---|---|---|---|---|
| (+)-3-carene | 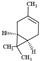 | 136.24 | To irritate pulmonary system and central nervous system | [33] |
| Camphene |  | 136.23 | To irritate serious eye | [45] |
| Camphor | 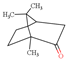 | 152.23 | To irritate gastrointestinal tract and central nervous system | [116] |
| 1,8-cineol | 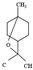 | 154.25 | Genotoxicity via inducing oxidative DNA damage | [35] |
| p-cymene |  | 134.22 | To induce neurochemical problem by 4 weeks of inhalation | [117] |
| Limonene | 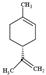 | 136.23 | Hepatotoxicity and neurotoxicity Oxidized form makes skin allergy. | [36,37] |
| Linalool |  | 154.25 | To induce skin allergy by oxidized linalool | [37] |
| Myrcene | 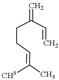 | 136.23 | It does not have unique toxicity but in 2018 US FDA decided to stop the usage of it as a food additive. | [118] |
| ɑ-phellandrene | 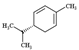 | 136.20 | To make contact allergy | [38] |
| ɑ-pinene | 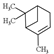 | 136.23 | To induce pulmonary inflammation | [119] |
| ß-pinene | 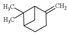 | 136.23 | To irritate skin and mucous system | [120] |
| ɑ-terpinene | 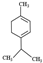 | 136.23 | Embryofoetal toxicity | [119] |
| Terpinolene | 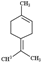 | 136.23 | Oxidized forms can make allergic contact dermatitis. | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-H.; Lee, S.-M.; An, K.-W.; Lee, M.-J.; Park, D.-H. Usage of Natural Volatile Organic Compounds as Biological Modulators of Disease. Int. J. Mol. Sci. 2021, 22, 9421. https://doi.org/10.3390/ijms22179421
Kim M-H, Lee S-M, An K-W, Lee M-J, Park D-H. Usage of Natural Volatile Organic Compounds as Biological Modulators of Disease. International Journal of Molecular Sciences. 2021; 22(17):9421. https://doi.org/10.3390/ijms22179421
Chicago/Turabian StyleKim, Min-Hee, Seung-Min Lee, Ki-Wan An, Min-Jae Lee, and Dae-Hun Park. 2021. "Usage of Natural Volatile Organic Compounds as Biological Modulators of Disease" International Journal of Molecular Sciences 22, no. 17: 9421. https://doi.org/10.3390/ijms22179421
APA StyleKim, M. -H., Lee, S. -M., An, K. -W., Lee, M. -J., & Park, D. -H. (2021). Usage of Natural Volatile Organic Compounds as Biological Modulators of Disease. International Journal of Molecular Sciences, 22(17), 9421. https://doi.org/10.3390/ijms22179421







