Canady Helios Cold Plasma Induces Breast Cancer Cell Death by Oxidation of Histone mRNA
Abstract
:1. Introduction
2. Results
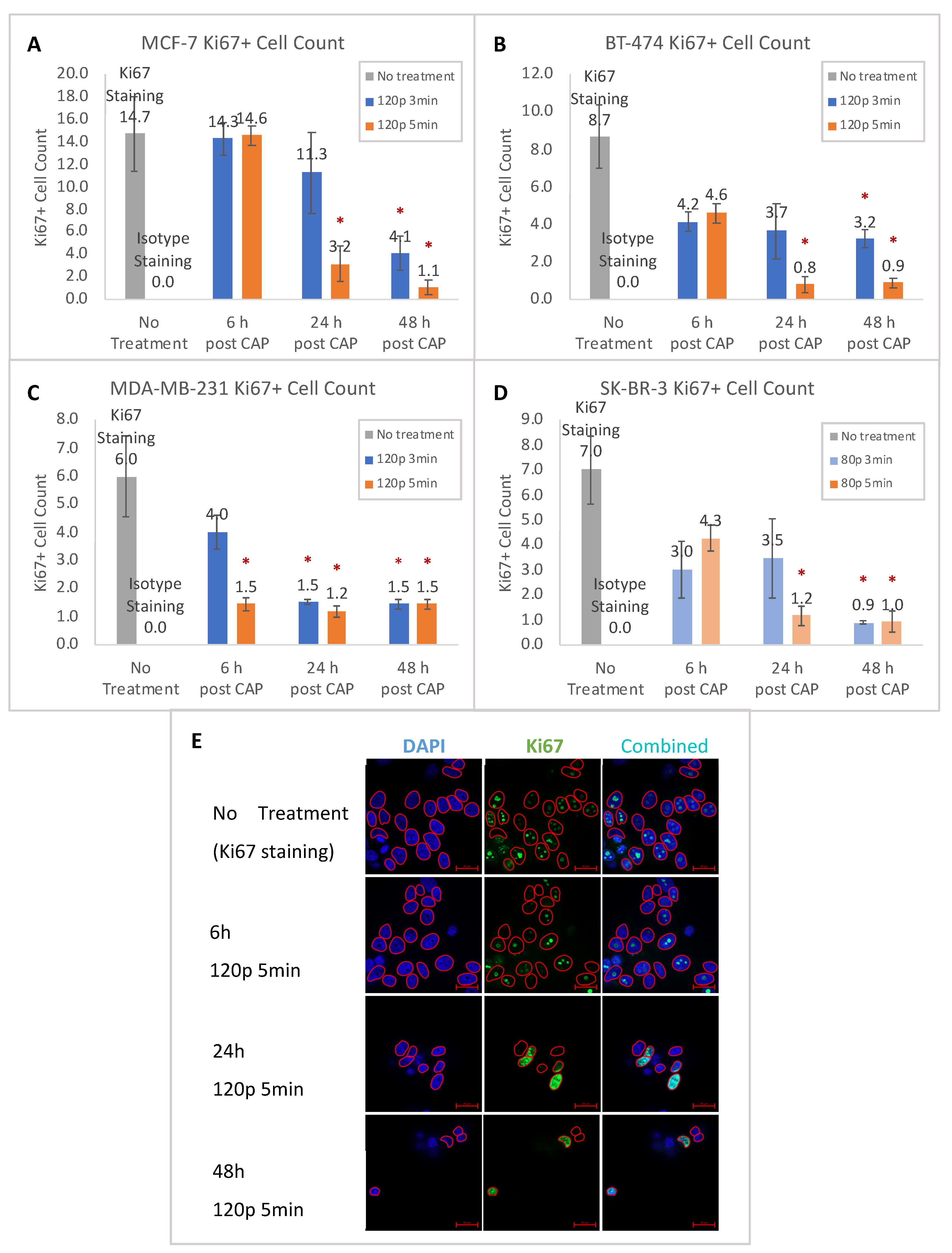
2.1. Proliferation Reduction after CHCP Treatment
2.2. Apoptosis Induced by CHCP Treatment
2.3. Temporal Progress of the Cell Cycle
2.4. Whole Transcriptome Analysis
2.5. Oxidation of RNA after CHCP Treatment
2.6. DNA Damage Induced by CHCP Comes after RNA Oxidation
3. Discussion
4. Materials and Methods
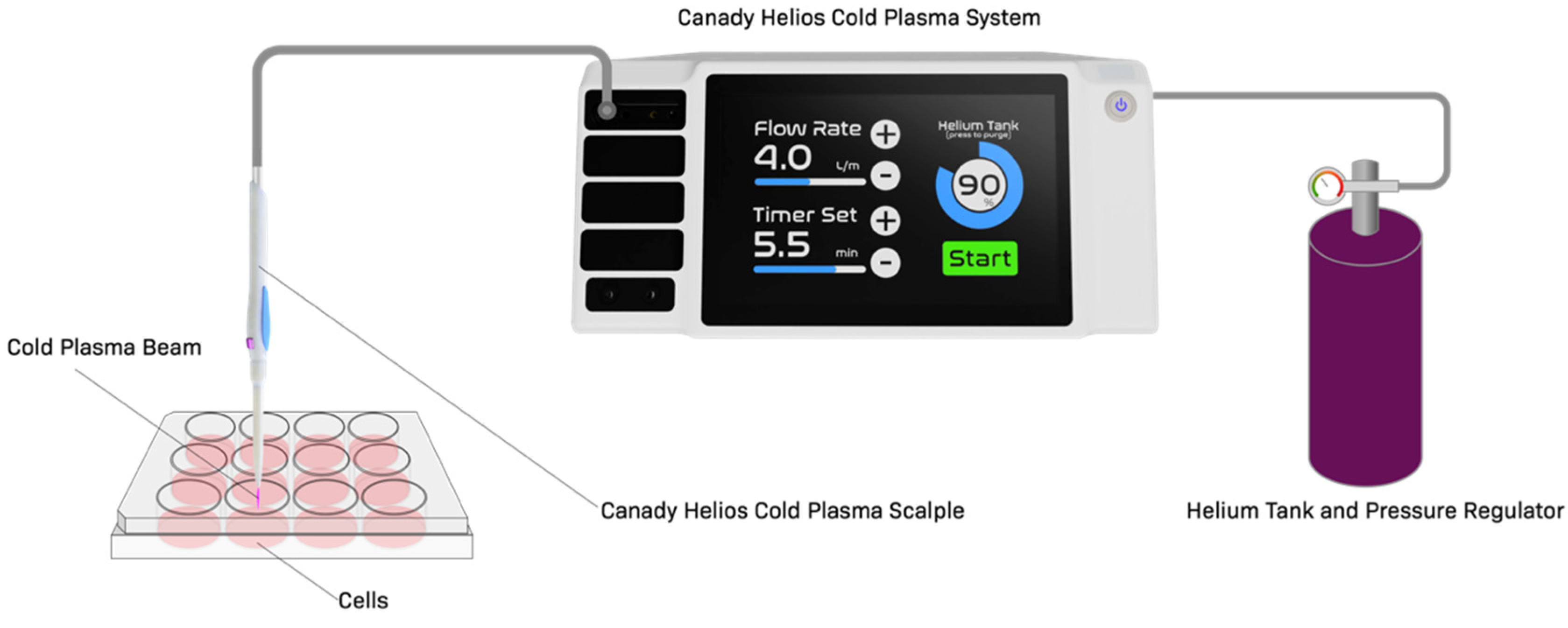
4.1. Cold Plasma Device
4.2. Cell Culture
4.3. IncuCyte Live Cell Imaging for Confluence, Caspase Activity, and Cell Cycle
4.4. Flow Cytometry
4.5. Confocal Microscopy
4.6. RNA-Seq Library Preparation
4.7. Real-Time qPCR Validation
4.8. Oxoguanine (8-oxoG) Modification of RNA
4.9. Pull-Down
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russnes, H.G.; Lingjaerde, O.C.; Borresen-Dale, A.L.; Caldas, C. Breast Cancer Molecular Stratification: From Intrinsic Subtypes to Integrative Clusters. Am. J. Pathol. 2017, 187, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [Green Version]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [Green Version]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, H.; Ji, H.W.; Kim, H.W.; Yun, S.H.; Choi, E.H.; Kim, S.J. Cold Atmospheric Plasma Restores Paclitaxel Sensitivity to Paclitaxel-Resistant Breast Cancer Cells by Reversing Expression of Resistance-Related Genes. Cancers 2019, 11, 22011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Jeong, D.; Ham, J.; Park, S.; Choi, E.H.; Kim, S.J. Cold atmospheric plasma restores tamoxifen sensitivity in resistant MCF-7 breast cancer cell. Free Radic. Biol. Med. 2017, 110, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Holmes, B.; Cheng, X.; Zhu, W.; Keidar, M.; Zhang, L.G. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS ONE 2013, 8, e73741. [Google Scholar] [CrossRef]
- Ly, L.; Cheng, X.; Murthy, S.R.K.; Zhuang, T.; Jones, O.Z.; Basadonna, G.; Keidar, M.; Canady, J. Canady cold plasma conversion system treatment: An effective inhibitor of cell viability in breast cancer molecular subtypes. Clin. Plasma Med. 2020. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, S.; Zhang, H.; Kong, X.; Ding, L.; Shen, J.; Lan, Y.; Cheng, C.; Zhu, T.; Xia, W. Selective effects of non-thermal atmospheric plasma on triple-negative breast normal and carcinoma cells through different cell signaling pathways. Sci. Rep. 2017, 7, 7980. [Google Scholar] [CrossRef]
- Canady, J.; Shashurin, A.; Keidar, M.; Zhuang, T. Integrated Cold Plasma and High Frequency Plasma Electrosurgical System and Method. U.S. Patent No. 9,999,462, 19 June 2018. [Google Scholar]
- Rowe, W.; Cheng, X.; Ly, L.; Zhuang, T.; Basadonna, G.; Trink, B.; Keidar, M.; Canady, J. The Canady Helios Cold Plasma Scalpel Significantly Decreases Viability in Malignant Solid Tumor Cells in a Dose-Dependent Manner. Plasma 2018, 1, 16. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Rowe, W.; Ly, L.; Shashurin, A.; Zhuang, T.; Wigh, S.; Basadonna, G.; Trink, B.; Keidar, M.; Canady, J. Treatment of Triple-Negative Breast Cancer Cells with the Canady Cold Plasma Conversion System: Preliminary Results. Plasma 2018, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Bazaka, K.; Thompson, E.W.; Ostrikov, K.K. Cold Atmospheric Plasma: A Promising Controller of Cancer Cell States. Cancers 2020, 12, 3360. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, M.; Evans, M.D.; Ostrikov, K.K. Atmospheric pressure gas plasma-induced colorectal cancer cell death is mediated by Nox2-ASK1 apoptosis pathways and oxidative stress is mitigated by Srx-Nrf2 anti-oxidant system. Biochim. Biophys. Acta 2014, 1843, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zeng, W.; Xia, Y.; Wang, B.; Xu, D.; Liu, D.; Kong, M.G.; Dong, Y. Cold atmospheric plasma induces apoptosis of melanoma cells via Sestrin2-mediated nitric oxide synthase signaling. J. Biophotonics 2019, 12, e201800046. [Google Scholar] [CrossRef] [Green Version]
- Park, S.B.; Kim, B.; Bae, H.; Lee, H.; Lee, S.; Choi, E.H.; Kim, S.J. Differential Epigenetic Effects of Atmospheric Cold Plasma on MCF-7 and MDA-MB-231 Breast Cancer Cells. PLoS ONE 2015, 10, e0129931. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Campos, A.; Azorin, F. RNA is an integral component of chromatin that contributes to its structural organization. PLoS ONE 2007, 2, e1182. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.K.; Roberts, J.T.; Balas, M.M.; Johnson, A.M. RNA matchmaking in chromatin regulation. Biochem. Soc. Trans. 2020, 48, 2467–2481. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, R.D.; Lorch, Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 1999, 98, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Khorasanizadeh, S. The nucleosome: From genomic organization to genomic regulation. Cell 2004, 116, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Heintz, N. The regulation of histone gene expression during the cell cycle. Biochim. Biophys. Acta 1991, 1088, 327–339. [Google Scholar] [CrossRef]
- Marzluff, W.F.; Duronio, R.J. Histone mRNA expression: Multiple levels of cell cycle regulation and important developmental consequences. Curr. Opin. Cell Biol. 2002, 14, 692–699. [Google Scholar] [CrossRef]
- Osley, M.A. The regulation of histone synthesis in the cell cycle. Annu. Rev. Biochem. 1991, 60, 827–861. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Stein, J.L.; Van Wijnen, A.J.; Lian, J.B. Transcriptional control of cell cycle progression: The histone gene is a paradigm for the G1/S phase and proliferation/differentiation transitions. Cell Biol. Int. 1996, 20, 41–49. [Google Scholar] [CrossRef]
- Gunjan, A.; Paik, J.; Verreault, A. Regulation of histone synthesis and nucleosome assembly. Biochimie 2005, 87, 625–635. [Google Scholar] [CrossRef]
- DeRan, M.; Pulvino, M.; Greene, E.; Su, C.; Zhao, J. Transcriptional activation of histone genes requires NPAT-dependent recruitment of TRRAP-Tip60 complex to histone promoters during the G1/S phase transition. Mol. Cell Biol. 2008, 28, 435–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miele, A.; Braastad, C.D.; Holmes, W.F.; Mitra, P.; Medina, R.; Xie, R.; Zaidi, S.K.; Ye, X.; Wei, Y.; Harper, J.W.; et al. HiNF-P directly links the cyclin E/CDK2/p220NPAT pathway to histone H4 gene regulation at the G1/S phase cell cycle transition. Mol. Cell Biol. 2005, 25, 6140–6153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, C.; Heintz, N.; Roeder, R.G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell 1987, 51, 773–781. [Google Scholar] [CrossRef]
- Mei, Q.; Huang, J.; Chen, W.; Tang, J.; Xu, C.; Yu, Q.; Cheng, Y.; Ma, L.; Yu, X.; Li, S. Regulation of DNA replication-coupled histone gene expression. Oncotarget 2017, 8, 95005–95022. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J. Coordination of DNA synthesis and histone gene expression during normal cell cycle progression and after DNA damage. Cell Cycle 2004, 3, 695–697. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Franco, A.A.; Santos, H.; Nelson, D.M.; Kaufman, P.D.; Adams, P.D. Defective S phase chromatin assembly causes DNA damage, activation of the S phase checkpoint, and S phase arrest. Mol. Cell 2003, 11, 341–351. [Google Scholar] [CrossRef]
- Meeks-Wagner, D.; Hartwell, L.H. Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission. Cell 1986, 44, 43–52. [Google Scholar] [CrossRef]
- Kurat, C.F.; Lambert, J.P.; van Dyk, D.; Tsui, K.; van Bakel, H.; Kaluarachchi, S.; Friesen, H.; Kainth, P.; Nislow, C.; Figeys, D.; et al. Restriction of histone gene transcription to S phase by phosphorylation of a chromatin boundary protein. Genes Dev. 2011, 25, 2489–2501. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Eswaran, J.; Cyanam, D.; Mudvari, P.; Reddy, S.D.; Pakala, S.B.; Nair, S.S.; Florea, L.; Fuqua, S.A.; Godbole, S.; Kumar, R. Transcriptomic landscape of breast cancers through mRNA sequencing. Sci. Rep. 2012, 2, 264. [Google Scholar] [CrossRef] [Green Version]
- Horvath, A.; Pakala, S.B.; Mudvari, P.; Reddy, S.D.; Ohshiro, K.; Casimiro, S.; Pires, R.; Fuqua, S.A.; Toi, M.; Costa, L.; et al. Novel insights into breast cancer genetic variance through RNA sequencing. Sci. Rep. 2013, 3, 2256. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Li, Y.; Qin, N.; Wang, F.; Du, J.; Wang, C.; Du, F.; Jiang, T.; Jiang, Y.; Dai, J.; et al. RNA-seq analysis identified hormone-related genes associated with prognosis of triple negative breast cancer. J. Biomed. Res. 2020, 34, 129–138. [Google Scholar] [CrossRef]
- Kurita, H.; Haruta, N.; Uchihashi, Y.; Seto, T.; Takashima, K. Strand breaks and chemical modification of intracellular DNA induced by cold atmospheric pressure plasma irradiation. PLoS ONE 2020, 15, e0232724. [Google Scholar] [CrossRef]
- Gonzalez-Rivera, J.C.; Sherman, M.W.; Wang, D.S.; Chuvalo-Abraham, J.C.L.; Hildebrandt Ruiz, L.; Contreras, L.M. RNA oxidation in chromatin modification and DNA-damage response following exposure to formaldehyde. Sci. Rep. 2020, 10, 16545. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.M.; Shin, J.I.; Kim, J.B.; Lee, K.; Chung, J.H.; Yang, H.W.; Kim, K.N.; Han, Y.S. Detection of 8-oxoguanine and apurinic/apyrimidinic sites using a fluorophore-labeled probe with cell-penetrating ability. BMC Mol. Cell Biol. 2019, 20, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Lu, D.; Hai, T.; Boyd, D.D. Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. EMBO J. 2005, 24, 2425–2435. [Google Scholar] [CrossRef] [Green Version]
- Guerrero Llobet, S.; van der Vegt, B.; Jongeneel, E.; Bense, R.D.; Zwager, M.C.; Schroder, C.P.; Everts, M.; Fehrmann, R.S.N.; de Bock, G.H.; van Vugt, M. Cyclin E expression is associated with high levels of replication stress in triple-negative breast cancer. NPJ Breast Cancer 2020, 6, 40. [Google Scholar] [CrossRef]
- Krones-Herzig, A.; Mittal, S.; Yule, K.; Liang, H.; English, C.; Urcis, R.; Soni, T.; Adamson, E.D.; Mercola, D. Early growth response 1 acts as a tumor suppressor in vivo and in vitro via regulation of p53. Cancer Res. 2005, 65, 5133–5143. [Google Scholar] [CrossRef] [Green Version]
- Soliman, N.A.; Yussif, S.M. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol. Med. 2016, 13, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Endl, E.; Gerdes, J. Posttranslational Modifications of the Ki-67 Protein Coincide with Two Major Checkpoints during Mitosis. J. Cell. Physiol. 2000, 182, 371–3880. [Google Scholar] [CrossRef]
- Kill, I.R. Localisation of the Ki-67 antigen within the nucleolus. Evidence for a fibrillarin-deficient region of the dense fibrillar component. J. Cell Sci. 1996, 109, 1253–1263. [Google Scholar] [CrossRef]
- Chierico, L.; Rizzello, L.; Guan, L.; Joseph, A.S.; Lewis, A.; Battaglia, G. The role of the two splice variants and extranuclear pathway on Ki-67 regulation in non-cancer and cancer cells. PLoS ONE 2017, 12, e0171815. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Kaushik, N.K.; Kaushik, N.; Hammerschmid, D.; Privat-Maldonado, A.; De Backer, J.; Shiratani, M.; Choi, E.H.; Bogaerts, A. Plasma treatment causes structural modifications in lysozyme, and increases cytotoxicity towards cancer cells. Int. J. Biol. Macromol. 2021, 182, 1724–1736. [Google Scholar] [CrossRef]
- Lafontaine, J.; Boisvert, J.S.; Glory, A.; Coulombe, S.; Wong, P. Synergy between Non-Thermal Plasma with Radiation Therapy and Olaparib in a Panel of Breast Cancer Cell Lines. Cancers 2020, 12, 348. [Google Scholar] [CrossRef] [Green Version]
- Semmler, M.L.; Bekeschus, S.; Schafer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.S.; Moon, E.; Baek, S.J.; Lee, K.; et al. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: Involvement of DNA-damage-triggering sub-G(1) arrest via the ATM/p53 pathway. Arch. Biochem. Biophys. 2014, 545, 133–140. [Google Scholar] [CrossRef]
- Kaushik, N.; Uddin, N.; Sim, G.B.; Hong, Y.J.; Baik, K.Y.; Kim, C.H.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Responses of solid tumor cells in DMEM to reactive oxygen species generated by non-thermal plasma and chemically induced ROS systems. Sci. Rep. 2015, 5, 8587. [Google Scholar] [CrossRef] [Green Version]
- Judee, F.; Fongia, C.; Ducommun, B.; Yousfi, M.; Lobjois, V.; Merbahi, N. Short and long time effects of low temperature Plasma Activated Media on 3D multicellular tumor spheroids. Sci. Rep. 2016, 6, 21421. [Google Scholar] [CrossRef]
- Sagwal, S.K.; Pasqual-Melo, G.; Bodnar, Y.; Gandhirajan, R.K.; Bekeschus, S. Combination of chemotherapy and physical plasma elicits melanoma cell death via upregulation of SLC22A16. Cell Death Dis. 2018, 9, 1179. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schutz, C.S.; Niessner, F.; Wende, K.; Weltmann, K.D.; Gelbrich, N.; von Woedtke, T.; Schmidt, A.; Stope, M.B. Elevated H2AX Phosphorylation Observed with kINPen Plasma Treatment Is Not Caused by ROS-Mediated DNA Damage but Is the Consequence of Apoptosis. Oxid. Med. Cell Longev. 2019, 2019, 8535163. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Li, X.; Guo, M.; Yu, J.; Yan, C. The common stress responsive transcription factor ATF3 binds genomic sites enriched with p300 and H3K27ac for transcriptional regulation. BMC Genom. 2016, 17, 335. [Google Scholar] [CrossRef] [Green Version]
- Stuart, J.R.; Kawai, H.; Tsai, K.K.; Chuang, E.Y.; Yuan, Z.M. c-Abl regulates early growth response protein (EGR1) in response to oxidative stress. Oncogene 2005, 24, 8085–8092. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, Z.; Long, C.; Ning, J.; Zhang, H.; Kuang, X.; Zhang, Q.; Shen, H. ID2 protects retinal pigment epithelium cells from oxidative damage through p-ERK1/2/ID2/NRF2. Arch. Biochem. Biophys. 2018, 650, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.M.; Ye, X.; Hall, C.; Santos, H.; Ma, T.; Kao, G.D.; Yen, T.J.; Harper, J.W.; Adams, P.D. Coupling of DNA synthesis and histone synthesis in S phase independent of cyclin/cdk2 activity. Mol. Cell Biol. 2002, 22, 7459–7472. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Chen, X.; Liu, Z.; Ye, W.; Li, L.; Qian, L.; Ding, H.; Li, P.; Aung, L.H.H. Recent Advances: Molecular Mechanism of RNA Oxidation and Its Role in Various Diseases. Front. Mol. Biosci. 2020, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cai, J.P.; Zhang, L.Q.; Sun, N.; Cui, J.; Wang, H.; Yang, J.F. The mechanism of RNA oxidation involved in the development of heart failure. Free Radic. Res. 2019, 53, 910–921. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Nunomura, A.; Lee, H.G.; Zhu, X.; Perry, G. Consequences of RNA oxidation on protein synthesis rate and fidelity: Implications for the pathophysiology of neuropsychiatric disorders. Biochem. Soc. Trans. 2017, 45, 1053–1066. [Google Scholar] [CrossRef]
- Han, D.; Cho, J.H.; Lee, R.H.; Bang, W.; Park, K.; Kim, M.S.; Shim, J.H.; Chae, J.I.; Moon, S.Y. Antitumorigenic effect of atmospheric-pressure dielectric barrier discharge on human colorectal cancer cells via regulation of Sp1 transcription factor. Sci. Rep. 2017, 7, 43081. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.W.; Jeong, D.; Ham, J.; Kim, H.; Ji, H.W.; Choi, E.H.; Kim, S.J. ZNRD1 and Its Antisense Long Noncoding RNA ZNRD1-AS1 Are Oppositely Regulated by Cold Atmospheric Plasma in Breast Cancer Cells. Oxid. Med. Cell Longev. 2020, 2020, 9490567. [Google Scholar] [CrossRef]
- Simms, C.L.; Hudson, B.H.; Mosior, J.W.; Rangwala, A.S.; Zaher, H.S. An active role for the ribosome in determining the fate of oxidized mRNA. Cell Rep. 2014, 9, 1256–1264. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Chock, P.B.; Stadtman, E.R. Oxidized messenger RNA induces translation errors. Proc. Natl. Acad. Sci. USA 2007, 104, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Chock, P.B. Oxidative Modifications of RNA and Its Potential Roles in Biosystem. Front. Mol. Biosci. 2021, 8, 685331. [Google Scholar] [CrossRef]
- Boo, S.H.; Kim, Y.K. The emerging role of RNA modifications in the regulation of mRNA stability. Exp. Mol. Med. 2020, 52, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyrick, J.J.; Parra, M.A. The role of histone H2A and H2B post-translational modifications in transcription: A genomic perspective. Biochim. Biophys. Acta 2009, 1789, 37–44. [Google Scholar] [CrossRef]
- Harris, M.E.; Bohni, R.; Schneiderman, M.H.; Ramamurthy, L.; Schumperli, D.; Marzluff, W.F. Regulation of histone mRNA in the unperturbed cell cycle: Evidence suggesting control at two posttranscriptional steps. Mol. Cell Biol. 1991, 11, 2416–2424. [Google Scholar] [CrossRef] [Green Version]
- Heintz, N.; Sive, H.L.; Roeder, R.G. Regulation of human histone gene expression: Kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol. Cell Biol. 1983, 3, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Braunstein, M.; Liao, L.; Lyttle, N.; Lobo, N.; Taylor, K.J.; Krzyzanowski, P.M.; Kalatskaya, I.; Yao, C.Q.; Stein, L.D.; Boutros, P.C.; et al. Downregulation of histone H2A and H2B pathways is associated with anthracycline sensitivity in breast cancer. Breast Cancer Res. 2016, 18, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzluff, W.F.; Koreski, K.P. Birth and Death of Histone mRNAs. Trends Genet. 2017, 33, 745–759. [Google Scholar] [CrossRef]
- Zhong, B.L.; Bian, L.J.; Wang, G.M.; Zhou, Y.F.; Chen, Y.Y.; Peng, F. Identification of key genes involved in HER2-positive breast cancer. Eur. Rev. Med. Pharm. Sci. 2016, 20, 664–672. [Google Scholar]
- Saleh, R.; Sasidharan Nair, V.; Toor, S.M.; Taha, R.Z.; Murshed, K.; Al-Dhaheri, M.; Khawar, M.; Petkar, M.A.; Abu Nada, M.; Al-Ejeh, F.; et al. Differential gene expression of tumor-infiltrating CD8(+) T cells in advanced versus early-stage colorectal cancer and identification of a gene signature of poor prognosis. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Parssinen, J.; Alarmo, E.L.; Khan, S.; Karhu, R.; Vihinen, M.; Kallioniemi, A. Identification of differentially expressed genes after PPM1D silencing in breast cancer. Cancer Lett. 2008, 259, 61–70. [Google Scholar] [CrossRef]
- Su, C.H.; Tzeng, T.Y.; Cheng, C.; Hsu, M.T. An H2A histone isotype regulates estrogen receptor target genes by mediating enhancer-promoter-3′-UTR interactions in breast cancer cells. Nucleic Acids Res. 2014, 42, 3073–3088. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lim, W.; You, D.; Jeong, Y.; Kim, S.; Lee, J.E.; Shin, T.H.; Lee, G.; Park, S. Chemoresistance in the Human Triple-Negative Breast Cancer Cell Line MDA-MB-231 Induced by Doxorubicin Gradient Is Associated with Epigenetic Alterations in Histone Deacetylase. J. Oncol. 2019, 2019, 1345026. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Xue, J.Y.; Chen, C.M.; Yang, B.L.; Xu, Q.H.; Wu, F.; Liu, F.; Ye, X.; Meng, X.; Liu, G.Y.; et al. PPAR signaling pathway may be an important predictor of breast cancer response to neoadjuvant chemotherapy. Cancer Chemother. Pharm. 2012, 70, 637–644. [Google Scholar] [CrossRef]
- Monteiro, F.L.; Baptista, T.; Amado, F.; Vitorino, R.; Jeronimo, C.; Helguero, L.A. Expression and functionality of histone H2A variants in cancer. Oncotarget 2014, 5, 3428–3443. [Google Scholar] [CrossRef] [Green Version]
- de Kok, J.B.; Roelofs, R.W.; Giesendorf, B.A.; Pennings, J.L.; Waas, E.T.; Feuth, T.; Swinkels, D.W.; Span, P.N. Normalization of gene expression measurements in tumor tissues: Comparison of 13 endogenous control genes. Lab. Investig. 2005, 85, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Murthy, S.R.K.; Zhuang, T.; Ly, L.; Jones, O.; Basadonna, G.; Keidar, M.; Kanaan, Y.; Canady, J. Canady Helios Cold Plasma Induces Breast Cancer Cell Death by Oxidation of Histone mRNA. Int. J. Mol. Sci. 2021, 22, 9578. https://doi.org/10.3390/ijms22179578
Cheng X, Murthy SRK, Zhuang T, Ly L, Jones O, Basadonna G, Keidar M, Kanaan Y, Canady J. Canady Helios Cold Plasma Induces Breast Cancer Cell Death by Oxidation of Histone mRNA. International Journal of Molecular Sciences. 2021; 22(17):9578. https://doi.org/10.3390/ijms22179578
Chicago/Turabian StyleCheng, Xiaoqian, Saravana R. K. Murthy, Taisen Zhuang, Lawan Ly, Olivia Jones, Giacomo Basadonna, Michael Keidar, Yasmine Kanaan, and Jerome Canady. 2021. "Canady Helios Cold Plasma Induces Breast Cancer Cell Death by Oxidation of Histone mRNA" International Journal of Molecular Sciences 22, no. 17: 9578. https://doi.org/10.3390/ijms22179578





