Investigating the Molecular Processes behind the Cell-Specific Toxicity Response to Titanium Dioxide Nanobelts
Abstract
:1. Introduction
2. Results and Discussion
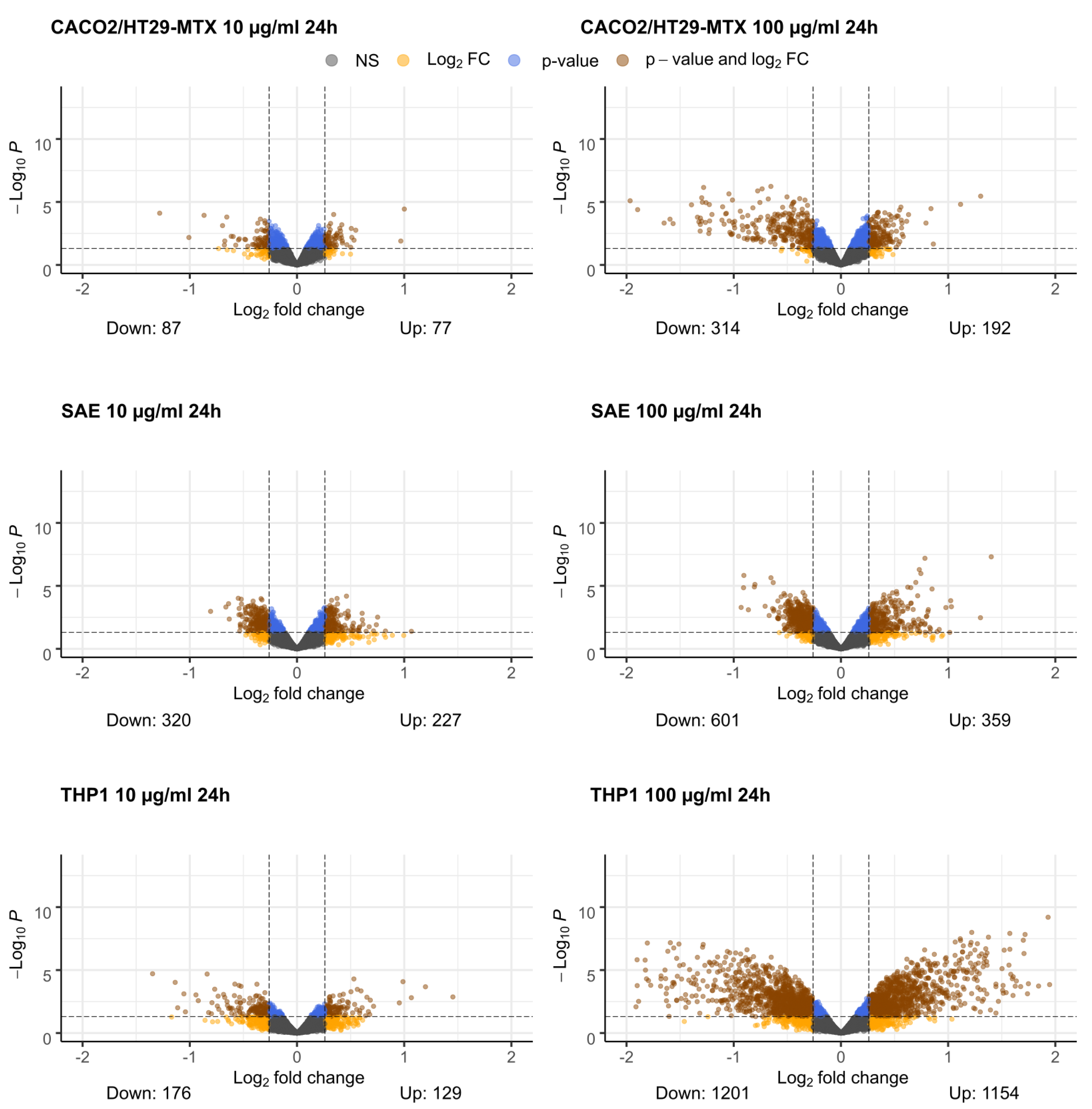
2.1. Differential Gene Expression
2.2. Pathway Analysis
2.3. Toxicity-Related Pathways
2.4. Study Effect on Toxicity-Related Pathways
2.5. Caco-2/HT29-MTX Cells
2.6. SAE Cells
2.7. THP1 Cells
2.8. Comparison between Cell Lines
2.9. The Advantage of Our Approach
3. Conclusions
4. Materials and Methods
4.1. Dataset
4.2. Pathway Analysis
4.3. Toxicity-Related Processes
4.4. Network Visualization
4.5. Reproducible Analysis Workflow
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TiO2 | Titanium dioxide |
| SAE | Small airway epithelial cells |
| THP1 | Human monocytic cells |
| Caco-2 | Human epithelial colorectal adenocarcinoma cells |
| GO | Gene Ontology |
Appendix A
| Number of Pathways Based on | |||||
|---|---|---|---|---|---|
| ORA (Adjust p Value < 0.05) | 80% Gene Overlap | 70% Gene Overlap | 60% Gene Overlap | 50% Gene Overlap | |
| apoptotic process (GO:0006915) | 401 | 11 | 15 | 31 | 66 |
| inflammatory response (GO:0006954) | 250 | 0 | 0 | 6 | 15 |
| DNA damage (GO:0006974) | 203 | 15 | 20 | 23 | 28 |
| oxidative stress (GO:0006979) | 205 | 1 | 1 | 1 | 1 |
| Abbreviation | Full Name | Category |
|---|---|---|
| IBA | Inferred from Biological aspect of Ancestor | Phylogenetically-inferred annotations |
| IC | Inferred by Curator | Curator statement evidence codes |
| IDA | Inferred from Direct Assay | Experimental evidence codes |
| IEP | Inferred from Expression Pattern | Experimental evidence codes |
| IGI | Inferred from Genetic Interaction | Experimental evidence codes |
| IMP | Inferred from Mutant Phenotype | Experimental evidence codes |
| IPI | Inferred from Physical Interaction | Experimental evidence codes |
| TAS | Traceable Author Statement | Author statement evidence codes |
| Removed annotations | ||
| ND | No Biological Data Available | Curator statement evidence codes |
| NAS | Non-traceable Author Statement | Author statement evidence codes |
| IEA | Inferred from Electronic Annotation | Electronic annotation evidence code |
| ISS | Inferred from Sequence or structural Similarity | Computational analysis evidence codes |
| ISO | Inferred from Sequence Orthology | Computational analysis evidence codes |
| ISA | Inferred from Sequence Alignment | Computational analysis evidence codes |
| ISM | Inferred from Sequence Model | Computational analysis evidence codes |
| IGC | Inferred from Genomic Context | Computational analysis evidence codes |
| RCA | Inferred from Reviewed Computational Analysis | Computational analysis evidence codes |



References
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Song, I.; Um, S. Role of Physicochemical Properties in Nanoparticle Toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visonà, S.D.; Villani, S.; Manzoni, F.; Chen, Y.; Ardissino, G.; Russo, F.; Moretti, M.; Javan, G.T.; Osculati, A. Impact of asbestos on public health: A retrospective study on a series of subjects with occupational and non-occupational exposure to asbestos during the activity of Fibronit plant (Broni, Italy). J. Public Health Res. 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Markowitz, S. Asbestos-Related Lung Cancer and Malignant Mesothelioma of the Pleura: Selected Current Issues. Semin. Respir. Crit. Care Med. 2015, 36, 334–346. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Wang, H.; Hnizdo, E.; Sun, Y.; Su, L.; Zhang, X.; Weng, S.; Bochmann, F.; Hearl, F.J.; et al. Long-Term Exposure to Silica Dust and Risk of Total and Cause-Specific Mortality in Chinese Workers: A Cohort Study. PLoS Med. 2012, 9, e1001206. [Google Scholar] [CrossRef]
- Arnoldussen, Y.; Ervik, T.K.; Eriksen, M.B.; Kero, I.; Skaug, V.; Zienolddiny, S. Cellular Responses of Industrially Relevant Silica Dust on Human Glial Cells In Vitro. Int. J. Mol. Sci. 2019, 20, 358. [Google Scholar] [CrossRef] [Green Version]
- Podila, R.; Brown, J.M. Toxicity of Engineered Nanomaterials: A Physicochemical Perspective. J. Biochem. Mol. Toxicol. 2012, 27, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M.a. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Stefano, D.D.; Carnuccio, R.; Maiuri, M.C. Nanomaterials Toxicity and Cell Death Modalities. J. Drug Deliv. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.M.; TeeSy, C.; Franzi, L.; Weir, A.; Westerhoff, P.; Evans, J.E.; Pinkerton, K.E. Biological Response to Nano-Scale Titanium Dioxide (TiO2): Role of Particle Dose, Shape, and Retention. J. Toxicol. Environ. Health Part A 2013, 76, 953–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atha, D.H.; Nagy, A.; Steinbrück, A.; Dennis, A.M.; Hollingsworth, J.A.; Dua, V.; Iyer, R.; Nelson, B.C. Quantifying engineered nanomaterial toxicity: Comparison of common cytotoxicity and gene expression measurements. J. Nanobiotechnol. 2017, 15, 79. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, K.; Stone, V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann. Dell’Istituto Super. Sanitã 2003, 39, 405–410. [Google Scholar]
- Sung, J.H.; Ji, J.H.; Yoon, J.U.; Kim, D.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H.; Chung, Y.H.; Kim, J.; et al. Lung Function Changes in Sprague-Dawley Rats After Prolonged Inhalation Exposure to Silver Nanoparticles. Inhal. Toxicol. 2008, 20, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Elle, R.E.; Gaillet, S.; Vidé, J.; Romain, C.; Lauret, C.; Rugani, N.; Cristol, J.; Rouanet, J. Dietary exposure to silver nanoparticles in Sprague—Dawley rats: Effects on oxidative stress and inflammation. Food Chem. Toxicol. 2013, 60, 297–301. [Google Scholar] [CrossRef]
- Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.; de la Torre, J.G.; Jiang, Y.; Valsami-Jones, E.; Langevin, D.; et al. Characterization of Nanoparticle Batch-To-Batch Variability. Nanomaterials 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliisa Saarimäki, L.; Federico, A.; Lynch, I.; Papadiamantis, A.G.; Tsoumanis, A.; Melagraki, G.; Afantitis, A.; Serra, A.; Greco, D. Manually curated transcriptomics data collection for toxicogenomic assessment of engineered nanomaterials. Sci. Data 2021, 8, 49. [Google Scholar] [CrossRef]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Elem. Res. 2015, 172, 1–36. [Google Scholar] [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef]
- L’Azou, B.; Jorly, J.; On, D.; Sellier, E.; Moisan, F.; Fleury-Feith, J.; Cambar, J.; Brochard, P.; Ohayon-Courtès, C. In vitro effects of nanoparticles on renal cells. Part. Fibre Toxicol. 2008, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free. Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, D.; Li, K.; Chen, Q.; Lai, W.; Tian, L.; Lin, B.; Tan, Y.; Liu, X.; Xi, Z. Toxic effects of the food additives titanium dioxide and silica on the murine intestinal tract: Mechanisms related to intestinal barrier dysfunction involved by gut microbiota. Environ. Toxicol. Pharmacol. 2020, 80, 103485. [Google Scholar] [CrossRef]
- Baisch, B.L.; Corson, N.M.; Wade-Mercer, P.; Gelein, R.; Kennell, A.J.; Oberdörster, G.; Elder, A. Equivalent titanium dioxide nanoparticle deposition by intratracheal instillation and whole body inhalation: The effect of dose rate on acute respiratory tract inflammation. Part. Fibre Toxicol. 2014, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Meena, R.; Kumar, S.; Paulraj, R. Titanium oxide (TiO2) nanoparticles in induction of apoptosis and inflammatory response in brain. J. Nanoparticle Res. 2015, 17, 49. [Google Scholar] [CrossRef]
- Rothen-Rutishauser, B.; Mühlfeld, C.; Blank, F.; Musso, C.; Gehr, P. Translocation of particles and inflammatory responses after exposure to fine particles and nanoparticles in an epithelial airway model. Part. Fibre Toxicol. 2007, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Déciga-Alcaraz, A.; Freyre-Fonseca, V.; Delgado-Buenrostro, N.L.; Flores-Flores, J.O.; Gutiérrez-López, G.F.; Sánchez-Pérez, Y.; García-Cuéllar, C.M.; Pedraza-Chaverri, J.; Chirino, Y.I. Titanium dioxide nanoparticles induce an adaptive inflammatory response and invasion and proliferation of lung epithelial cells in chorioallantoic membrane. Environ. Res. 2015, 136, 424–434. [Google Scholar] [CrossRef]
- Zhang, Y. Cell toxicity mechanism and biomarker. Clin. Transl. Med. 2018, 7, e34. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Tang, M. Toxic effects and involved molecular pathways of nanoparticles on cells and subcellular organelles. J. Appl. Toxicol. 2019, 40, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.; Sirota, M.; Butte, A.J. Ten Years of Pathway Analysis: Current Approaches and Outstanding Challenges. PLoS Comput. Biol. 2012, 8, e1002375. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.; Ammar, A.; Riutta, A.; Waagmeester, A.; Slenter, D.N.; Hanspers, K.; Miller, R.A.; Digles, D.; Lopes, E.N.; Ehrhart, F.; et al. WikiPathways: Connecting communities. Nucleic Acids Res. 2020, 49, D613–D621. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2017, 46, D649–D655. [Google Scholar] [CrossRef]
- Tilton, S.C.; Karin, N.J.; Tolic, A.; Xie, Y.; Lai, X.; Hamilton, R.F.; Waters, K.M.; Holian, A.; Witzmann, F.A.; Orr, G. Three human cell types respond to multi-walled carbon nanotubes and titanium dioxide nanobelts with cell-specific transcriptomic and proteomic expression patterns. Nanotoxicology 2013, 8, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E. Nanoparticle Exposure and Hormetic Dose–Responses: An Update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Stilwell, J.; Zhang, T.; Elboudwarej, O.; Jiang, H.; Selegue, J.P.; Cooke, P.A.; Gray, J.W.; Chen, F.F. Molecular Characterization of the Cytotoxic Mechanism of Multiwall Carbon Nanotubes and Nano-Onions on Human Skin Fibroblast. Nano Lett. 2005, 5, 2448–2464. [Google Scholar] [CrossRef] [Green Version]
- Bohler, A.; Weitz, E.; Kutmon, M.; Reactome Team. Detoxification of Reactive Oxygen Species (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP2824_r117697 (accessed on 28 August 2021).
- Reyes, I.; Hanspers, K.; Pico, A.; Schmidt, H.; Willighagen, E.; Rashid, K.; van Iersel, M.; Reyes, N.; Deng, X. Oxidative Stress (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP408_r106567 (accessed on 28 August 2021).
- Pandey, A.; Hanspers, K.; Pico, A.; Waagmeester, A.; Chichester, C.; Willighagen, E.; Weitz, E.; Dupuis, L.J.; van Iersel, M.; NetPath; et al. IL-5 Signaling Pathway (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP127_r116634 (accessed on 28 August 2021).
- Pandey, A.; Hanspers, K.; Pico, A.; Kutmon, M.; Willighagen, E.; Ehrhart, F.; Chichester, C.; Weitz, E.; van Iersel, M.; NetPath. IL-2 Signaling Pathway (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP49_r116633 (accessed on 28 August 2021).
- Hanspers, K.; Pico, A.; Ehrhart, F. Endometrial Cancer (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP4155_r119086 (accessed on 28 August 2021).
- Weitz, E.; Kutmon, M.; Reactome Team. HDR through Homologous Recombination (HRR) or Single Strand Annealing (SSA) (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP3567_r117752 (accessed on 28 August 2021).
- Miller, R.; Reactome Team. Nonhomologous End-Joining (NHEJ) (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP3550_r114784 (accessed on 28 August 2021).
- Kutmon, M.; Reactome Team. DNA Double Strand Break Response (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP3543_r114907 (accessed on 28 August 2021).
- Zambon, A.C.; Hanspers, K.; Pico, A.; Kutmon, M.; Kalafati, M.; van Iersel, M.; Nunes, N.; Riutta, A.; Willighagen, E.; Anam, A.; et al. Apoptosis (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP254_r106302 (accessed on 28 August 2021).
- Pandey, A.; Hanspers, K.; Willighagen, E.; Kutmon, M.; Chichester, C.; Dupuis, L.J.; van Iersel, M.; Coort, S.; Roudbari, Z.; NetPath. IL-6 signaling pathway (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP364_r115943 (accessed on 28 August 2021).
- Miller, R.; Reactome Team. Regulation of TP53 Activity through Phosphorylation (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP3838_r114823 (accessed on 28 August 2021).
- Manzo-Fontes, A.; Slenter, D.; Willighagen, E.; Hu, F.; Ehrhart, F.; Hanspers, K. DNA IR-Damage and Cellular Response via ATR (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP4016_r119294 (accessed on 28 August 2021).
- Manzo-Fontes, A.; Willighagen, E.; Hanspers, K. DNA IR-Double Strand Breaks (DSBs) and Cellular Response via ATM (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP3959_r101991 (accessed on 28 August 2021).
- Kelder, T.; Hanspers, K.; Pico, A.; Willighagen, E.; van Iersel, M. DNA Mismatch Repair (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP531_r113644 (accessed on 28 August 2021).
- Rückert, F.; Hanspers, K.; Willighagen, E.; Ehrhart, F.; Kalafati, M.; Pico, A.; Riutta, A.; Domingo-Fdez, D.; Weitz, E.; van Iersel, M.; et al. Apoptosis Modulation and Signaling (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP1772_r116807 (accessed on 28 August 2021).
- Reyes, I.; Hanspers, K.; Simons, L.; Pico, A.; Chichester, C.; Willighagen, E.; Weitz, E.; Bouwman, J.; Romano, P. Apoptosis Modulation by HSP70 (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP384_r117650 (accessed on 28 August 2021).
- Willighagen, E.; Ehrhart, F.; Reactome Team. TNF Signaling (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP3380_r114940 (accessed on 28 August 2021).
- Bohler, A.; Pico, A.; van Iersel, M.; Reactome Team. Apoptotic Execution Phase (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP1784_r114845 (accessed on 28 August 2021).
- Pandey, A.; Hanspers, K.; Pico, A.; Evelo, C.; Willighagen, E.; Roudbari, Z.; Digles, D.; Weitz, E.; Dupuis, L.J.; Kutmon, M.; et al. TNF-Alpha Signaling Pathway (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP231_r116472 (accessed on 28 August 2021).
- Hanspers, K.; Willighagen, E.; Pico, A.; Evelo, C.; Digles, D.; Weitz, E.; Kalafati, M.; Roudbari, Z.; NetPath. TNF-Related Weak Inducer of Apoptosis (TWEAK) Signaling Pathway (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP2036_r117848 (accessed on 28 August 2021).
- Brzicova, T.; Javorkova, E.; Vrbova, K.; Zajicova, A.; Holan, V.; Pinkas, D.; Philimonenko, V.; Sikorova, J.; Klema, J.; Topinka, J.; et al. Molecular Responses in THP-1 Macrophage-Like Cells Exposed to Diverse Nanoparticles. Nanomaterials 2019, 9, 687. [Google Scholar] [CrossRef] [Green Version]
- Willighagen, E.; Smeets, B.; Hanspers, K.; Verbruggen, T.; Winckers, L.; Kutmon, M. Nanoparticle Triggered Regulated Necrosis (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP2513_r110691 (accessed on 28 August 2021).
- Miller, R.; Reactome Team. Mismatch Repair (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP3381_r113324 (accessed on 28 August 2021).
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef] [Green Version]
- Weijer, R.; Hanspers, K.; Pico, A.; Ehrhart, F. Photodynamic Therapy-Induced NF-kB Survival Signaling (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP3617_r106541 (accessed on 28 August 2021).
- Reactome Team. Interleukin-10 Signaling (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP4063_r115095 (accessed on 28 August 2021).
- Hanspers, K.; Akassoglou, K.; Pico, A.; Mendiola, A.; Willighagen, E.; Weitz, E.; Kutmon, M. Fibrin Complement Receptor 3 Signaling Pathway (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP4136_r118430 (accessed on 28 August 2021).
- Hanspers, K.; Slenter, D.; Willighagen, E. Platelet-Mediated Interactions with Vascular and Circulating Cells (Homo Sapiens). 2020. Available online: https://wikipathways.org/instance/WP4462_r102079 (accessed on 28 August 2021).
- Åkerlund, E.; Islam, S.; McCarrick, S.; Alfaro-Moreno, E.; Karlsson, H.L. Inflammation and (secondary) genotoxicity of Ni and NiO nanoparticles. Nanotoxicology 2019, 13, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Tada-Oikawa, S.; Ichihara, G.; Fukatsu, H.; Shimanuki, Y.; Tanaka, N.; Watanabe, E.; Suzuki, Y.; Murakami, M.; Izuoka, K.; Chang, J.; et al. Titanium Dioxide Particle Type and Concentration Influence the Inflammatory Response in Caco-2 Cells. Int. J. Mol. Sci. 2016, 17, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, D.; Bramini, M.; Hristov, D.R.; Wan, S.; Salvati, A.; Åberg, C.; Dawson, K.A. Low uptake of silica nanoparticles in Caco-2 intestinal epithelial barriers. Beilstein J. Nanotechnol. 2017, 8, 1396–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, T.; Hamilton, R.F.; Bonner, J.C.; Crandall, E.D.; Elder, A.; Fazlollahi, F.; Girtsman, T.A.; Kim, K.; Mitra, S.; Ntim, S.A.; et al. Interlaboratory Evaluation of in Vitro Cytotoxicity and Inflammatory Responses to Engineered Nanomaterials: The NIEHS Nano GO Consortium. Environ. Health Perspect. 2013, 121, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urso, M.L.; Clarkson, P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Bouchier-Hayes, L.; Green, D.R. Caspase-2: The orphan caspase. Cell Death Differ. 2011, 19, 51–57. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.D. Caspase-7: A protease involved in apoptosis and inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Stang, M.T.; Armstrong, M.J.; Watson, G.A.; Sung, K.Y.; Liu, Y.; Ren, B.; Yim, J.H. Interferon regulatory factor-1-induced apoptosis mediated by a ligand-independent fas-associated death domain pathway in breast cancer cells. Oncogene 2007, 26, 6420–6430. [Google Scholar] [CrossRef] [Green Version]
- Fanzo, J.C.; Hu, C.M.; Jang, S.Y.; Pernis, A.B. Regulation of Lymphocyte Apoptosis by Interferon Regulatory Factor 4 (IRF-4). J. Exp. Med. 2003, 197, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Barnes, B.J. IRF-5 Is a Mediator of the Death Receptor-induced Apoptotic Signaling Pathway. J. Biol. Chem. 2009, 284, 2767–2777. [Google Scholar] [CrossRef] [Green Version]
- Mota, M.B.S.; Carvalho, M.A.; Monteiro, A.N.A.; Mesquita, R.D. DNA damage response and repair in perspective: Aedes aegypti, Drosophila melanogaster and Homo sapiens. Parasites Vectors 2019, 12, 533. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Winckers, L.A.; Willighagen, E.L.; Kutmon, M. Laurent2207/TiO2-Scripts: TiO2-NB Analysis Scripts (Version v2.2). 2021. Available online: https://doi.org/10.5281/zenodo.5137467 (accessed on 28 August 2021).
- Eijssen, L.M.T.; Jaillard, M.; Adriaens, M.E.; Gaj, S.; de Groot, P.J.; Müller, M.; Evelo, C.T. User-friendly solutions for microarray quality control and pre-processing on ArrayAnalysis.org. Nucleic Acids Res. 2013, 41, W71–W76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winckers, L.A.; Willighagen, E.L.; Kutmon, M. Laurent2207/TiO2-Scripts: TiO2-NB Analysis Scripts 1h (Version v1.0). 2021. Available online: https://doi.org/10.5281/zenodo.5156769 (accessed on 28 August 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Blighe, K.; Rana, S.; Lewis, M. Enhanced Volcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. 2020. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 28 August 2021).
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Carbon, S.; Douglass, E.; Good, B.M.; Unni, D.R.; Harris, N.L.; Mungall, C.J.; Basu, S.; Chisholm, R.L.; Dodson, R.J.; Hartline, E.; et al. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2020, 49, D325–D334. [Google Scholar] [CrossRef]
- Durinck, S.; Moreau, Y.; Kasprzyk, A.; Davis, S.; Moor, B.D.; Brazma, A.; Huber, W. BioMart and Bioconductor: A powerful link between biological databases and microarray data analysis. Bioinformatics 2005, 21, 3439–3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveros, J.C.; Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2020. Available online: https://bioinfogp.cnb.csic.es/tools/venny/ (accessed on 28 August 2021).
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. Int. J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]


| Significant | Toxicity | Apoptosis | Inflammation | DNA Damage | Oxidative Stress | |
|---|---|---|---|---|---|---|
| Pathways | 1076 | 101 | 66 | 15 | 28 | 2 |
| Caco-2/HT29-MTX low | 56 | 10 | 5 | 0 | 5 | 0 |
| Caco-2/HT29-MTX high | 58 | 10 | 10 | 1 | 0 | 0 |
| SAE low | 28 | 3 | 1 | 0 | 2 | 0 |
| SAE high | 50 | 15 | 6 | 1 | 8 | 0 |
| THP1 low | 38 | 10 | 9 | 4 | 0 | 0 |
| THP1 high | 201 | 39 | 31 | 9 | 3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winckers, L.A.; Evelo, C.T.; Willighagen, E.L.; Kutmon, M. Investigating the Molecular Processes behind the Cell-Specific Toxicity Response to Titanium Dioxide Nanobelts. Int. J. Mol. Sci. 2021, 22, 9432. https://doi.org/10.3390/ijms22179432
Winckers LA, Evelo CT, Willighagen EL, Kutmon M. Investigating the Molecular Processes behind the Cell-Specific Toxicity Response to Titanium Dioxide Nanobelts. International Journal of Molecular Sciences. 2021; 22(17):9432. https://doi.org/10.3390/ijms22179432
Chicago/Turabian StyleWinckers, Laurent A., Chris T. Evelo, Egon L. Willighagen, and Martina Kutmon. 2021. "Investigating the Molecular Processes behind the Cell-Specific Toxicity Response to Titanium Dioxide Nanobelts" International Journal of Molecular Sciences 22, no. 17: 9432. https://doi.org/10.3390/ijms22179432
APA StyleWinckers, L. A., Evelo, C. T., Willighagen, E. L., & Kutmon, M. (2021). Investigating the Molecular Processes behind the Cell-Specific Toxicity Response to Titanium Dioxide Nanobelts. International Journal of Molecular Sciences, 22(17), 9432. https://doi.org/10.3390/ijms22179432






