Protective Role of a Donepezil-Huprine Hybrid against the β-Amyloid (1-42) Effect on Human Erythrocytes
Abstract
:1. Introduction
2. Results
2.1. Transmission Electron Microscopy (TEM) of Aβ(1-42) Oligomeric Aggregates and Fibers
2.2. X-ray Diffraction of DMPC and DMPE Multibilayers
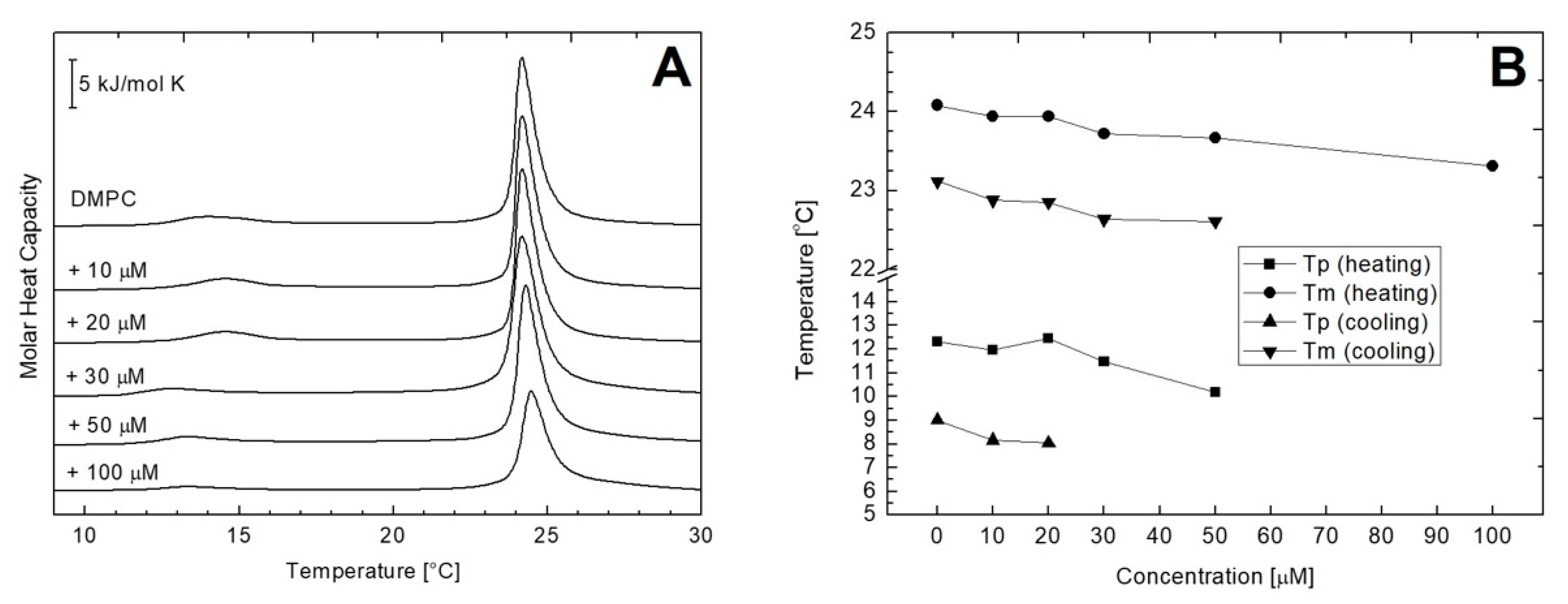
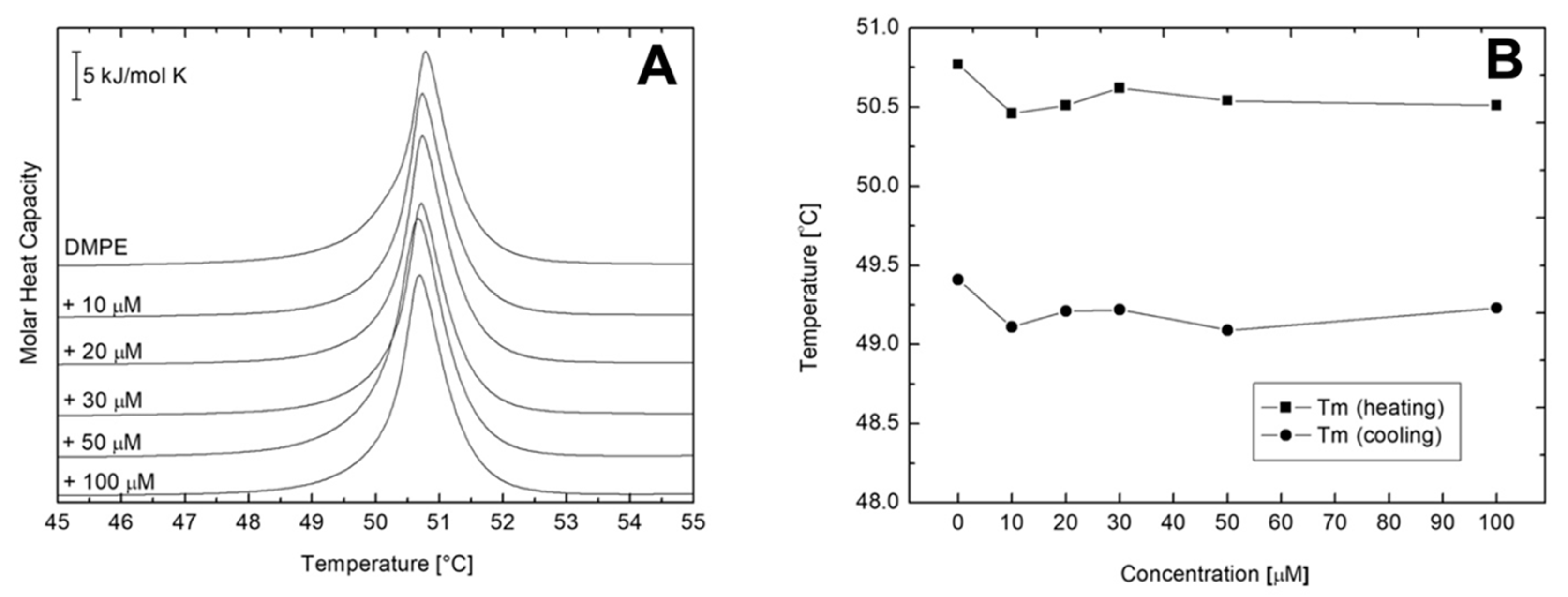
2.3. Differential Scanning Calorimetry (DSC) of Multilamellar Vesicles (MLV) of DMPC and DMPE
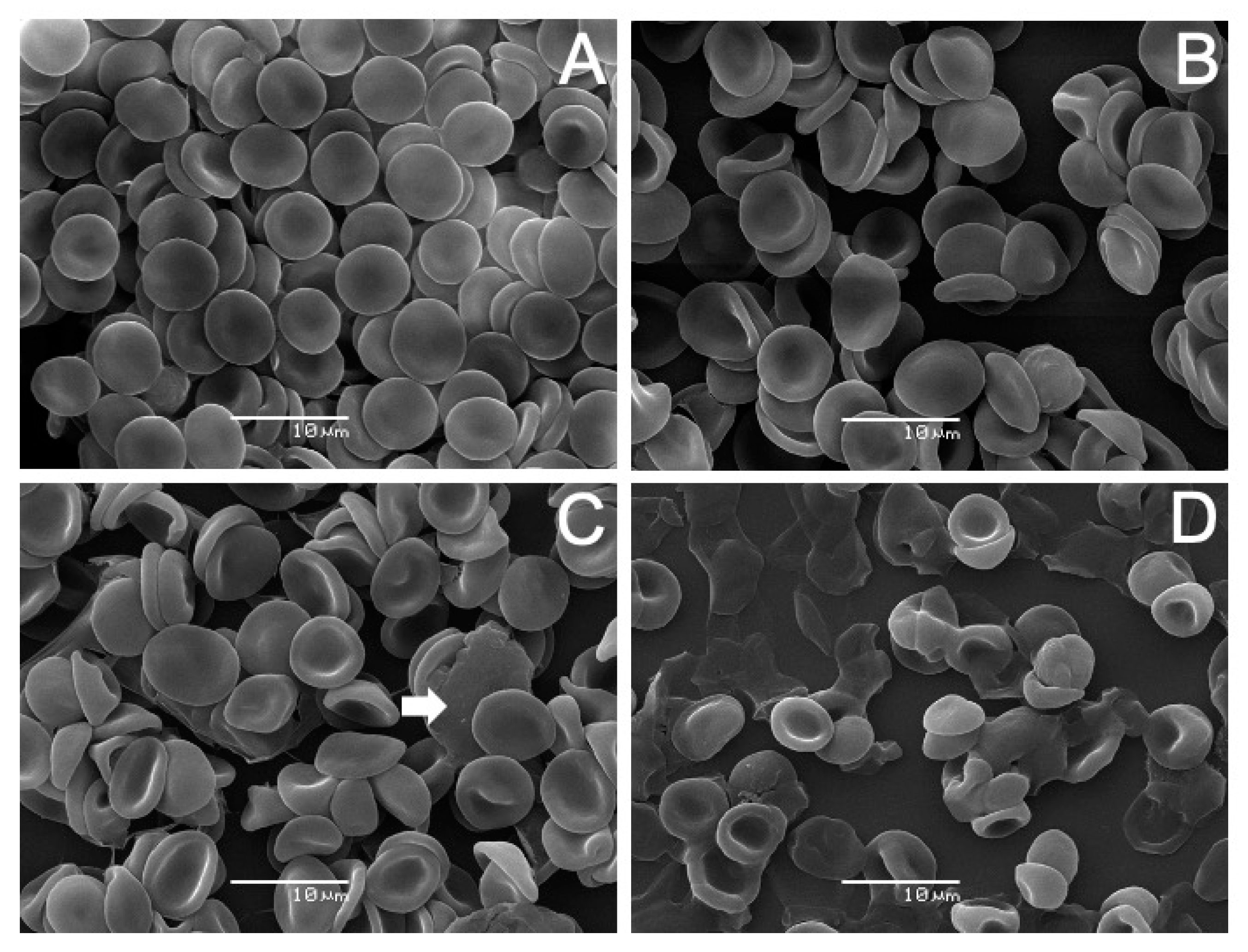
2.4. Scanning Electron Microscopy (SEM) Analysis on Human Erythrocytes
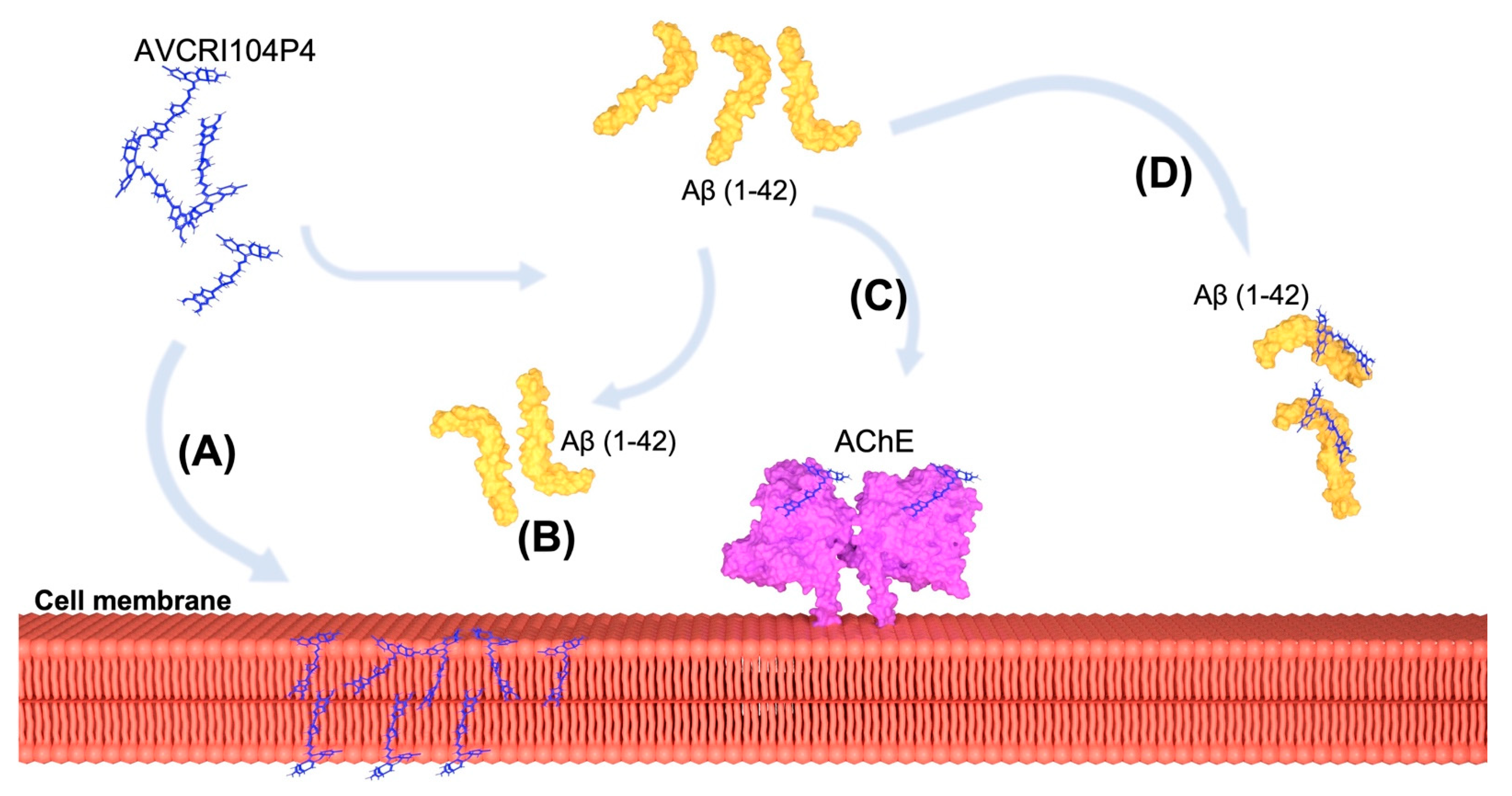
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Oligomeric Aggregates of Aβ(1-42)
4.3. Transmission Electron Microscopy of Aβ(1-42) Oligomeric Aggregates and Fibers
4.4. X-ray Diffraction of DMPC and DMPE Multibilayers
4.5. Differential Scanning Calorimetry (DSC) of Multilamellar Vesicles (MLV) of DMPC and DMPE
4.6. Scanning Electron Microscopy (SEM) of Human Erythrocytes
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, X.J.; Hu, Y.Y.; Yang, Z.J.; Jiang, L.P.; Shi, S.L.; Li, Y.R.; Guo, M.Y.; Wu, H.L.; Wan, Y.Y. Amyloid β-42 induces neuronal apoptosis by targeting mitochondria. Mol. Med. Rep. 2017, 16, 4521–4528. [Google Scholar] [CrossRef] [Green Version]
- Cha, M.Y.; Han, S.H.; Son, S.M.; Hong, H.S.; Choi, Y.J.; Byun, J.; Mook-Jung, I. Mitochondria-specific accumulation of amyloid β induces mitochondrial dysfunction leading to apoptotic cell death. PLoS ONE 2012, 7, e34929. [Google Scholar] [CrossRef]
- Kametani, F.; Hasegawa, M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Korczyn, A.D. The amyloid cascade hypothesis. Alzheimer’s Dement. 2008, 4, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Gertsik, N.; Chiu, D.; Li, Y.M. Complex regulation of γ-secretase: From obligatory to modulatory subunits. Front. Aging Neurosci. 2015, 7, 342. [Google Scholar] [CrossRef] [Green Version]
- Hussain, I.; Fabrègue, J.; Anderes, L.; Ousson, S.; Borlat, F.; Eligert, V.; Berger, S.; Dimitrov, M.; Alattia, J.R.; Fraering, P.C.; et al. The role of γ-secretase activating protein (GSAP) and imatinib in the regulation of γ-secretase activity and amyloid-β generation. J. Biol. Chem. 2013, 288, 2521–2531. [Google Scholar] [CrossRef] [Green Version]
- Xia, W. Amyloid metabolism and secretases in Alzheimer’s disease. Curr. Neurol. Neurosci. Rep. 2001, 1, 422–427. [Google Scholar] [CrossRef]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Coburger, I.; Hoefgen, S.; Than, M.E. The structural biology of the amyloid precursor protein APP-A complex puzzle reveals its multi-domain architecture. Biol. Chem. 2014, 395, 485–498. [Google Scholar] [CrossRef]
- Tyan, S.H.; Shih, A.Y.J.; Walsh, J.J.; Maruyama, H.; Sarsoza, F.; Ku, L.; Eggert, S.; Hof, P.R.; Koo, E.H.; Dickstein, D.L. Amyloid precursor protein (APP) regulates synaptic structure and function. Mol. Cell. Neurosci. 2012, 51, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Reinhard, C.; Hébert, S.S.; De Strooper, B. The amyloid-β precursor protein: Integrating structure with biological function. EMBO J. 2005, 24, 3996–4006. [Google Scholar] [CrossRef] [Green Version]
- Dulubova, I.; Ho, A.; Huryeva, I.; Südhof, T.C.; Rizo, J. Three-dimensional structure of an independently folded extracellular domain of human amyloid-β precursor protein. Biochemistry 2004, 43, 9583–9588. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, Q.; Chen, Y.X.; Zhao, Y.F.; Li, Y.M. Aβ42 and Aβ40: Similarities and differences. J. Pept. Sci. 2015, 21, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Sachse, C.; Richter, W.; Xu, C.; Fandrich, M.; Grigorieff, N. Comparison of Alzheimer A (1-40) and A (1-42) amyloid fibrils reveals similar protofilament structures. Proc. Natl. Acad. Sci. USA 2009, 106, 19813–19818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Horsley, J.R.; Jovcevski, B.; Wegener, K.L.; Yu, J.; Pukala, T.L.; Abell, A.D. Rationally designed peptide-based inhibitor of Aβ42fibril formation and toxicity: A potential therapeutic strategy for Alzheimer’s disease. Biochem. J. 2020, 477, 2039–2054. [Google Scholar] [CrossRef]
- Ochiishi, T.; Kaku, M.; Kiyosue, K.; Doi, M.; Urabe, T.; Hattori, N.; Shimura, H.; Ebihara, T. New Alzheimer’s disease model mouse specialized for analyzing the function and toxicity of intraneuronal Amyloid β oligomers. Sci. Rep. 2019, 9, 17368. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Samanta, S.; Bagoband, V.; Murugan, N.A.; Govindaraju, T. Antioxidant Berberine-Derivative Inhibits Multifaceted Amyloid Toxicity. iScience 2020, 23, 101005. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussman, J.L. Acetylcholinesterase: From 3D structure to function. Chem. Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Carvajal, F.J.; Inestrosa, N.C. Interactions of AChE with Aβ Aggregates in Alzheimer´s Brain: Therapeutic Relevance of IDN 5706. Front. Mol. Neurosci. 2011, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajmohan, R.; Reddy, P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimer’s Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Tanzi, R.E.; Choi, S.H.; Tanzi, R.E. Alzheimer’s Disease: Causes, Mechanisms, and Steps Toward Prevention. In The Oxford Handbook of the Neurobiology of Learning and Memory; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Inestrosa, N.C.; Alvarez, A.; Dinamarca, M.C.; Pérez-acle, T.; Colombres, M. Acetylcholinesterase-Amyloid-β-peptide Interaction: Effect of Congo Red and the Role of the Wnt Pathway. Curr. Alzheimer Res. 2005, 2, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.L.; Bartolini, M.; Cavalli, A.; Andrisano, V.; Rosini, M.; Minarini, A.; Melchiorre, C. Design, synthesis, and biological evaluation of conformationally restricted rivastigmine analogues. J. Med. Chem. 2004, 47, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, M.; Shentu, J.; Huang, C.; Bai, Y.; Pan, H.; Zhang, D.; Yuan, Z.; Zhang, H.; Xiao, X.; et al. Inhibition of acetylcholinesterase activity and β-amyloid oligomer formation by 6-bromotryptamine A, a multi-target anti-Alzheimer’s molecule. Oncol. Lett. 2020, 19, 1593–1601. [Google Scholar] [CrossRef] [Green Version]
- Mohsin, N.U.A.; Ahmad, M. Donepezil: A review of the recent structural modifications and their impact on anti-alzheimer activity. Brazilian J. Pharm. Sci. 2020, 56. [Google Scholar] [CrossRef]
- Pérez-Areales, F.J.; Betari, N.; Viayna, A.; Pont, C.; Espargaró, A.; Bartolini, M.; De Simone, A.; Rinaldi Alvarenga, J.F.; Pérez, B.; Sabate, R.; et al. Design, synthesis and multitarget biological profiling of second-generation anti-Alzheimer rhein-huprine hybrids. Future Med. Chem. 2017, 9, 965–981. [Google Scholar] [CrossRef]
- Serrano, F.G.; Tapia-Rojas, C.; Carvajal, F.J.; Cisternas, P.; Viayna, E.; Sola, I.; Munoz-Torrero, D.; Inestrosa, N.C. Rhein-Huprine Derivatives Reduce Cognitive Impairment, Synaptic Failure and Amyloid Pathology in AβPPswe/PS-1 Mice of Different Ages. Curr. Alzheimer Res. 2016, 13, 1017–1029. [Google Scholar] [CrossRef]
- Viayna, E.; Sola, I.; Bartolini, M.; De Simone, A.; Tapia-Rojas, C.; Serrano, F.G.; Sabaté, R.; Juárez-Jiménez, J.; Pérez, B.; Luque, F.J.; et al. Synthesis and multitarget biological profiling of a novel family of rhein derivatives as disease-modifying anti-Alzheimer agents. J. Med. Chem. 2014, 57, 2549–2567. [Google Scholar] [CrossRef] [Green Version]
- Sola, I.; Viayna, E.; Gómez, T.; Galdeano, C.; Cassina, M.; Camps, P.; Romeo, M.; Diomede, L.; Salmona, M.; Franco, P.; et al. Multigram synthesis and in Vivo efficacy studies of a novel multitarget anti-alzheimer’s compound. Molecules 2015, 20, 4492–4515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viayna, E.; Gómez, T.; Galdeano, C.; Ramírez, L.; Ratia, M.; Badia, A.; Clos, M.V.; Verdaguer, E.; Junyent, F.; Camins, A.; et al. Novel huprine derivatives with inhibitory activity toward β-amyloid aggregation and formation as disease-modifying anti-Alzheimer drug candidates. ChemMedChem 2010, 5, 1855–1870. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood–brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Ratia, M.; Pérez, B.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Clos, M.V. AVCRI104P3, a novel multitarget compound with cognition-enhancing and anxiolytic activities: Studies in cognitively poor middle-aged mice. Behav. Brain Res. 2015, 286, 97–103. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Santana-Santana, M.; Ratia, M.; Pérez, B.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Clos, M.V. Clock/Sleep-Dependent Learning and Memory in Male 3xTg-AD Mice at Advanced Disease Stages and Extrinsic Effects of Huprine X and the Novel Multitarget Agent AVCRI104P3. Brain Sci. 2021, 11, 426. [Google Scholar] [CrossRef]
- Relat, J.; Come, J.; Perez, B.; Camps, P.; Muñoz-Torrero, D.; Badia, A.; Gimenez-Llort, L.; Clos, M.V. Neuroprotective Effects of the Multitarget Agent AVCRI104P3 in Brain of Middle-Aged Mice. Int. J. Mol. Sci. 2018, 19, 2615. [Google Scholar] [CrossRef] [Green Version]
- Bartolini, M.; Bertucci, C.; Cavrini, V.; Andrisano, V. β-Amyloid aggregation induced by human acetylcholinesterase: Inhibition studies. Biochem. Pharmacol. 2003, 65, 407–416. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Bolognesi, M.L.; Cavalli, A.; Melchiorre, C.; Andrisano, V. Insight Into the Kinetic of Amyloid β (1–42) Peptide Self-Aggregation: Elucidation of Inhibitors’ Mechanism of Action. ChemBioChem 2007, 8, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.K.; Cappai, R.; Pham, C.L.L.; Ciccotosto, G.D. Membrane-bound tetramer and trimer Aβ oligomeric species correlate with toxicity towards cultured neurons. J. Neurochem. 2016, 136, 594–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe-Nakayama, T.; Ono, K.; Itami, M.; Takahashi, R.; Teplow, D.B.; Yamada, M. High-speed atomic force microscopy reveals structural dynamics of amyloid β1-42 aggregates. Proc. Natl. Acad. Sci. USA 2016, 113, 5835–5840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tristram-Nagle, S.; Liu, Y.; Legleiter, J.; Nagle, J.F. Structure of gel phase DMPC determined by x-ray diffraction. Biophys. J. 2002, 83, 3324–3335. [Google Scholar] [CrossRef] [Green Version]
- Petit, K.; Suwalsky, M.; Colina, J.R.; Aguilar, L.F.; Jemiola-Rzeminska, M.; Strzalka, K. In vitro effects of the antitumor drug miltefosine on human erythrocytes and molecular models of its membrane. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 17–25. [Google Scholar] [CrossRef]
- Pentak, D.; Sułkowski, W.W.; Sułkowska, A. Calorimetric and EPR studies of the thermotropic phase behavior of phospholipid membranes. J. Therm. Anal. Calorim. 2008, 93, 471–477. [Google Scholar] [CrossRef]
- Salamone, J.C. Polymeric Materials Encyclopedia, Twelve Volume Set; CRC Press: Boca Raton, FL, USA, 1996; ISBN 9780849324703-CAT# 2470. [Google Scholar]
- Akabori, K.; Nagle, J.F. Structure of the DMPC lipid bilayer ripple phase. Soft Matter 2015, 11, 918–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeagle, P.L. The Structure of Biological Membranes: Third Edition; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781439809587. [Google Scholar]
- Wu, F.G.; Jia, Q.; Wu, R.G.; Yu, Z.W. Regional cooperativity in the phase transitions of dipalmitoylphosphatidylcholine bilayers: The lipid tail triggers the isothermal crystallization process. J. Phys. Chem. B 2011, 115, 8559–8568. [Google Scholar] [CrossRef]
- ChemAxon. ChemAxon Chemicalize Was Used for logP and logD Calculations. Available online: https://chemicalize.com/ (accessed on 11 November 2020).
- Sheetz, M.P.; Singer, S.J. Biological Membranes as Bilayer Couples. A Molecular Mechanism of Drug-Erythrocyte Interactions. Proc. Natl. Acad. Sci. USA 1974, 71, 4457–4461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, W. Letter to the editor: Which concentrations are optimal for in vitro testing? EXCLI J. 2020, 19, 1172–1173. [Google Scholar] [PubMed]
- Paulick, M.G.; Bertozzi, C.R. The glycosylphosphatidylinositol anchor: A complex membrane-anchoring structure for proteins. Biochemistry 2008, 47, 6991–7000. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Ali, M.T.; Shawan, M.M.A.K.; Sarwar, M.G.; Khan, M.A.K.; Halim, M.A. Halogen-directed drug design for Alzheimer’s disease: A combined density functional and molecular docking study. Springerplus 2016, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zambrano, P.; Suwalsky, M.; Jemiola-Rzeminska, M.; Strzalka, K.; Sepúlveda, B.; Gallardo, M.J. The acetylcholinesterase (AChE) inhibitor and anti-Alzheimer drug donepezil interacts with human erythrocytes. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Rohou, A.; Lasker, K.; Yadav, J.K.; Schiene-Fischer, C.; Fändrich, M.; Grigorieff, N.; Petsko, G.A. Peptide dimer structure in an Aβ(1-42) fibril visualized with cryo-EM. Proc. Natl. Acad. Sci. USA 2015, 112, 11858–11863. [Google Scholar] [CrossRef] [Green Version]
- Ambroggio, E.E.; Kim, D.H.; Separovic, F.; Barrow, C.J.; Barnham, K.J.; Bagatolli, L.A.; Fidelio, G.D. Surface behavior and lipid interaction of Alzheimer β-amyloid peptide 1-42: A membrane-disrupting peptide. Biophys. J. 2005, 88, 2706–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, D.C.; Baker, M.D.; Viles, J.H. Ion channel formation by amyloid-β42 oligomers but not amyloid-β40 in cellular membranes. J. Biol. Chem. 2017, 292, 1404–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.N.; Long, H.; Mu, Y.; Chew, L.Y. The toxicity of amyloid β oligomers. Int. J. Mol. Sci. 2012, 13, 7303–7327. [Google Scholar] [CrossRef] [Green Version]
- Müller, W.E.; Koch, S.; Eckert, A.; Hartmann, H.; Scheuer, K. β-amyloid peptide decreases membrane fluidity. Brain Res. 1995, 674, 133–136. [Google Scholar] [CrossRef]
- Müller, W.E.; Eckert, G.P.; Scheuer, K.; Cairns, N.J.; Maras, A.; Gattaz, W.F. Effects of β-amyloid peptides on the fluidity of membranes from frontal and parietal lobes of human brain. High potencies of Aβ1-42 and Aβ1-43. Amyloid 1998, 5, 10–15. [Google Scholar] [CrossRef]
- Allan Butterfield, D.; Castegna, A.; Lauderback, C.M.; Drake, J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol. Aging 2002, 23, 655–664. [Google Scholar] [CrossRef]
- Shirwany, N.A.; Payette, D.; Xie, J.; Guo, Q. The amyloid beta ion channel hypothesis of Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2007, 3, 597–612. [Google Scholar]
- Kagan, B.L. Membrane pores in the pathogenesis of neurodegenerative disease. Prog. Mol. Biol. Transl. Sci. 2012, 107, 295–325. [Google Scholar] [CrossRef] [PubMed]
- Bate, C.; Williams, A. Squalestatin protects neurons and reduces the activation of cytoplasmic phospholipase A2 by Aβ1-42. Neuropharmacology 2007, 53, 222–231. [Google Scholar] [CrossRef]
- Press-Sandler, O.; Miller, Y. Molecular mechanisms of membrane-associated amyloid aggregation: Computational perspective and challenges. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 1889–1905. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.H.; Berkowitz, M.L. Interaction between amyloid-β (1-42) peptide and phospholipid bilayers: A molecular dynamics study. Biophys. J. 2009, 96, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Dante, S.; Hauß, T.; Steitz, R.; Canale, C.; Dencher, N.A. Nanoscale structural and mechanical effects of beta-amyloid (1-42) on polymer cushioned membranes: A combined study by neutron reflectometry and AFM Force Spectroscopy. Biochim. Biophys. Acta-Biomembr. 2011, 1808, 2646–2655. [Google Scholar] [CrossRef] [Green Version]
- Dies, H.; Toppozini, L.; Rheinstädter, M.C. The interaction between amyloid-β peptides and anionic lipid membranes containing cholesterol and melatonin. PLoS ONE 2014, 9, e99124. [Google Scholar] [CrossRef] [Green Version]
- Suwalsky, M.; Hernandez, P.L.; Sotomayor, C.P. Aluminum increases toxic effects of amyloid β-peptides on the human erythrocyte membrane and molecular models. In Metal Ions in Neurological Systems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 9783709110, pp. 125–135. ISBN 9783709110010. [Google Scholar]
- Suwalsky, M.; Hernández, P. Aluminum enhances the toxic effects of amyloid β-peptide on cell membranes and a molecular model. Mon. Chem. 2011, 142, 431–437. [Google Scholar] [CrossRef]
- Sepulveda, F.J.; Parodi, J.; Peoples, R.W.; Opazo, C.; Aguayo, L.G. Synaptotoxicity of Alzheimer beta amyloid can be explained by its membrane perforating property. PLoS ONE 2010, 5, e11820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, C.; Bascuñán, D.; Opazo, C.; Aguayo, L.G. Differential Membrane Toxicity of amyloid-β Fragments by Pore Forming Mechanisms. J. Alzheimer’s Dis. 2016, 51, 689–699. [Google Scholar] [CrossRef]






| AVCRI104P4 | Area Under Curve Decrease (%) | |||
|---|---|---|---|---|
| Concentration [µM] | DMPC | DMPE | ||
| SA | WA | SA | WA | |
| 5 | 9.5 ± 0.4 | 4.6 ± 0.2 | 4.2 ± 0.1 | 15.7 ± 0.7 |
| 10 | 10.0 ± 0.5 | 4.7 ± 0.8 | 11.7 ± 0.3 | 21.4 ± 0.3 |
| 20 | 11.1 ± 0.7 | 5.2 ± 0.9 | 26.7 ± 0.8 | 34.5 ± 0.7 |
| 50 | 13.4 ± 0.7 | 8.2 ± 0.3 | 41.3 ± 1.1 | 43.6 ± 0.9 |
| Aβ (1-42) | Area Under Curve Decrease (%) | |||
|---|---|---|---|---|
| Concentration [µM] | DMPC | DMPE | ||
| SA | WA | SA | WA | |
| 5 | 83.1 ± 0.4 | 83.6 ± 0.9 | 2.1 ± 0.2 | 1.7 ± 0.1 |
| 10 | 92.1 ± 1.5 | 99.9 ± 0.1 | 2.9 ± 0.1 | 3.1 ± 0.1 |
| 20 | 94.5 ± 1.6 | 99.9 ± 0.6 | 2.8 ± 0.4 | 3.2 ± 0.4 |
| 30 | 99.9 ± 0.9 | 99.9 ± 0.3 | 3.0 ± 0.2 | 3.3 ± 0.2 |
| Area under Curve Decrease (%) | ||
|---|---|---|
| Concentration [µM] | DMPC | |
| SA | WA | |
| Aβ(1-42) 20 µM | 99.3 ± 0.3 | 99.1 ± 0.4 |
| +AVCRI104P4 | ||
| 10 µM | 81.4 ± 0.9 | 95.2 ± 0.1 |
| 20 µM | 73.6 ± 0.3 | 94.6 ± 0.5 |
| 50 µM | 64.2 ± 0.7 | 92.1 ± 0.1 |
| 80 µM | 61.7 ± 0.5 | 89.5 ± 0.7 |
| AVCRI104P4 Concentration [μM] | Pre-Transition (Heating) | Main Transition (Heating) | ||||
| DMPC + AVCRI104P4 | ΔH [kJ/mol] | ΔS [J/mol K] | Tp [°C] | ΔH [kJ/mol] | ΔS [J/mol K] | Tm [°C] |
| 0 | 1.98 | 0.67 | 12.31 | 13.01 | 6.01 | 24.08 |
| 10 | 0.78 | 0.51 | 11.96 | 15.05 | 5.07 | 23.94 |
| 20 | 0.35 | 0.20 | 12.45 | 14.35 | 4.83 | 23.94 |
| 30 | 0.32 | 0.11 | 11.48 | 11.96 | 4.03 | 23.72 |
| 50 | 0.23 | 0.12 | 10.19 | 12.78 | 4.31 | 23.67 |
| 100 | - | - | - | 9.91 | 3.34 | 23.31 |
| AVCRI104P4 Concentration [μM] | Pre-Transition (Cooling) | Main Transition (Cooling) | ||||
| DMPC + AVCRI104P4 | ΔH [kJ/mol] | ΔS [J/mol K] | Tp [°C] | ΔH [kJ/mol] | ΔS [J/mol K] | Tm [°C] |
| 0 | 1.03 | 0.35 | 9.01 | 20.32 | 6.61 | 23.12 |
| 10 | 0.09 | 0.03 | 8.17 | 14.92 | 5.04 | 22.88 |
| 20 | 0.04 | 0.10 | 8.05 | 13.21 | 4.46 | 22.85 |
| 30 | - | - | - | 12.14 | 4.11 | 22.64 |
| 50 | - | - | - | 11.73 | 4.64 | 22.61 |
| 100 | - | - | - | 10.10 | 3.79 | 19.88 |
| AVCRI104P4 Concentration [μM] | Main Transition (Heating) | Main Transition (Cooling) | ||||
|---|---|---|---|---|---|---|
| DMPE + AVCRI104P4 | ΔH [kJ/mol] | ΔS [J/mol K] | Tm [°C] | ΔH [kJ/mol] | ΔS [J/mol K] | Tm [°C] |
| 0 | 20.73 | 6.01 | 50.77 | 19.17 | 6.06 | 49.41 |
| 10 | 14.97 | 4.64 | 50.46 | 17.18 | 4.13 | 49.11 |
| 20 | 13.16 | 4.07 | 50.51 | 17.84 | 5.81 | 49.21 |
| 30 | 10.51 | 3.25 | 50.62 | 18.43 | 4.52 | 49.22 |
| 50 | 12.46 | 3.86 | 50.54 | 18.53 | 6.23 | 49.09 |
| 100 | 6.49 | 2.01 | 50.51 | 19.17 | 5.18 | 49.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrano, P.; Suwalsky, M.; Jemiola-Rzeminska, M.; Gallardo-Nelson, M.J.; Strzalka, K.; Muñoz-Torrero, D. Protective Role of a Donepezil-Huprine Hybrid against the β-Amyloid (1-42) Effect on Human Erythrocytes. Int. J. Mol. Sci. 2021, 22, 9563. https://doi.org/10.3390/ijms22179563
Zambrano P, Suwalsky M, Jemiola-Rzeminska M, Gallardo-Nelson MJ, Strzalka K, Muñoz-Torrero D. Protective Role of a Donepezil-Huprine Hybrid against the β-Amyloid (1-42) Effect on Human Erythrocytes. International Journal of Molecular Sciences. 2021; 22(17):9563. https://doi.org/10.3390/ijms22179563
Chicago/Turabian StyleZambrano, Pablo, Mario Suwalsky, Malgorzata Jemiola-Rzeminska, María José Gallardo-Nelson, Kazimierz Strzalka, and Diego Muñoz-Torrero. 2021. "Protective Role of a Donepezil-Huprine Hybrid against the β-Amyloid (1-42) Effect on Human Erythrocytes" International Journal of Molecular Sciences 22, no. 17: 9563. https://doi.org/10.3390/ijms22179563
APA StyleZambrano, P., Suwalsky, M., Jemiola-Rzeminska, M., Gallardo-Nelson, M. J., Strzalka, K., & Muñoz-Torrero, D. (2021). Protective Role of a Donepezil-Huprine Hybrid against the β-Amyloid (1-42) Effect on Human Erythrocytes. International Journal of Molecular Sciences, 22(17), 9563. https://doi.org/10.3390/ijms22179563








