Circulating microRNAs Signature for Predicting Response to GLP1-RA Therapy in Type 2 Diabetic Patients: A Pilot Study
Abstract
:1. Introduction
2. Results
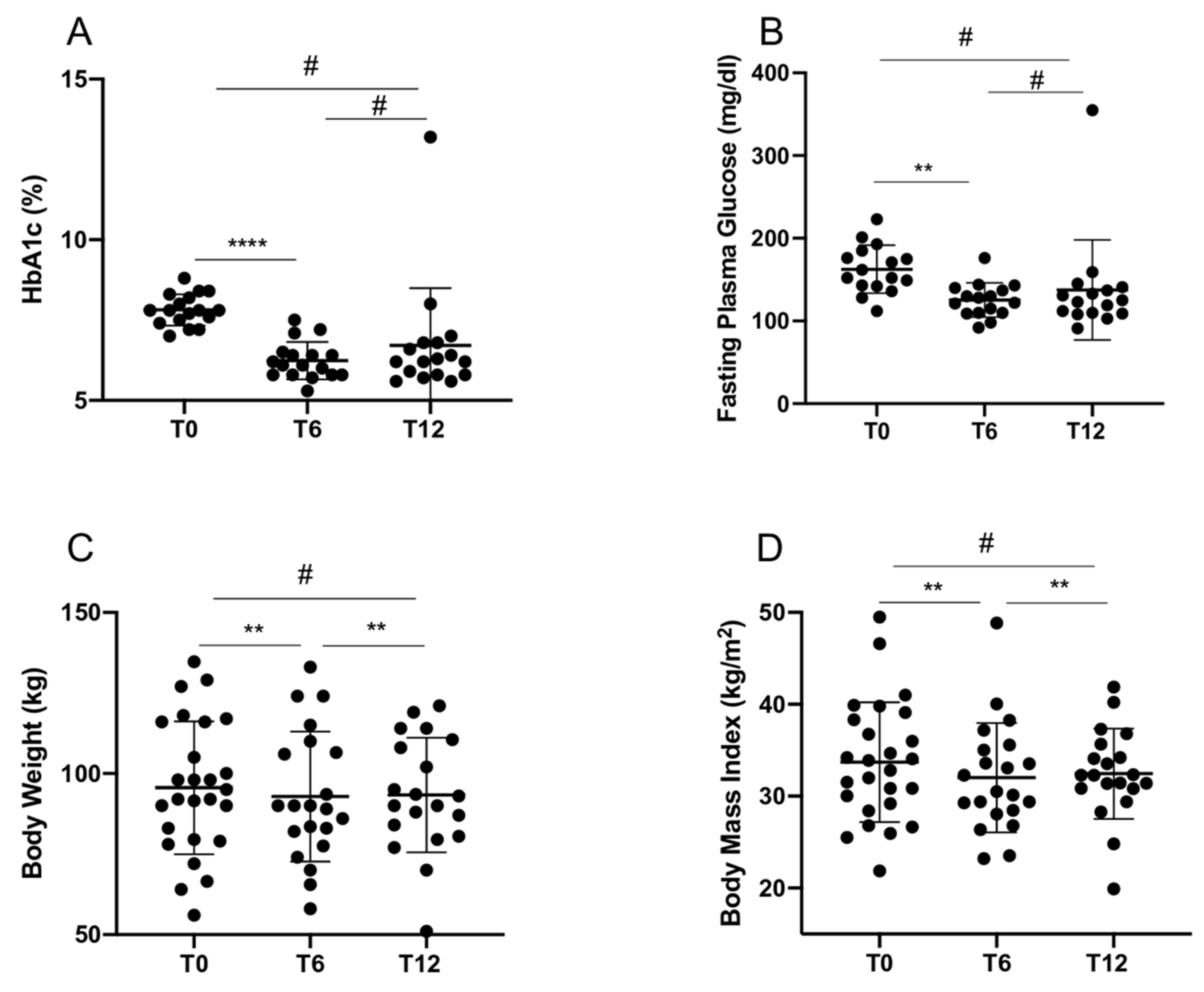
2.1. GLP1-RA Treatment Outcomes in T2D Patients
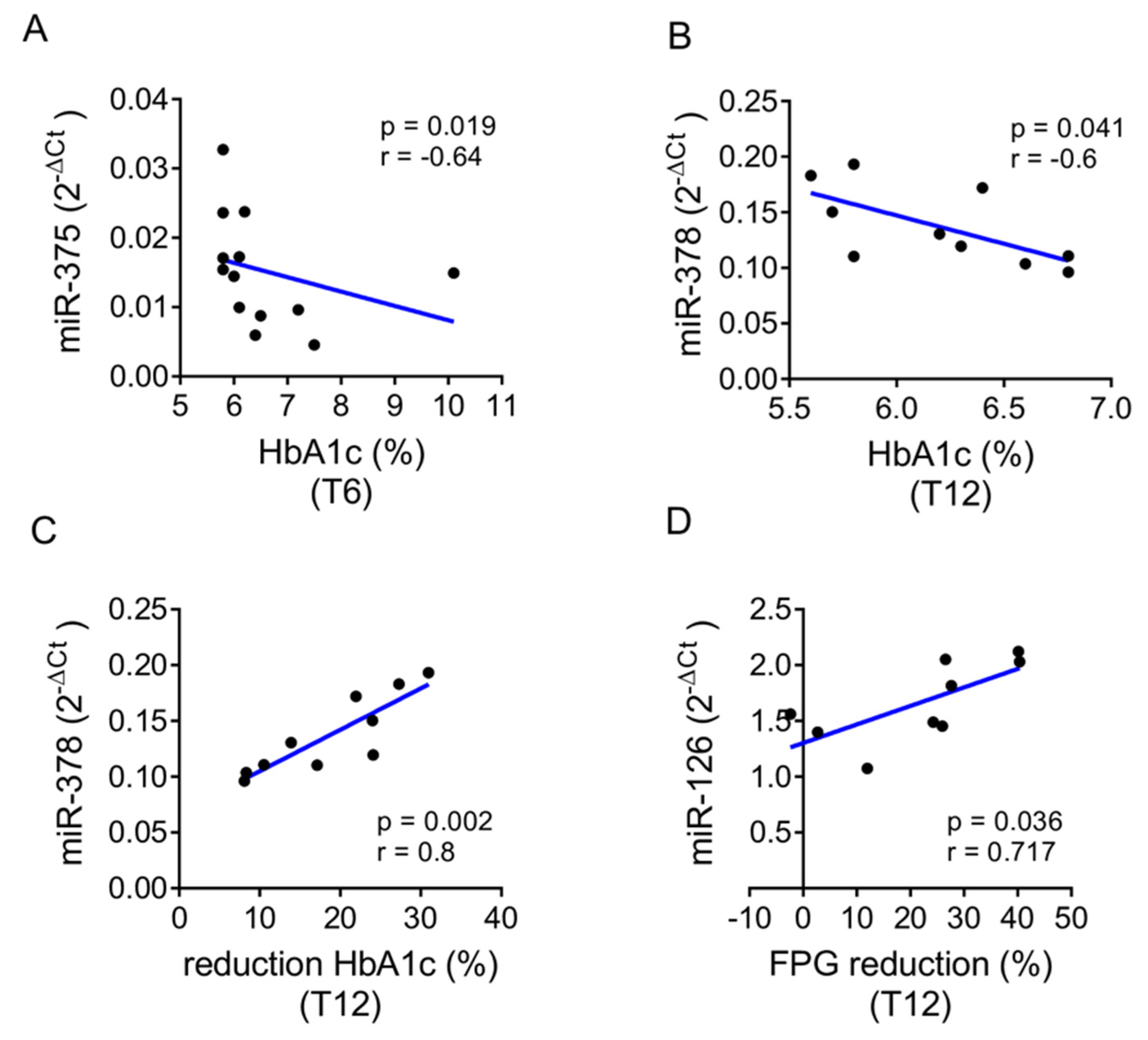
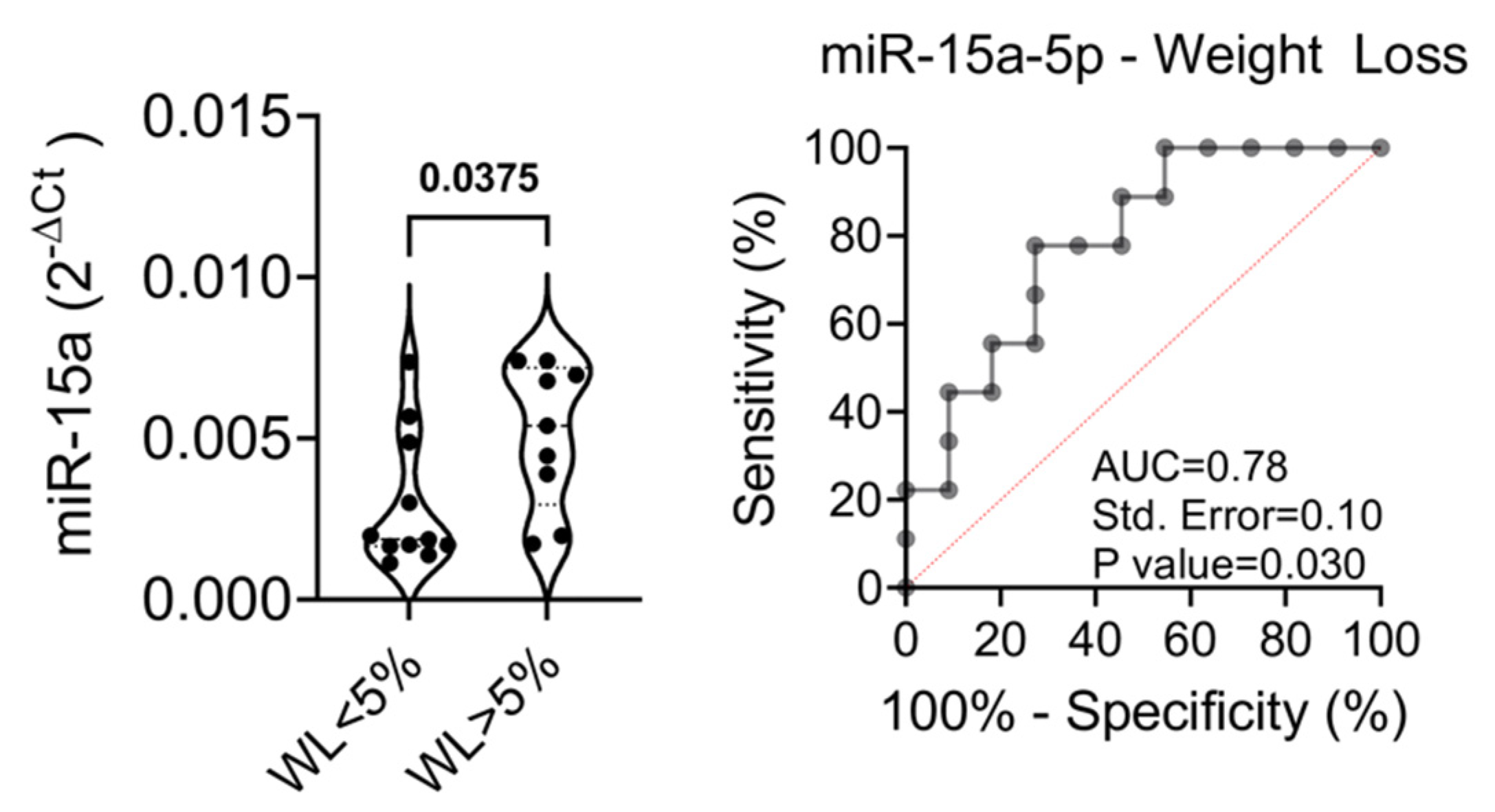
2.2. Baseline Levels of the Selected miRNAs Distinguish Patients with Better Treatment Outcomes
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Clinical and Biochemical Parameters
4.3. Blood Collection Procedure
4.4. RNA Extraction from Plasma Samples
4.5. Circulating microRNAs qRT-Real-Time PCR Analysis
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Huang, S.-Y.; Sun, T. Pharmacogenomic studies of current antidiabetic agents and potential new drug targets for precision medicine of diabetes. Diabetes Ther. 2020, 11, 2521–2538. [Google Scholar] [CrossRef]
- Nasykhova, Y.A.; Tonyan, Z.N.; Mikhailova, A.A.; Danilova, M.M.; Glotov, A.S. Pharmacogenetics of type 2 diabetes—Progress and prospects. Int. J. Mol. Sci. 2020, 21, 6842. [Google Scholar] [CrossRef]
- IDF—International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; IDF: Brussels, Belgium, 2019. [Google Scholar]
- American Diabetes Association. 6. Glycemic targets: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S73–S84. [Google Scholar] [CrossRef]
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S111–S124. [Google Scholar] [CrossRef]
- Chung, W.K.; Erion, K.; Florez, J.C.; Hattersley, A.T.; Hivert, M.-F.; Lee, C.G.; McCarthy, M.I.; Nolan, J.J.; Norris, J.M.; Pearson, E.R.; et al. Precision medicine in diabetes: A consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 1617–1635. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012, 35, 1364–1379. [Google Scholar] [CrossRef] [Green Version]
- Mohan, V.; Radha, V. Precision diabetes is slowly becoming a reality. Med. Princ. Pract. 2019, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rathmann, W.; Bongaerts, B. Pharmacogenetics of novel glucose-lowering drugs. Diabetologia 2021, 64, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Hébert, H.L.; Shepherd, B.; Milburn, K.; Veluchamy, A.; Meng, W.; Carr, F.; Donnelly, L.A.; Tavendale, R.; Leese, G.; Colhoun, H.M.; et al. Cohort profile: Genetics of Diabetes Audit and Research in Tayside Scotland (GoDARTS). Int. J. Epidemiol. 2018, 47, 380–381j. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Donnelly, L.A.; Kimber, C.H.; Donnan, P.T.; Doney, A.S.F.; Leese, G.; Hattersley, A.T.; McCarthy, M.I.; Morris, A.D.; Palmer, C.N.A.; et al. Reduced-function SLC22A1 polymorphisms encoding organic cation transporter 1 and glycemic response to metformin: A GoDARTS study. Diabetes 2009, 58, 1434–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dujic, T.; Zhou, K.; Donnelly, L.A.; Tavendale, R.; Palmer, C.N.A.; Pearson, E.R. Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: A GoDARTS study. Diabetes 2015, 64, 1786–1793. [Google Scholar] [CrossRef] [Green Version]
- Dawed, A.Y.; Donnelly, L.; Tavendale, R.; Carr, F.; Leese, G.; Palmer, C.N.A.; Pearson, E.R.; Zhou, K. CYP2C8 and SLCO1B1 variants and therapeutic response to thiazolidinediones in patients with type 2 diabetes. Diabetes Care 2016, 39, 1902–1908. [Google Scholar] [CrossRef] [Green Version]
- Kyriakidou, A.; Koufakis, T.; Goulis, D.G.; Vasilopoulos, Y.; Zebekakis, P.; Kotsa, K. Pharmacogenetics of the glucagon-like peptide-1 receptor agonist liraglutide: A step towards personalized type 2 diabetes management. Curr. Pharm. Des. 2021, 27, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Wilding, J.P.H.; Barber, T.M.; Alam, U.; Cuthbertson, D.J. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes. Rev. 2019, 20, 816–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigi, L.; Grieco, G.; Ventriglia, G.; Brusco, N.; Mancarella, F.; Formichi, C.; Dotta, F.; Sebastiani, G. MicroRNAs as regulators of insulin signaling: Research updates and potential therapeutic perspectives in type 2 diabetes. Int. J. Mol. Sci. 2018, 19, 3705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastiani, G.; Nigi, L.; Grieco, G.E.; Mancarella, F.; Ventriglia, G.; Dotta, F. Circulating MicroRNAs and diabetes mellitus: A novel tool for disease prediction, diagnosis, and staging? J. Endocrinol. Investig. 2017, 40, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Demirsoy, İ.H.; Ertural, D.Y.; Balci, Ş.; Çınkır, Ü.; Sezer, K.; Tamer, L.; Aras, N. Profiles of circulating MiRNAs following metformin treatment in patients with type 2 diabetes. J. Med. Biochem. 2018, 37, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, G.; Besharat, Z.M.; Chiacchiarini, M.; Abballe, L.; Sabato, C.; Vacca, A.; Borgiani, P.; Dotta, F.; Tesauro, M.; Po, A.; et al. Circulating MicroRNAs in elderly type 2 diabetic patients. Int. J. Endocrinol. 2018, 2018, 6872635. [Google Scholar] [CrossRef]
- Flowers, E.; Aouizerat, B.E.; Abbasi, F.; Lamendola, C.; Grove, K.M.; Fukuoka, Y.; Reaven, G.M. Circulating MicroRNA-320a and MicroRNA-486 predict thiazolidinedione response: Moving towards precision health for diabetes prevention. Metabolism 2015, 64, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Nunez Lopez, Y.O.; Retnakaran, R.; Zinman, B.; Pratley, R.E.; Seyhan, A.A. Predicting and understanding the response to short-term intensive insulin therapy in people with early type 2 diabetes. Mol. Metab. 2019, 20, 63–78. [Google Scholar] [CrossRef]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma MicroRNA profiling reveals loss of endothelial MiR-126 and other MicroRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef]
- Wang, X.; Sundquist, J.; Zöller, B.; Memon, A.A.; Palmér, K.; Sundquist, K.; Bennet, L. Determination of 14 circulating MicroRNAs in Swedes and Iraqis with and without diabetes mellitus type 2. PLoS ONE 2014, 9, e86792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lv, C.; Li, L.; Chen, S.; Liu, S.; Wang, C.; Su, B. Plasma MiR-126 is a potential biomarker for early prediction of type 2 diabetes mellitus in susceptible individuals. BioMed Res. Int. 2013, 2013, 761617. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, G.; Yang, C.; Zhou, K.; Shen, B.; Liang, H.; Jiang, X. The role of circulating MicroRNA-126 (MiR-126): A novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int. J. Mol. Sci. 2014, 15, 10567–10577. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Lucena, R.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; López-Moreno, J.; Roncero-Ramos, I.; Molina-Abril, H.; Yubero-Serrano, E.M.; Caballero-Villarraso, J.; Delgado-Lista, J.; Castaño, J.P.; et al. Circulating MiRNAs as predictive biomarkers of type 2 diabetes mellitus development in coronary heart disease patients from the CORDIOPREV study. Mol. Ther.-Nucleic Acids 2018, 12, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, L.; Shang, Q.; Lv, C.; Wang, C.; Su, B. Circulating MiR-126 is a potential biomarker to predict the onset of type 2 diabetes mellitus in susceptible individuals. Biochem. Biophys. Res. Commun. 2015, 463, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Spazzafumo, L.; Bonafè, M.; Recchioni, R.; Prattichizzo, F.; Marcheselli, F.; Micolucci, L.; Mensà, E.; Giuliani, A.; Santini, G.; et al. MiR-21-5p and MiR-126a-3p levels in plasma and circulating angiogenic cells: Relationship with type 2 diabetes complications. Oncotarget 2015, 6, 35372–35382. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, M.; Weithauser, A.; Tabaraie, T.; Steffens, D.; Kränkel, N.; Witkowski, M.; Stratmann, B.; Tschoepe, D.; Landmesser, U.; Rauch-Kroehnert, U. Micro–RNA-126 reduces the blood thrombogenicity in diabetes mellitus via targeting of tissue factor. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1263–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amr, K.; Abdelmawgoud, H.; Ali, Z.; Shehata, S.; Raslan, H. Potential value of circulating MicroRNA-126 and MicroRNA-210 as biomarkers for type 2 diabetes with coronary artery disease. Br. J. Biomed. Sci. 2018, 75, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Mahdavi, R.; Alipoor, B.; Panahi, G.; Nasli Esfahani, E.; Razi, F.; Taghikhani, M.; Meshkani, R. Decreased serum MicroRNA-21 level is associated with obesity in healthy and type 2 diabetic subjects. Arch. Physiol. Biochem. 2018, 124, 300–305. [Google Scholar] [CrossRef]
- Seyhan, A.A.; Nunez Lopez, Y.O.; Xie, H.; Yi, F.; Mathews, C.; Pasarica, M.; Pratley, R.E. Pancreas-enriched MiRNAs are altered in the circulation of subjects with diabetes: A pilot cross-sectional study. Sci. Rep. 2016, 6, 31479. [Google Scholar] [CrossRef] [Green Version]
- Ghai, V.; Kim, T.-K.; Etheridge, A.; Nielsen, T.; Hansen, T.; Pedersen, O.; Galas, D.; Wang, K. Extracellular vesicle encapsulated MicroRNAs in patients with type 2 diabetes are affected by metformin treatment. J. Clin. Med. 2019, 8, 617. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum MicroRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2011, 48, 61–69. [Google Scholar] [CrossRef]
- Nunez Lopez, Y.O.; Garufi, G.; Seyhan, A.A. Altered levels of circulating cytokines and MicroRNAs in lean and obese individuals with prediabetes and type 2 diabetes. Mol. BioSyst. 2017, 13, 106–121. [Google Scholar] [CrossRef]
- Rong, Y.; Bao, W.; Shan, Z.; Liu, J.; Yu, X.; Xia, S.; Gao, H.; Wang, X.; Yao, P.; Hu, F.B.; et al. Increased MicroRNA-146a levels in plasma of patients with newly diagnosed type 2 diabetes mellitus. PLoS ONE 2013, 8, e73272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poy, M.N.; Eliasson, L.; Krutzfeldt, J.; Kuwajima, S.; Ma, X.; MacDonald, P.E.; Pfeffer, S.; Tuschl, T.; Rajewsky, N.; Rorsman, P.; et al. A pancreatic islet-specific MicroRNA regulates insulin secretion. Nature 2004, 432, 226–230. [Google Scholar] [CrossRef]
- Willeit, P.; Skroblin, P.; Kiechl, S.; Fernández-Hernando, C.; Mayr, M. Liver MicroRNAs: Potential mediators and biomarkers for metabolic and cardiovascular disease? Eur. Heart J. 2016, 37, 3260–3266. [Google Scholar] [CrossRef] [Green Version]
- EU-CardioRNA COST Action CA17129; Barbalata, T.; Zhang, L.; Dulceanu, M.D.; Stancu, C.S.; Devaux, Y.; Sima, A.V.; Niculescu, L.S. Regulation of MicroRNAs in high-fat diet induced hyperlipidemic hamsters. Sci. Rep. 2020, 10, 20549. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ceinos, J.; Rangel-Zuñiga, O.A.; Clemente-Postigo, M.; Podadera-Herreros, A.; Camargo, A.; Alcalá-Diaz, J.F.; Guzmán-Ruiz, R.; López-Miranda, J.; Malagón, M.M. MiR-223-3p as a potential biomarker and player for adipose tissue dysfunction preceding type 2 diabetes onset. Mol. Ther.-Nucleic Acids 2021, 23, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhong, J.; Wang, Y.; Xie, W. Proteomic and MicroRNA-omic profiles and potential mechanisms of dysfunction in pancreatic islet cells primed by inflammation. Exp. Ther. Med. 2020, 21, 122. [Google Scholar] [CrossRef]
- Carls, G.; Huynh, J.; Tuttle, E.; Yee, J.; Edelman, S.V. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther. 2017, 8, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Grieco, G.E.; Brusco, N.; Licata, G.; Fignani, D.; Formichi, C.; Nigi, L.; Sebastiani, G.; Dotta, F. The landscape of MicroRNAs in βCell: Between phenotype maintenance and protection. Int. J. Mol. Sci. 2021, 22, 803. [Google Scholar] [CrossRef]
- Gaborit, B.; Julla, J.-B.; Besbes, S.; Proust, M.; Vincentelli, C.; Alos, B.; Ancel, P.; Alzaid, F.; Garcia, R.; Mailly, P.; et al. Glucagon-like peptide 1 receptor agonists, diabetic retinopathy and angiogenesis: The AngioSafe type 2 diabetes study. J. Clin. Endocrinol. Metab. 2020, 105, e1549–e1560. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Mercader, J.M.; Moreno-Navarrete, J.M.; Rovira, O.; Guerra, E.; Esteve, E.; Xifra, G.; Martínez, C.; Ricart, W.; Rieusset, J.; et al. Profiling of Circulating MicroRNAs Reveals Common MicroRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care 2014, 37, 1375–1383. [Google Scholar] [CrossRef] [Green Version]
- Moeez, S.; Riaz, S.; Masood, N.; Kanwal, N.; Arif, M.A.; Niazi, R.; Khalid, S. Evaluation of the Rs3088442 G>A SLC22A3 Gene polymorphism and the role of MicroRNA 147 in groups of adult Pakistani populations with type 2 diabetes in response to metformin. Can. J. Diabetes 2019, 43, 128–135.E3. [Google Scholar] [CrossRef]
- Li, Y.; Deng, S.; Peng, J.; Wang, X.; Essandoh, K.; Mu, X.; Peng, T.; Meng, Z.-X.; Fan, G.-C. MicroRNA-223 is essential for maintaining functional β-cell mass during diabetes through inhibiting both FOXO1 and SOX6 pathways. J. Biol. Chem. 2019, 294, 10438–10448. [Google Scholar] [CrossRef]
- Runtsch, M.C.; Nelson, M.C.; Lee, S.-H.; Voth, W.; Alexander, M.; Hu, R.; Wallace, J.; Petersen, C.; Panic, V.; Villanueva, C.J.; et al. Anti-inflammatory MicroRNA-146a protects mice from diet-induced metabolic disease. PLoS Genet. 2019, 15, e1007970. [Google Scholar] [CrossRef]
- Iacomino, G.; Siani, A. Role of MicroRNAs in obesity and obesity-related diseases. Genes Nutr. 2017, 12, 23. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.; Dehghani Ashkezari, M.; Seifati, S.M.; Vahidi Mehrjardi, M.Y.; Dehghan Tezerjani, M.; Sadeghzadeh, S.; Ladan, S.A.B. Circulating MiR-15a and MiR-222 as potential biomarkers of type 2 diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3461–3469. [Google Scholar] [CrossRef]
- Kamalden, T.A.; Macgregor-Das, A.M.; Kannan, S.M.; Dunkerly-Eyring, B.; Khaliddin, N.; Xu, Z.; Fusco, A.P.; Yazib, S.A.; Chow, R.C.; Duh, E.J.; et al. Exosomal MicroRNA-15a transfer from the pancreas augments diabetic complications by inducing oxidative stress. Antioxid. Redox Signal. 2017, 27, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Krist, B.; Florczyk, U.; Pietraszek-Gremplewicz, K.; Józkowicz, A.; Dulak, J. The role of MiR-378a in metabolism, angiogenesis, and muscle biology. Int. J. Endocrinol. 2015, 2015, 281756. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Cao, H.; Ye, C.; Chang, C.; Lu, M.; Jing, Y.; Zhang, D.; Yao, X.; Duan, Z.; Xia, H.; et al. Hepatic MiR-378 targets P110α and controls glucose and lipid homeostasis by modulating hepatic insulin signalling. Nat. Commun. 2014, 5, 5684. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Y.; Zhou, Y.; Zhang, Y.; Zhang, T.; Li, Y.; You, W.; Chang, X.; Yuan, L.; Han, X. MicroRNA-24 promotes pancreatic Beta cells toward dedifferentiation to avoid endoplasmic reticulum stress-induced apoptosis. J. Mol. Cell Biol. 2019, 11, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Hromadka, M.; Motovska, Z.; Hlinomaz, O.; Kala, P.; Tousek, F.; Jarkovsky, J.; Beranova, M.; Jansky, P.; Svoboda, M.; Krepelkova, I.; et al. MiR-126-3p and MiR-223-3p as biomarkers for prediction of thrombotic risk in patients with acute myocardial infarction and primary angioplasty. J. Pers. Med. 2021, 11, 508. [Google Scholar] [CrossRef]
- Pishavar, E.; Behravan, J. MiR-126 as a therapeutic agent for diabetes mellitus. Curr. Pharm. Des. 2017, 23, 3309–3314. [Google Scholar] [CrossRef]
- Deng, X.; Liu, Y.; Luo, M.; Wu, J.; Ma, R.; Wan, Q.; Wu, J. Circulating MiRNA-24 and its target YKL-40 as potential biomarkers in patients with coronary heart disease and type 2 diabetes mellitus. Oncotarget 2017, 8, 63038–63046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieco, G.E.; Sebastiani, G.; Fignani, D.; Brusco, N.; Nigi, L.; Formichi, C.; Licata, G.; Bruttini, M.; D’Aurizio, R.; Mathieu, C.; et al. Protocol to analyze circulating small non-coding RNAs by high-throughput RNA sequencing from human plasma samples. STAR Protoc. 2021, 2, 100606. [Google Scholar] [CrossRef] [PubMed]


| T0 (n = 26) | T6 (n = 21) | T12 (n = 20) | |
|---|---|---|---|
| Age (years) | 60.3 ± 10.3 (35–79) | / | / |
| Disease Duration (years) | 10.2 ± 8.7 (1–32) | / | / |
| Weight (kg) | 95.6 ± 20.6 (56–134.7) | 92.9 ± 20.2 (58–133) * | 93.3 ± 17.8 (51–121) § |
| BMI (kg/m2) | 33.7 ± 6.5 (21.9–49.5) | 32.0 ± 5.9 (23.2–48.5) * | 32.4 ± 4.9 (19.9–41.9) § |
| HbA1c (%) | 7.7 ± 0.58 (6–8.8) | 6.4 ± 1.0 (5.3–10.1) * | 6.7 ± 1.7 (5.5–13.2) |
| FPG (mg/dL) | 159.1 ± 27.9 (112–223) | 128.9 ± 37.5 (86–251) * | 134.7 ± 56.3 (91–355) |
| Total Cholesterol (mg/dL) | 157.5 ± 42.4 (54–258) | 155.6 ± 26.5 (106–208) | 150.2 ± 24.8 (104–200) |
| Triglycerides (mg/dL) | 138.7 ± 63.1 (56–313) | 129.9 ± 49.3 (60–257) | 132.6 ± 58.5 (61–232) |
| HDL (mg/dL) | 46.3 ± 11.7 (28–72) | 44.9 ± 10.9 (28–71) | 46.5 ± 11.8 (29–76) |
| LDL (mg/dL) | 92.2 ± 34.9 (50–173) | 84.7 ± 22.6 (49–130) | 76.2 ± 24.1 (45–131) |
| Creatinine (mg/dL) | 0.91 ± 0.23 (0.64–1.34) | 0.93 ± 0.19 (0.67–1.27) | 0.93 ± 0.26 (0.59–1.56) |
| eGFR (mL/min/1.73 m2) | 84.7 ± 21.1 (44–116) | 85.0 ± 17.4 (47–112) | 84.3 ± 21.5 (37–122) |
| Albuminuria (mg/dL) | 46.0 ± 104.4 (0–462) | 199.0 ± 681.2 (0–2834) | 73.8 ± 171.4 (0–575) |
| %WL | / | 4.9 ± 5.8 (−5.2–23.3) | 5.5 ± 6.1 (−2.9–18.1) |
| %EWL | / | 18.9 ± 26.8 (−41.2–81.2) | 15.5 ± 16.4 (−9.7–50) |
| HbA1c reduction (%) | / | 16.5 ± 14.4 (−31.2–36.9) | 12.7 ± 22.3 (−69.2–33.3) |
| Dulaglutide | Liraglutide | p Value | |||||
|---|---|---|---|---|---|---|---|
| T0 (n = 18) | T6 (n = 14) | T12 (n = 13) | T0 (n = 8) | T6 (n = 7) | T12 (n = 7) | ||
| Age (years) | 60.1 ± 12.1 (35–79) | / | / | 61.0 ± 4.9 (55–67) | / | / | * 0.83 |
| Disease Duration (years) | 10.8 ± 9.4 (1–32) | / | / | 8.8 ± 7.3 (1–23) | / | / | * 0.65 |
| Weight (kg) | 93.6 ± 19.3 (56–127) | 91.4 ± 17.6 (58–124) | 93.4 ± 17.6 (51–119) | 100.0 ± 24.2 (72–134.7) | 95.8 ± 26.0 (65.5–133) | 93.4 ± 19.5 (70–121) | * 0.61; § 0.87; # 0.8 |
| BMI (kg/m2) | 32.5 ± 5.1 (21.9–41) | 32.4 ± 4.5 (23.5–40.0) | 32.4 ± 4.6 (19.9–40.2) | 36.3 ± 8.7 (25.5–49.5) | 33.3 ± 8.5 (23.2–48.9) | 32.4 ± 5.8 (24.8–41.9) | * 0.53; § 0.91; # 0.81 |
| HbA1c (%) | 7.6 ± 0.6 (6.0–8.4) | 6.6 ± 1.2 (5.3–10.1) | 6.2 ± 0.6 (5.5–7.4) | 7.8 ± 0.6 (7.0–8.8) | 6.2 ± 0.6 (5.7–7.5) | 7.5 ± 2.6 (5.8–13.2) | * 0.77; § 0.42; # 0.23 |
| FPG (mg/dL) | 156.9 ± 30.9 (112–223) | 133.7 ± 41.4 (86–251) | 120.2 ± 14.4 (94–141) | 164.0 ± 20.7 (143–201) | 119.3 ± 28.4 (92–176) | 166.0 ± 95.8 (91–355) | * 0.45; § 0.36; # 0.23 |
| Total Cholesterol (mg/dL) | 145.9 ± 36.8 (54–220) | 157.7 ± 27.7 (106–208) | 144.8 ± 18.7 (112–178) | 182 ± 45.3 (123–258) | 151.6 ± 25.5 (119–187) | 161.8 ± 33.8 (104–200) | * 0.1; § 0.57; # 0.14 |
| Triglycerides (mg/dL) | 138.1 ± 68.1 (56–313) | 129.9 ± 56.0 (60–257) | 120.8 ± 55.0 (61–222) | 140.0 ± 55. (77–229) | 129.7 ± 37.8 (88–187) | 163.2 ± 61.9 (110–232) | * 0.74; § 0.89; # 0.11 |
| HDL (mg/dL) | 46.3 ± 12.8 (28–72) | 44.8 ± 11.9 (28–71) | 48.5 ± 13.0 (31–76) | 46.4 ± 9.6 (30–61) | 45.1 ± 9.6 (28–57) | 42.5 ± 8.3 (29–52) | * 0.76; § 0.86; # 0.43 |
| LDL (mg/dL) | 83.0 ± 29.3 (51–147) | 87.0 ± 22.9 (54–130) | 71.9 ± 20.7 (47–110) | 114.5 ± 39.44 (50–173) | 80.5 ± 23.0 (49–106) | 86.4 ± 31.1 (45–131) | * 0.08; § 0.65; # 0.31 |
| Creatinine (mg/dL) | 0.94 ± 0.2 (0.64–1.34) | 0.96 ± 0.2 (0.67–1.27) | 0.94 ± 0.3 (0.59–1.56) | 0.9 ± 0.2 (0.64–1.23) | 0.87 ± 0.2 (0.67–1.06) | 0.89 ± 0.3 (0.66–1.37) | * 0.5; § 0.52; # 0.61 |
| eGFR (mL/min/1.73 m2) | 85.1 ± 23.9 (44–116) | 84.5 ± 20.9 (47–112) | 84.5 ± 23.7 (37–122) | 83.8 ± 14.1 (61–97) | 86.0 ± 9.3 (72–98) | 83.6 ± 16.7 (54–93) | * 0.69; § 0.83; # 0.75 |
| Albuminuria (mg/dL) | 51.6 ± 120.3 (0–462) | 314.0 ± 887.5 (0–2834) | 88.9 ± 187.9 (0− 575) | 30.5 ± 39.6 (4–97.6) | 34.9 ± 51.3 (3.2–143) | 6.0 ± 1.4 (5–7) | * 0.89; § 0.98; # 0.54 |
| %WL | / | 5.0 ± 6.9 (−5.3–23.3) | 5.3 ± 6.3 (−2.9–18.1) | / | 4.7 ± 3.2 (1.3–9.0) | 5.8 ± 6.2 (−1.9–15.4) | § 0.74; # 0.81 |
| %EWL | / | 17.2 ± 27.7 (−41.2–64.3) | 14.3 ± 18.5 (−9.7–50) | / | 22.5 ± 26.7 (2.3–81.2) | 17.5 ± 13.4 (−6.5–34.1) | § 0.7; # 0.53 |
| HbA1c reduction (%) | / | 14.8 ± 16.3 (−31.2–36.9) | 18.0 ± 11. (−8.3–33.3) | / | 19.8 ± 9.8 (3.8–35.2) | 2.9 ± 33.5 (−69.2–29.5) | § 0.59; # 0.35 |
| Low Expressing (n = 13) | High Expressing (n = 13) | p Value | |||||
|---|---|---|---|---|---|---|---|
| T0 | T6 | T12 | T0 | T6 | T12 | ||
| Age (years) | 59.5 ± 10.7 (35–74) | / | / | 61.9 ± 10.1 (41–79) | / | / | * 0.65 |
| Disease Duration (years) | 9.3 ± 10.5 (1–32) | / | / | 11.1 ± 6.7 (2–23) | / | / | * 0.24 |
| Weight (kg) | 97.3 ± 19.7 (56–129) | 97.4 ± 17.1 (74–124) | 93.3 ± 20.8 (51–121) | 93.9 ± 22.2 (64–134.7) | 90.1 ± 22.1 (58–133) | 93.4 ± 15.4 (70–114) | * 0.65 § 0.3 ¶ 0.69 |
| BMI (kg/m2) | 34.1 ± 6.3 (21.8–46.6) | 32.1 ± 4.2 (26.3–38.3) | 32.1 ± 5.3 (19.9–40.2) | 33.3 ± 6.9 (25.5–49.5) | 31.9 ± 6.9 (23.2–48.8) | 32.8 ± 4.7 (24.8–41.9) | * 0.67 § 0.74 ¶ > 0.9 |
| HbA1c (%) | 7.7 ± 0.7 (6–8.8) | 6.2 ± 0.75 (5.3–7.5) | 7.1 ± 2.3 (5.5–13.2) | 7.7 ± 0.4 (7–8.4) | 6.7 ± 1.2 (5.8–10.1) | 6.2 ± 0.4 (5.6–6.8) | * 0.24 § 0.5 ¶ 0.4 |
| FPG (mg/dL) | 160.4 ± 32.0 (112–201) | 123.0 ± 30.3 (86–176) | 146.5 ± 75.5 (94–355) | 157.8 ± 24.5 (128–223) | 132.5 ± 42.0 (93–251) | 121.6 ± 17.5 (91–145) | * 0.62 § 0.76 ¶ 0.7 |
| Total Cholesterol (mg/dL) | 148.6 ± 49.3 (54–258) | 147.3 ± 31.4 (106–187) | 143.8 ± 23.7 (104–190) | 167.1 ± 32.7 (124–220) | 160.0 ± 23.6 (131–208) | 157.3 ± 25.4 (112–200) | * 0.17 § 0.3 ¶ 0.19 |
| Triglycerides (mg/dL) | 140.0 ± 72.6 (73–313) | 151.4 ± 61.8 (72–257) | 127.0 ± 72.5 (54–232) | 137.3 ± 54.1 (56–245) | 118.2 ± 39.0 (60–188) | 129.3 ± 48.6 (62–219) | * 0.75 § 0.3 ¶ 0.56 |
| HDL (mg/dL) | 47.6 ± 11.5 (30–68) | 42.4 ± 10.2 (28–54) | 56.7 ± 31.7 (29–136) | 45.1 ± 12.3 (28–72) | 46.2 ± 11.4 (28–71) | 45.4 ± 12.8 (31–76) | * 0.5 § 0.49 ¶ 0.49 |
| LDL (mg/dL) | 89.7 ± 40.3 (51–173) | 74.7 ± 19.6 (49–99) | 68.9 ± 18.9 (45–96) | 94.8 ± 30.2 (50–149.8) | 90.9 ± 23.7 (55.4–130) | 84.9 ± 28.4 (47–131) | * 0.47 § 0.29 ¶ 0.86 |
| Creatinine (mg/dL) | 0.94 ± 0.26 (0.64–1.34) | 0.96 ± 0.18 (0.67–1.27) | 0.98 ± 0.31 (0.59–1.56) | 0.88 ± 0.21 (0.67–1.34) | 0.90 ± 0.19 (0.67–1.24) | 0.85 ± 0.18 (0.66–1.19) | * 0.69 § 0.46 ¶ 0.28 |
| eGFR (mL/min/1.73 m2) | 83.1 ± 25.0 (44–116) | 83.9 ± 20.4 (47–113) | 81.7 ± 26.8 (37–123) | 85.9 ± 16.9 (56–111) | 84.3 ± 18.1 (57–112) | 88.4 ± 13.9 (65 – 110) | * 0.9 § 0.95 ¶ 0.7 |
| Albuminuria (mg/dL) | 81.1 ± 157.1 (0–462) | 534.3 ± 1130 (2–2834) | 128.5 ± 225.4 (5–575) | 20.5 ± 25.0 (2–67) | 16.1 ± 17.9 (0–57) | 8.2 ± 9.68 (0–23) | * 0.59 § 0.2 ¶ 0.09 |
| %WL | / | 6.36 ± 7.2 (0.86–23.3) | 4.4 ± 6.1 (−1.9–18.1) | / | 14.2 ± 15.7 (−31.2–29.3) | 6.5 ± 6.2 (−2.8–15.4) | § 0.79 ¶ 0.4 |
| %EWL | / | 22.9 ± 23.2 (3.2–64.3) | 11.5 ± 17.7 (−7.8–50) | / | 16.5 ± 29.5 (−41.2–81.2) | 19.0 ± 15.1 (−9.7–42.4) | § 0.68 ¶ 0.3 |
| HbA1c reduction (%) | / | 20.2 ± 12.0 (3.85–36.9) | 6.8 ± 30.1 (−69.2–33.3) | / | 14.2 ± 15.7 (−31.2–29.3) | 18.6 ± 8.2 (8.1–30.9) | § 0.69 ¶ 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formichi, C.; Fignani, D.; Nigi, L.; Grieco, G.E.; Brusco, N.; Licata, G.; Sabato, C.; Ferretti, E.; Sebastiani, G.; Dotta, F. Circulating microRNAs Signature for Predicting Response to GLP1-RA Therapy in Type 2 Diabetic Patients: A Pilot Study. Int. J. Mol. Sci. 2021, 22, 9454. https://doi.org/10.3390/ijms22179454
Formichi C, Fignani D, Nigi L, Grieco GE, Brusco N, Licata G, Sabato C, Ferretti E, Sebastiani G, Dotta F. Circulating microRNAs Signature for Predicting Response to GLP1-RA Therapy in Type 2 Diabetic Patients: A Pilot Study. International Journal of Molecular Sciences. 2021; 22(17):9454. https://doi.org/10.3390/ijms22179454
Chicago/Turabian StyleFormichi, Caterina, Daniela Fignani, Laura Nigi, Giuseppina Emanuela Grieco, Noemi Brusco, Giada Licata, Claudia Sabato, Elisabetta Ferretti, Guido Sebastiani, and Francesco Dotta. 2021. "Circulating microRNAs Signature for Predicting Response to GLP1-RA Therapy in Type 2 Diabetic Patients: A Pilot Study" International Journal of Molecular Sciences 22, no. 17: 9454. https://doi.org/10.3390/ijms22179454






