Hypoxic and Hyperoxic Breathing as a Complement to Low-Intensity Physical Exercise Programs: A Proof-of-Principle Study
Abstract
1. Introduction
2. Results
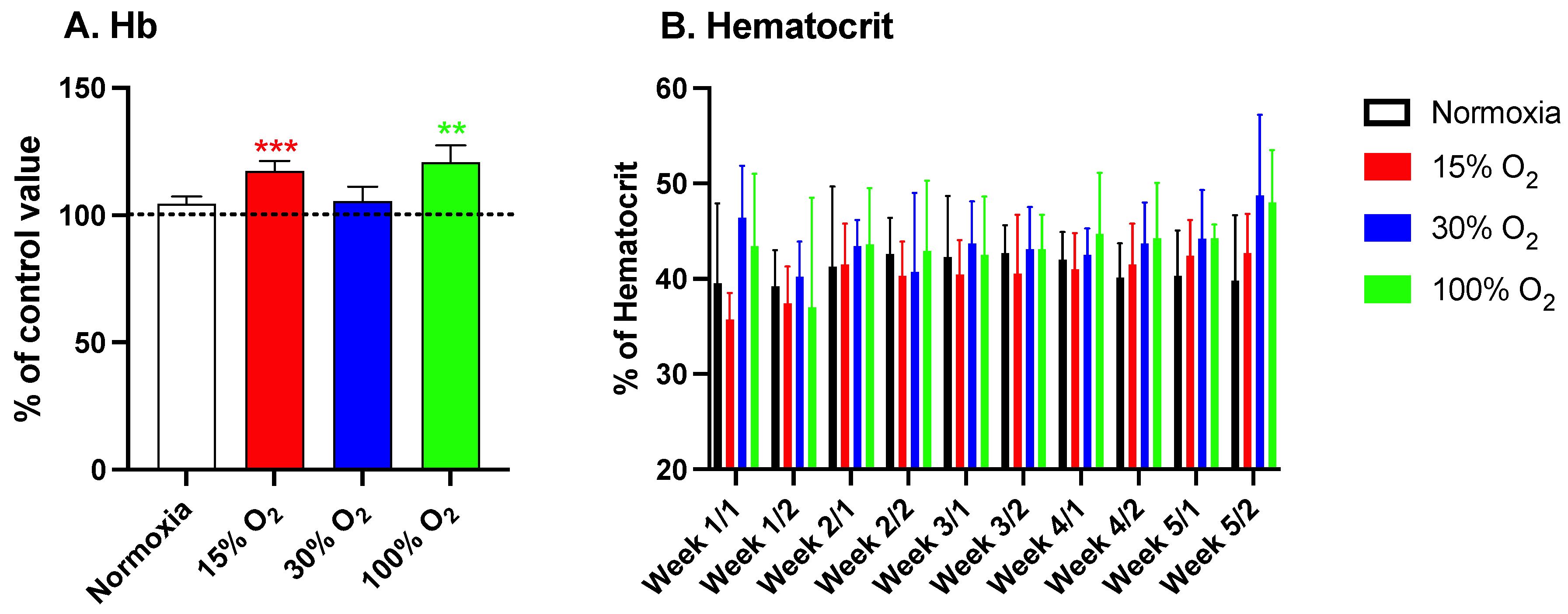
2.1. Hypoxia and High Hyperoxia Induce the Hb Synthesis Similar to an Hypoxic Stimulus
2.2. Low-Level Exercise Performed under Hypoxia or Hyperoxia Is Associated with an Increased Production of Reactive Oxygen Species (ROS) along with a Decreased Total Antioxidant Capacity (TAC)
2.3. Physical Exercise Performed under Modified Oxygen Concentration Affects iNOS Enzyme Expression and NOx Metabolites Levels
2.4. Low-Intensity Physical Excercise in Modified Oxygen Concentration Modulates the Inflammatory State
2.5. A 5-Week Mild Exercise Plan under Altered Oxygen Concentrations Affects Functional Kidney Biomarkers
2.6. Mild Exercise, Independently of Administered pO2, Does Not Affect the Maximal Oxygen Uptake although Hypoxia Significantly Affects Borg’s Ratings of Perceived Exertion
3. Discussion and Conclusions
4. Materials and Methods
4.1. Experimental Protocol
4.2. Cycle Ergometer Stress Test
4.3. Venous Blood Samples Analysis
4.3.1. Redox State
4.3.2. Inflammatory Status
4.3.3. Nitric Oxide Measurements
4.4. Urine Sample Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PO2 | oxygen partial pressure |
| Cys | cysteine |
| CysGly | cysteinyl-glycine |
| GSH | glutathione |
| Hcy | homocysteine |
| HPLC | high-pressure liquid chromatography |
| IL-6 | interleukin-6 |
| IL-10 | interleukin-10 |
| EPR | electron paramagnetic resonance |
| iNOS | inducible nitric oxide synthase |
| NOx | nitric oxide metabolites |
| ROS | reactive oxygen species |
| TAC | total antioxidant capacity |
| TNF-α | tumor necrosis factor-alpha |
References
- Schuster, R.; Rockel, J.S.; Kapoor, M.; Hinz, B. The inflammatory speech of fibroblasts. Immunol. Rev. 2021, 302, 126–146. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Kanso, F.; Khalil, A.; Noureddine, H.; El-Makhour, Y. Therapeutic perspective of thiosemicarbazones derivatives in inflammatory pathologies: A summary of in vitro/in vivo studies. Int. Immunopharmacol. 2021, 96, 107778. [Google Scholar] [CrossRef]
- Higashijima, Y.; Kanki, Y. Potential roles of super enhancers in inflammatory gene transcription. FEBS J. 2021. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Walder, K.; O’Neil, A.; Maes, M.; Puri, B.K. The lipid paradox in neuroprogressive disorders: Causes and consequences. Neurosci. Biobehav. Rev. 2021, 128, 35–57. [Google Scholar] [CrossRef]
- Mehta, O.P.; Bhandari, P.; Raut, A.; Kacimi, S.E.O.; Huy, N.T. Coronavirus Disease (COVID-19): Comprehensive Review of Clinical Presentation. Front. Public Health 2020, 8, 582932. [Google Scholar] [CrossRef]
- Shekhawat, J.; Gauba, K.; Gupta, S.; Purohit, P.; Mitra, P.; Garg, M.; Misra, S.; Sharma, P.; Banerjee, M. Interleukin-6 Perpetrator of the COVID-19 Cytokine Storm. Indian J. Clin. Biochem. 2021, 1–11. [Google Scholar] [CrossRef]
- Ardoin, S.P.; Sundy, J.S. Update on nonsteriodal anti-inflammatory drugs. Curr. Opin. Rheumatol. 2006, 18, 221–226. [Google Scholar] [CrossRef]
- Hemati, A.Y.; Rahimi, M.; Zilaei, B.S.; Shirivani, H.; Hemati, A.Z.; Bazgir, B. Inflammatory Immune System Response to Short Term Altitude Exposure and Recreational Physical Activity. Int. J. Sport Stud. 2014, 4, 1383–1387. [Google Scholar]
- Batista, M.L., Jr.; Lopes, R.D.; Seelaender, M.C.; Lopes, A.C. Anti-inflammatory effect of physical training in heart failure: Role of TNF-alpha and IL-10. Arq. Bras. Cardiol. 2009, 93, 643–651, 692–700. [Google Scholar]
- Nimmo, M.A.; Leggate, M.; Viana, J.L.; King, J.A. The effect of physical activity on mediators of inflammation. Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 51–60. [Google Scholar] [CrossRef]
- Philippou, A.; Maridaki, M.; Psarros, C.; Koutsilieris, M. Systemic responses of inflammation-related factors following eccentric exercise in humans. Am. J. Sports Sci. 2018, 6, 32–37. [Google Scholar] [CrossRef]
- Suzuki, K. Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int. J. Sports Exerc. Med. 2019, 5, 1–13. [Google Scholar] [CrossRef]
- Pham, K.; Parikh, K.; Heinrich, E.C. Hypoxia and inflammation: Insights from high-altitude physiology. Front. Physiol. 2021, 12, 676782. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Okita, K.; Suga, T.; Omokawa, M.; Kadoguchi, T.; Sato, T.; Takahashi, M.; Yokota, T.; Hirabayashi, K.; Morita, N.; et al. Low-intensity exercise can increase muscle mass and strength proportionally to enhanced metabolic stress under ischemic conditions. J. Appl. Physiol. 2012, 113, 199–205. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 2009, 24, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafère, P.; Germonpré, P.; Balestra, C. Increasing oxygen partial pressures induce a distinct transcriptional response in human PBMC: A pilot study on the “normobaric oxygen paradox”. Int. J. Mol. Sci. 2021, 22, 458. [Google Scholar] [CrossRef] [PubMed]
- Germonpre, P.; Van der Eecken, P.; Van Renterghem, E.; Germonpre, F.L.; Balestra, C. First impressions: Use of the azoth systems O’Dive subclavian bubble monitor on a liveaboard dive vessel. Diving Hyperb. Med. 2020, 50, 405–412. [Google Scholar] [CrossRef]
- Balestra, C.; Germonpré, P.; Poortmans, J.R.; Marroni, A. Serum erythropoietin levels in healthy humans after a short period of normobaric and hyperbaric oxygen breathing: The “normobaric oxygen paradox”. J. Appl. Physiol. 2006, 100, 512–518. [Google Scholar] [CrossRef]
- Cimino, F.; Balestra, C.; Germonpré, P.; De Bels, D.; Tillmans, F.; Saija, A.; Speciale, A.; Virgili, F. Pulsed high oxygen induces a hypoxic-like response in human umbilical endothelial cells and in humans. J. Appl. Physiol. 2012, 113, 1684–1689. [Google Scholar] [CrossRef]
- Hadanny, A.; Efrati, S. The hyperoxic-hypoxic paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef]
- Balestra, C.; Germonpre, P. Hypoxia, a multifaceted phenomenon: The example of the “normobaric oxygen paradox”. Eur. J. Appl. Physiol. 2012, 112, 4173–4175. [Google Scholar] [CrossRef] [PubMed]
- De Bels, D.; Theunissen, S.; Devriendt, J.; Germonpre, P.; Lafere, P.; Valsamis, J.; Snoeck, T.; Meeus, P.; Balestra, C. The “normobaric oxygen paradox”: Does it increase haemoglobin? Diving Hyperb. Med. J. South. Pac. Underw. Med. Soc. 2012, 42, 67–71. [Google Scholar]
- Lafere, P.; Schubert, T.; De Bels, D.; Germonpre, P.; Balestra, C. Can the normobaric oxygen paradox (NOP) increase reticulocyte count after traumatic hip surgery? J. Clin. Anesth. 2013, 25, 129–134. [Google Scholar] [CrossRef][Green Version]
- Fratantonio, D.; Cimino, F.; Speciale, A.; Virgili, F. Need (more than) two to Tango: Multiple tools to adapt to changes in oxygen availability. Biofactors 2018, 44, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible nitric oxide synthase: Good or bad? Biomed. Pharm. 2017, 93, 370–375. [Google Scholar] [CrossRef]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchala, M.; Guzik, P. Effect of various exercise regimens on selected exercise-induced cytokines in healthy people. Int. J. Environ. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Vezzoli, A.; D’Alessandro, F.; Paganini, M.; Dellanoce, C.; Cialoni, D.; Bosco, G. Change in oxidative stress biomarkers during 30 days in saturation dive: A pilot study. Int. J. Environ. Res. Public Health 2020, 17, 7118. [Google Scholar] [CrossRef]
- Vittori, L.N.; Romasco, J.; Tarozzi, A.; Latessa, P.M. Urinary markers and chronic effect of physical exercise. Methods Mol. Biol. 2021, 2292, 193–200. [Google Scholar] [CrossRef]
- Lewthwaite, H.; Jensen, D.; Ekström, M. How to assess breathlessness in chronic obstructive pulmonary disease. Int. J. Chron Obs. Pulmon. Dis 2021, 16, 1581–1598. [Google Scholar] [CrossRef] [PubMed]
- Balestra, C.; Germonpre, P. Increasing EPO using the normobaric oxygen paradox: A ’not so simple’ task. Acta Physiol. 2011, 203, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Calzia, E.; Asfar, P.; Hauser, B.; Matejovic, M.; Ballestra, C.; Radermacher, P.; Georgieff, M. Hyperoxia may be beneficial. Crit. Care Med. 2010, 38, S559–S568. [Google Scholar] [CrossRef] [PubMed]
- De Bels, D.; Corazza, F.; Balestra, C. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011, 365, 1845; author reply 1846. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.; D’Itri, L.; De Bels, D.; Corazza, F.; Balestra, C. The “normobaric oxygen paradox”: A new tool for the anesthetist? Minerva. Anestesiol. 2014, 80, 366–372. [Google Scholar] [PubMed]
- Moasefi, N.; Fouladi, M.; Norooznezhad, A.H.; Yarani, R.; Rahmani, A.; Mansouri, K. How could perfluorocarbon affect cytokine storm and angiogenesis in coronavirus disease 2019 (COVID-19): Role of hypoxia-inducible factor 1α. Inflamm. Res. 2021, 70, 749–752. [Google Scholar] [CrossRef]
- Görlach, A.; Dimova, E.Y.; Petry, A.; Martínez-Ruiz, A.; Hernansanz-Agustín, P.; Rolo, A.P.; Palmeira, C.M.; Kietzmann, T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015, 6, 372–385. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 2010, 23, 75–93. [Google Scholar] [CrossRef]
- Bredt, D.S. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic. Res. 1999, 31, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Webber, R.J.; Sweet, R.M.; Webber, D.S. Inducible nitric oxide synthase in circulating microvesicles: Discovery, evolution, and evidence as a novel biomarker and the probable causative agent for sepsis. J. Appl. Lab. Med. 2019, 3, 698–711. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, F.; Thioub, S.; Goanvec, C.; Theunissen, S.; Feray, A.; Balestra, C.; Mansourati, J. Effect of tetrahydrobiopterin and exercise training on endothelium-dependent vasorelaxation in SHR. J. Physiol. Biochem. 2013, 69, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Reihmane, D.; Dela, F. Interleukin-6: Possible biological roles during exercise. Eur. J. Sport Sci. 2014, 14, 242–250. [Google Scholar] [CrossRef]
- Brasier, A.R. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc. Res. 2010, 86, 211–218. [Google Scholar] [CrossRef]
- Mosser, D.M.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Jarosz-Griffiths, H.H.; Holbrook, J.; Lara-Reyna, S.; McDermott, M.F. TNF receptor signalling in autoinflammatory diseases. Int. Immunol. 2019, 31, 639–648. [Google Scholar] [CrossRef]
- Falvo, J.V.; Tsytsykova, A.V.; Goldfeld, A.E. Transcriptional control of the TNF gene. Curr. Dir. Autoimmun. 2010, 11, 27–60. [Google Scholar] [CrossRef]
- Elyasi, A.; Voloshyna, I.; Ahmed, S.; Kasselman, L.J.; Behbodikhah, J.; De Leon, J.; Reiss, A.B. The role of interferon-γ in cardiovascular disease: An update. Inflamm. Res. 2020, 69, 975–988. [Google Scholar] [CrossRef]
- Deng, S.Y.; Zhang, L.M.; Ai, Y.H.; Pan, P.H.; Zhao, S.P.; Su, X.L.; Wu, D.D.; Tan, H.Y.; Zhang, L.N.; Tsung, A. Role of interferon regulatory factor-1 in lipopolysaccharide-induced mitochondrial damage and oxidative stress responses in macrophages. Int. J. Mol. Med. 2017, 40, 1261–1269. [Google Scholar] [CrossRef]
- Calles-Escandon, J.; Cunningham, J.J.; Snyder, P.; Jacob, R.; Huszar, G.; Loke, J.; Felig, P. Influence of exercise on urea, creatinine, and 3-methylhistidine excretion in normal human subjects. Am. J. Physiol. 1984, 246, E334–E338. [Google Scholar] [CrossRef]
- Baker-Fulco, C.J.; Fulco, C.S.; Kellogg, M.D.; Glickman, E.; Young, A.J. Voluntary muscle function after creatine supplementation in acute hypobaric hypoxia. Med. Sci. Sports Exerc. 2006, 38, 1418–1424. [Google Scholar] [CrossRef]
- Jeffries, O.; Patterson, S.D.; Waldron, M. The effect of severe and moderate hypoxia on exercise at a fixed level of perceived exertion. Eur. J. Appl. Physiol. 2019, 119, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Hatamoto, Y.; Sudo, M.; Kiyonaga, A.; Tanaka, H.; Higaki, Y. The effects of exercise under hypoxia on cognitive function. PLoS ONE 2013, 8, e63630. [Google Scholar] [CrossRef]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a marker for immune system activation. Curr. Drug. Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Gieseg, S.P.; Baxter-Parker, G.; Lindsay, A. Neopterin, inflammation, and oxidative stress: What could we be missing? Antioxidants 2018, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Wachter, H.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Werner, E.R. Neopterin as marker for activation of cellular immunity: Immunologic basis and clinical application. Adv. Clin. Chem. 1989, 27, 81–141. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Díaz, J.; Caballero, A.; Córdova, A. The training of strength-resistance in hypoxia: Effect on muscle hypertrophy. Biomedica 2019, 39, 212–220. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, W.S.; Chung, S.; Park, H.Y. Exercise intervention under hypoxic condition as a new therapeutic paradigm for type 2 diabetes mellitus: A narrative review. World J. Diabetes 2021, 12, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.A.; Ekblom, B. Hyperoxia for performance and training. J. Sports Sci. 2018, 36, 1515–1522. [Google Scholar] [CrossRef]
- Hailemichael, W.; Kiros, M.; Akelew, Y.; Getu, S.; Andualem, H. Neopterin: A promising candidate biomarker for severe COVID-19. J. Inflamm. Res. 2021, 14, 245–251. [Google Scholar] [CrossRef]
- Natarelli, L.; Parca, L.; Mazza, T.; Weber, C.; Virgili, F.; Fratantonio, D. MicroRNAs and long non-coding RNAs as potential candidates to target specific motifs of SARS-CoV-2. Noncoding RNA 2021, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Green, H.J.; Fraser, I.G. Differential effects of exercise intensity on serum uric acid concentration. Med. Sci. Sports Exerc. 1988, 20, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.B.; Warburton, D.E.; Hodges, A.N.; Lyster, D.M.; McKenzie, D.C. Cardiovascular and splenic responses to exercise in humans. J. Appl. Physiol. 2003, 94, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. (1985) 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Schagatay, E.; Lunde, A.; Nilsson, S.; Palm, O.; Lodin-Sundstrom, A. Spleen contraction elevates hemoglobin concentration at high altitude during rest and exercise. Eur. J. Appl. Physiol. 2020, 120, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Pogliaghi, S.; Bellotti, C.; Paterson, D.H. “Tailored” submaximal step test for VO2max prediction in healthy older adults. J. Aging Phys. Act. 2014, 22, 261–268. [Google Scholar] [CrossRef]
- Meyer, T.; Lucía, A.; Earnest, C.P.; Kindermann, W. A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters--theory and application. Int. J. Sports Med. 2005, 26 (Suppl. S1), S38–S48. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, J.G.; Morán-Navarro, R.; Ortega, J.F.; Fernández-Elías, V.E.; Mora-Rodriguez, R. Validity and reliability of ventilatory and blood lactate thresholds in well-trained cyclists. PLoS ONE 2016, 11, e0163389. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxid. Med. Cell Longev. 2012, 2012, 973927. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. A quantitative method to monitor reactive oxygen species production by electron paramagnetic resonance in physiological and pathological conditions. Oxid. Med. Cell Longev. 2014, 2014, 306179. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative stress assessment in response to ultraendurance exercise: Thiols redox status and ROS production according to duration of a competitive race. Oxid. Med. Cell Longev. 2016, 2016, 6439037. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef]
- Vezzoli, A.; Mrakic-Sposta, S.; Montorsi, M.; Porcelli, S.; Vago, P.; Cereda, F.; Longo, S.; Maggio, M.; Narici, M. Moderate intensity resistive training reduces oxidative stress and improves muscle mass and function in older individuals. Antioxidants 2019, 8, 431. [Google Scholar] [CrossRef]
- Zang, S.; Tian, S.; Jiang, J.; Han, D.; Yu, X.; Wang, K.; Li, D.; Lu, D.; Yu, A.; Zhang, Z. Determination of antioxidant capacity of diverse fruits by electron spin resonance (ESR) and UV-vis spectrometries. Food Chem. 2017, 221, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Moretti, S.; Pratali, L.; Giardini, G.; Tacchini, P.; Dellanoce, C.; Tonacci, A.; Mastorci, F.; Borghini, A.; et al. Effects of mountain ultra-marathon running on ROS production and oxidative damage by micro-invasive analytic techniques. PLoS ONE 2015, 10, e0141780. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestra, C.; Lambrechts, K.; Mrakic-Sposta, S.; Vezzoli, A.; Levenez, M.; Germonpré, P.; Virgili, F.; Bosco, G.; Lafère, P. Hypoxic and Hyperoxic Breathing as a Complement to Low-Intensity Physical Exercise Programs: A Proof-of-Principle Study. Int. J. Mol. Sci. 2021, 22, 9600. https://doi.org/10.3390/ijms22179600
Balestra C, Lambrechts K, Mrakic-Sposta S, Vezzoli A, Levenez M, Germonpré P, Virgili F, Bosco G, Lafère P. Hypoxic and Hyperoxic Breathing as a Complement to Low-Intensity Physical Exercise Programs: A Proof-of-Principle Study. International Journal of Molecular Sciences. 2021; 22(17):9600. https://doi.org/10.3390/ijms22179600
Chicago/Turabian StyleBalestra, Costantino, Kate Lambrechts, Simona Mrakic-Sposta, Alessandra Vezzoli, Morgan Levenez, Peter Germonpré, Fabio Virgili, Gerardo Bosco, and Pierre Lafère. 2021. "Hypoxic and Hyperoxic Breathing as a Complement to Low-Intensity Physical Exercise Programs: A Proof-of-Principle Study" International Journal of Molecular Sciences 22, no. 17: 9600. https://doi.org/10.3390/ijms22179600
APA StyleBalestra, C., Lambrechts, K., Mrakic-Sposta, S., Vezzoli, A., Levenez, M., Germonpré, P., Virgili, F., Bosco, G., & Lafère, P. (2021). Hypoxic and Hyperoxic Breathing as a Complement to Low-Intensity Physical Exercise Programs: A Proof-of-Principle Study. International Journal of Molecular Sciences, 22(17), 9600. https://doi.org/10.3390/ijms22179600









