Cilia, Centrosomes and Skeletal Muscle
Abstract
1. Introduction
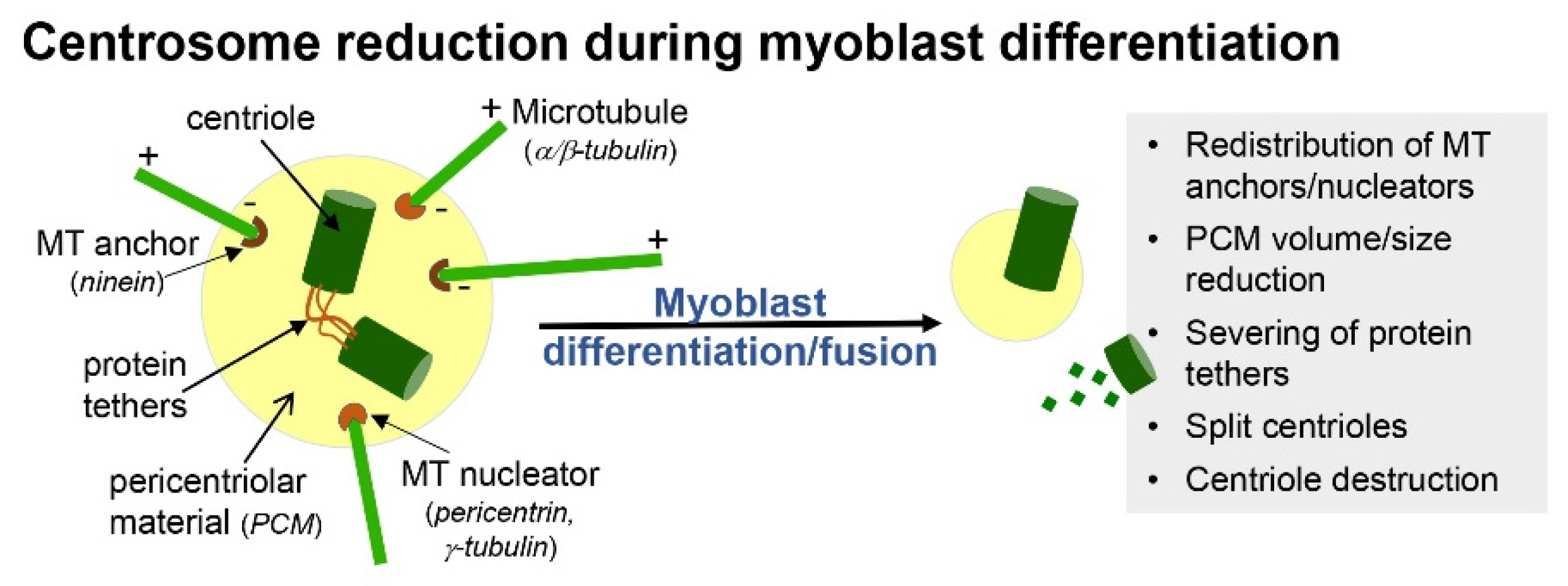
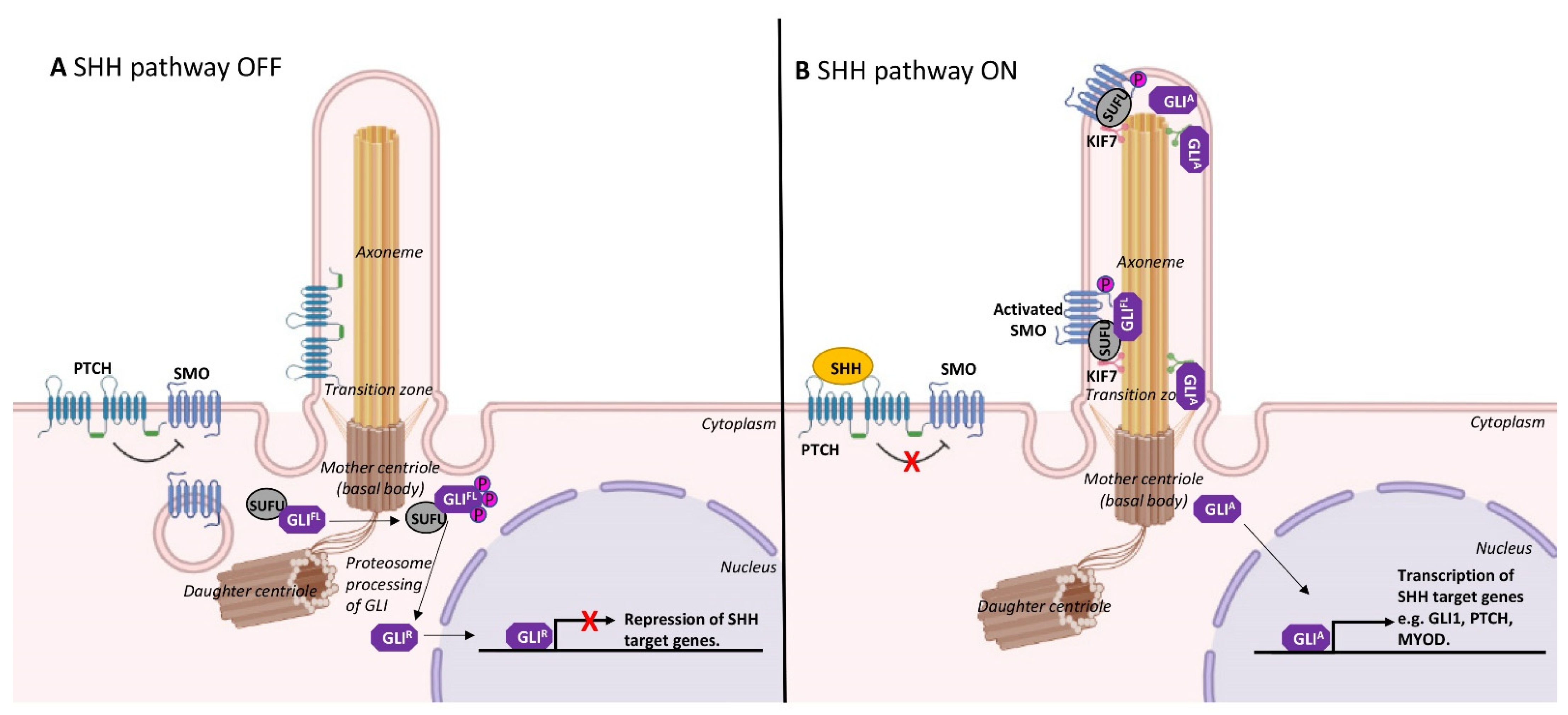
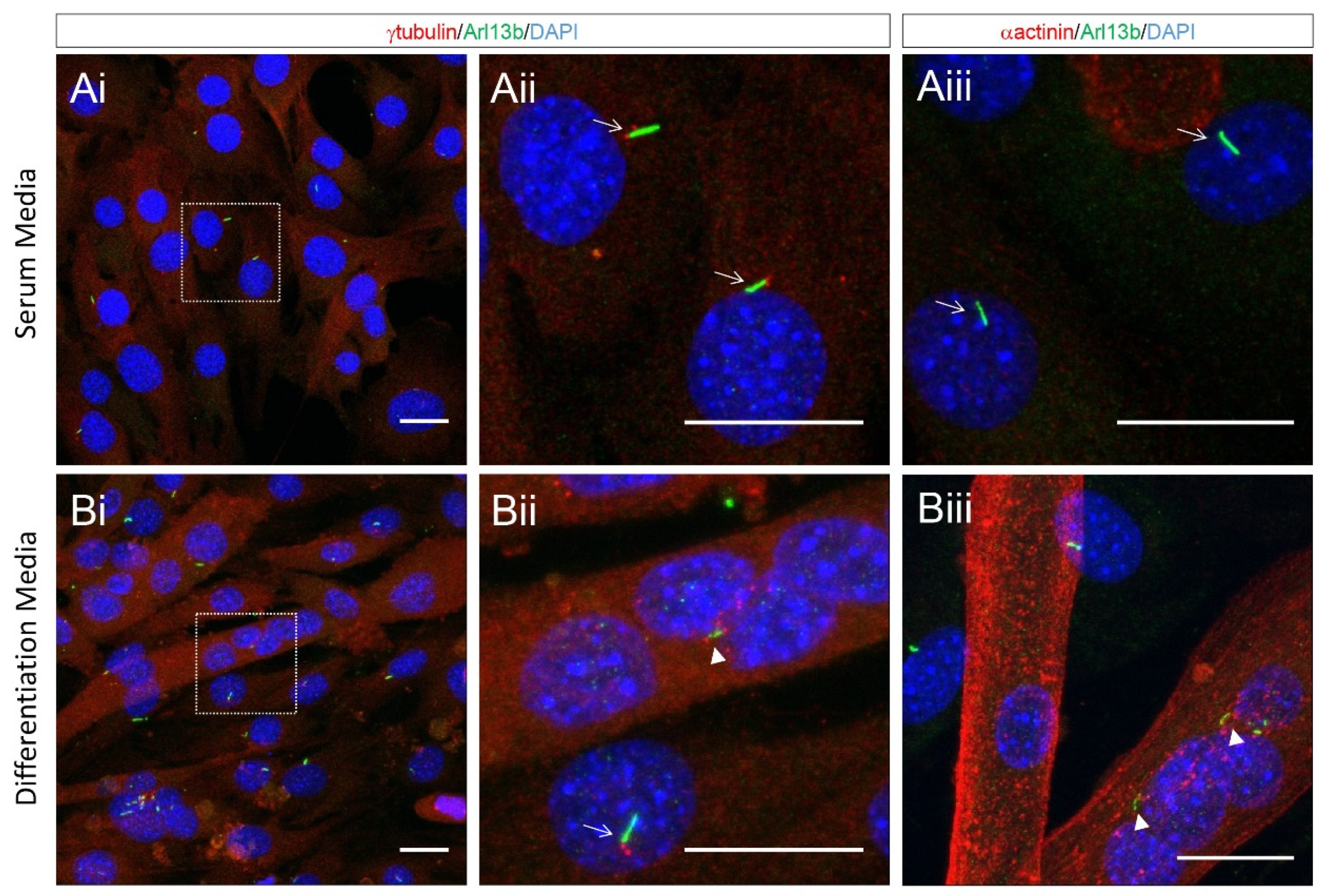
2. Formation and Resorption of Cilia during Skeletal Myogenesis
3. Cilia and Cell Cycle Regulation in Myoblasts
4. Cilia Maintenance and Satellite Cell Fate
5. Consequences of Ciliary Dysfunction in Satellite Cells
6. Cilia and Myoblast Fusion
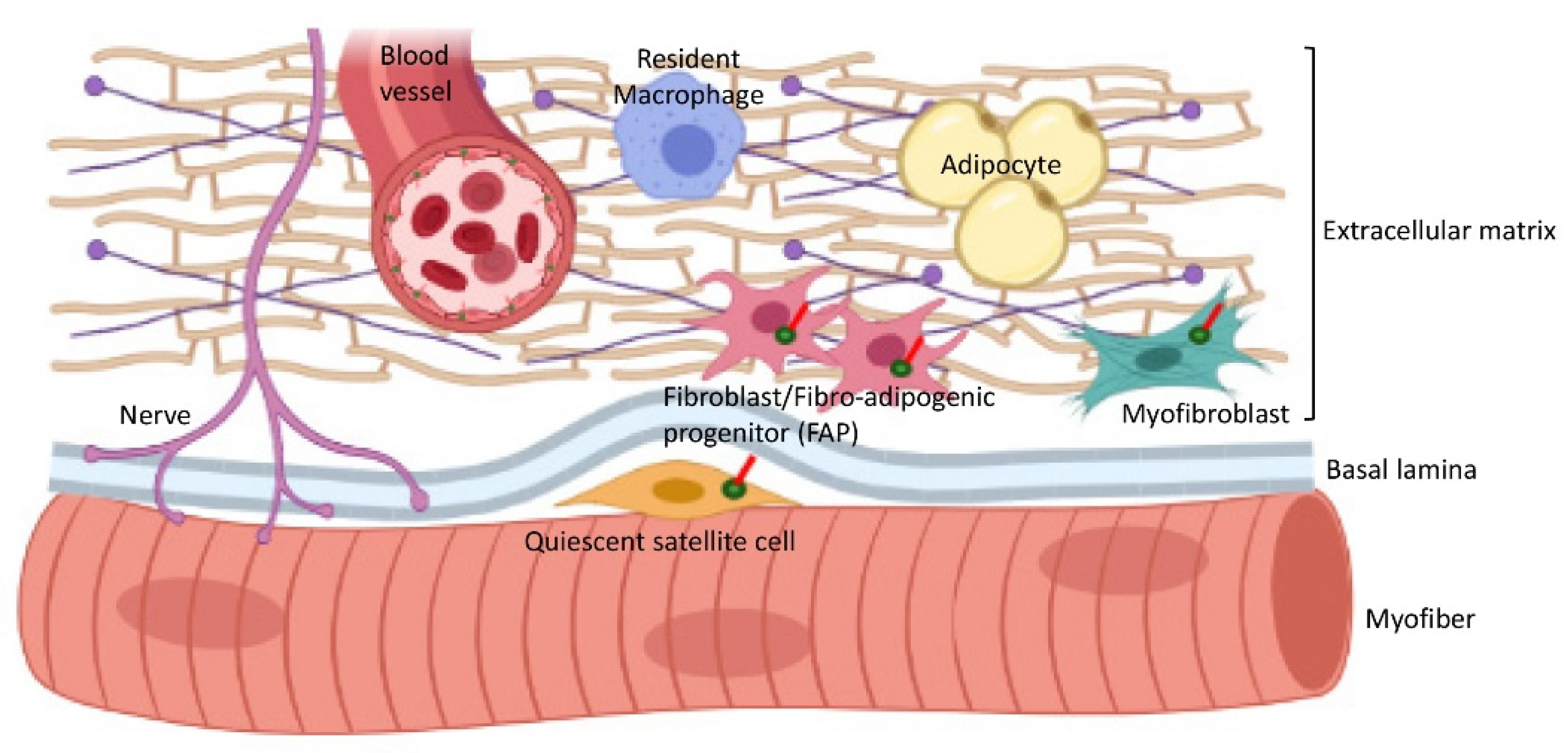
7. Cilia Interactions with the Extracellular Matrix (ECM) in Skeletal Muscle
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CEP | Centrosomal protein |
| DMD | Duchenne muscular dystrophy |
| ECM | Extracellular matrix |
| FAP | Fibro-adipogenic Progenitor |
| GLI2/3 | Gli family zinc finger 2/3 |
| IFT | Intraflagellar transport |
| MRF | Muscle regulatory family |
| PAX3/7 | Paired box 3/7 |
| RMS | Rhabdomyosarcoma |
| SHH | Sonic hedgehog |
| SMO | Smoothened |
| TGF-β | Transforming Growth Factor-beta |
References
- Hoey, D.A.; Downs, M.E.; Jacobs, C.R. The mechanics of the primary cilium: An intricate structure with complex function. J. Biomech. 2012, 45, 17–26. [Google Scholar] [CrossRef]
- Nigg, E.A.; Raff, J.W. Centrioles, centrosomes, and cilia in health and disease. Cell 2009, 139, 663–678. [Google Scholar] [CrossRef]
- Kim, S.; Dynlacht, B.D. Assembling a primary cilium. Curr. Opin. Cell Biol. 2013, 25, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Ovalle, W.K. Fine structure of the avian muscle spindle capsule. Cell Tissue Res. 1976, 166, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Kopinke, D.; Norris, A.M.; Mukhopadhyay, S. Developmental and regenerative paradigms of cilia regulated hedgehog signaling. Semin. Cell Dev. Biol. 2021, 110, 89–103. [Google Scholar] [CrossRef]
- Lyu, R.; Zhou, J. The multifaceted roles of primary cilia in the regulation of stem cell properties and functions. J. Cell Physiol. 2017, 232, 935–938. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Gurevich, D.B.; Nguyen, P.D.; Siegel, A.L.; Ehrlich, O.V.; Sonntag, C.; Phan, J.M.; Berger, S.; Ratnayake, D.; Hersey, L.; Berger, J.; et al. Asymmetric division of clonal muscle stem cells coordinates muscle regeneration in vivo. Science 2016, 353, aad9969. [Google Scholar] [CrossRef]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Marican, N.H.J.; Cruz-Migoni, S.B.; Borycki, A.G. Asymmetric distribution of primary cilia allocates satellite cells for self-renewal. Stem Cell Rep. 2016, 6, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Kopinke, D.; Roberson, E.C.; Reiter, J.F. Ciliary hedgehog signaling restricts injury-induced adipogenesis. Cell 2017, 170, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, R.J. Occurrence of centrioles during skeletal and cardiac myogenesis. J. Cell Biol. 1971, 49, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Roelofs, R.I.; Engel, W.K. Ultrastructural development of explanted human skeletal muscle in tissue culture. J. Neuropathol. Exp. Neurol. 1972, 31, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.A.; Grounds, M.D.; Mitchell, C.A.; Papadimitriou, J.M. Fusion between myogenic cells in vivo: An ultrastructural study in regenerating murine skeletal muscle. J. Struct. Biol. 1990, 105, 170–182. [Google Scholar] [CrossRef]
- Fu, W.; Asp, P.; Canter, B.; Dynlacht, B.D. Primary cilia control hedgehog signaling during muscle differentiation and are deregulated in rhabdomyosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, 9151–9156. [Google Scholar] [CrossRef] [PubMed]
- Breslow, D.K.; Holland, A.J. Mechanism and regulation of centriole and cilium biogenesis. Ann. Rev. Biochem. 2019, 88, 691–724. [Google Scholar] [CrossRef]
- Plotnikova, O.V.; Nikonova, A.S.; Loskutov, Y.V.; Kozyulina, P.Y.; Pugacheva, E.N.; Golemis, E.A. Calmodulin activation of Aurora-A kinase (AURKA) is required during ciliary disassembly and in mitosis. Mol. Biol. Cell 2012, 23, 2658–2670. [Google Scholar] [CrossRef]
- Bugnard, E.; Zaal, K.J.; Ralston, E. Reorganization of microtubule nucleation during muscle differentiation. Cell Motil. Cytoskelet. 2005, 60, 1–13. [Google Scholar] [CrossRef]
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.B.; Christensen, S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef]
- Quarmby, L.M.; Parker, J.D. Cilia and the cell cycle? J. Cell Biol. 2005, 169, 707–710. [Google Scholar] [CrossRef]
- Robert, A.; Margall-Ducos, G.; Guidotti, J.E.; Brégerie, O.; Celati, C.; Bréchot, C.; Desdouets, C. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J. Cell Sci. 2007, 120, 628–637. [Google Scholar] [CrossRef]
- Liu, H.; Kiseleva, A.A.; Golemis, E.A. Ciliary signalling in cancer. Nat. Rev. Cancer 2018, 18, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Plotnikova, O.V.; Golemis, E.A.; Pugacheva, E.N. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008, 68, 2058–2061. [Google Scholar] [CrossRef] [PubMed]
- Plotnikova, O.V.; Pugacheva, E.N.; Golemis, E.A. Primary cilia and the cell cycle. Methods Cell Biol. 2009, 94, 137–160. [Google Scholar] [CrossRef] [PubMed]
- Phua, S.C.; Chiba, S.; Suzuki, M.; Su, E.; Roberson, E.C.; Pusapati, G.V.; Pusapati, G.V.; Setou, M.; Rohatgi, R.; Reiter, J.F.; et al. Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell 2017, 168, 264–279. [Google Scholar] [CrossRef]
- Mirvis, M.; Siemers, K.A.; Nelson, W.J.; Stearns, T.P. Primary cilium loss in mammalian cells occurs predominantly by whole-cilium shedding. PLoS Biol. 2019, 17, e3000381. [Google Scholar] [CrossRef]
- Goto, H.; Inoko, A.; Inagaki, M. Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cell Mol. Life Sci. 2013, 70, 3893–3905. [Google Scholar] [CrossRef]
- Pugacheva, E.N.; Jablonski, S.A.; Hartman, T.R.; Henske, E.P.; Golemis, E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 2007, 129, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, N.; Ghosh, A.; Gala, H.; Aloysius, A.; Vyas, N.; Dhawan, J. The primary cilium dampens proliferative signaling and represses a G2/M transcriptional network in quiescent myoblasts. BMC Mol. Cell Biol. 2020, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, N.; Lypovetska, K.; Moratal, C.; Giorgetti-Peraldi, S.; Dechesne, C.A.; Dani, C.; Peraldi, P. The primary cilium is necessary for the differentiation and the maintenance of human adipose progenitors into myofibroblasts. Sci. Rep. 2017, 7, 15248. [Google Scholar] [CrossRef]
- Borycki, A.G.; Brunk, B.; Tajbakhsh, S.; Buckingham, M.; Chiang, C.; Emerson, C.P., Jr. Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development 1999, 126, 4053–4063. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, R.; Milenkovic, L.; Scott, M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science 2007, 317, 372–376. [Google Scholar] [CrossRef]
- Buckingham, M.; Rigby, P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Koleva, M.; Kappler, R.; Vogler, M.; Herwig, A.; Fulda, S.; Hahn, H. Pleiotropic effects of sonic hedgehog on muscle satellite cells. Cell Mol. Life Sci. 2005, 62, 1863–1870. [Google Scholar] [CrossRef]
- Flynt, A.S.; Li, N.; Thatcher, E.J.; Solnica-Krezel, L.; Patton, J.G. Zebrafish miR-214 modulates Hedgehog signaling to specify muscle cell fate. Nat. Genet. 2007, 39, 259–263. [Google Scholar] [CrossRef]
- Li, X.; Blagden, C.S.; Bildsoe, H.; Bonnin, M.A.; Duprez, D.; Hughes, S.M. Hedgehog can drive terminal differentiation of amniote slow skeletal muscle. BMC Dev. Biol. 2004, 4, 9. [Google Scholar] [CrossRef]
- Anderson, C.T.; Stearns, T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr. Biol. 2009, 19, 1498–1502. [Google Scholar] [CrossRef]
- Conboy, I.M.; Rando, T.A. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell. 2002, 3, 397–409. [Google Scholar] [CrossRef]
- Troy, A.; Cadwallader, A.B.; Fedorov, Y.; Tyner, K.; Tanaka, K.K.; Olwin, B.B. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38alpha/beta MAPK. Cell Stem Cell 2012, 11, 541–553. [Google Scholar] [CrossRef]
- Paridaen, J.T.; Wilsch-Brauninger, M.; Huttner, W.B. Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell 2013, 155, 333–344. [Google Scholar] [CrossRef]
- Anderson, C.; Williams, V.C.; Moyon, B.; Daubas, P.; Tajbakhsh, S.; Buckingham, M.E.; Shiroishi, T.; Hughes, S.M.; Borycki, A.-G. Sonic hedgehog acts cell-autonomously on muscle precursor cells to generate limb muscle diversity. Genes Dev. 2012, 26, 2103–2117. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Migoni, S.B.; Imran, K.M.; Wahid, A.; Rahman, O.; Briscoe, J.; Borycki, A.-G. A switch in cilia-mediated Hedgehog signaling controls muscle stem cell quiescence and cell cycle progression. bioRxiv 2019. [Google Scholar] [CrossRef]
- Brun, C.; Sincennes, M.-C.; Lin, A.Y.T.; Hall, D.; Jarassier, W.; Feige, P.; Ritso, M.; Le Grand, F.; Rudnicki, M.A. GLI3 processing by the primary cilium regulates muscle stem cell entry into GAlert. bioRxiv 2020. [Google Scholar] [CrossRef]
- Voronova, A.; Coyne, E.; Al Madhoun, A.; Fair, J.V.; Bosiljcic, N.; St-Louis, C.; Li, G.; Thurig, S.; Wallace, V.A.; Wiper-Bergeron, N.; et al. Hedgehog signaling regulates MyoD expression and activity. J. Biol. Chem. 2013, 288, 4389–4404. [Google Scholar] [CrossRef] [PubMed]
- Palla, A.R.; Hilgendorf, K.I.; Yang, A.V.; Kerr, J.P.; Hinken, A.C.; Demeter, J.; Kraft, P.; Mooney, N.A.; Yucel, N.; Jackson, P.K.; et al. Ciliation of muscle stem cells is critical to maintain regenerative capacity and is lost during aging. bioRxiv 2020. [Google Scholar] [CrossRef]
- Grounds, M.D. Therapies for sarcopenia and regeneration of old skeletal muscles: More a case of old tissue architecture than old stem cells. Bioarchitecture 2014, 4, 81–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Novak, J.S.; Mazala, D.A.G.; Nearing, M.; Hindupur, R.; Uapinyoying, P.; Habib, N.F.; Dickson, T.; Ioffe, O.B.; Harris, B.T.; Fidelia-Lambert, M.N.; et al. Human muscle stem cells are refractory to aging. Aging Cell 2021, 20, e13411. [Google Scholar] [CrossRef] [PubMed]
- Contreras, O.; Rossi, F.M.V.; Theret, M. Origins, potency, and heterogeneity of skeletal muscle fibro-adipogenic progenitors-time for new definitions. Skelet. Muscle 2021, 11, 16. [Google Scholar] [CrossRef]
- Joe, A.W.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M.V. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef]
- Lemos, D.R.; Babaeijandaghi, F.; Low, M.; Chang, C.K.; Lee, S.T.; Fiore, D.; Zhang, R.-H.; Natarajan, A.; Nedospasov, S.A.; Rossi, F.M.V. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat. Med. 2015, 21, 786–794. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.J.; Du, H.; Wu, J.; Jansen, D.A.; Jordan, K.L.; Xu, N.; Sieck, G.; Qian, Q. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res. 2008, 31, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Mohieldin, A.M.; Pala, R.; Beuttler, R.; Moresco, J.J.; Yates, J.R., III; Nauli, S.M. Ciliary extracellular vesicles are distinct from the cytosolic extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12086. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Takeda, S. Hedgehog signaling regulates myelination in the peripheral nervous system through primary cilia. Differentiation 2012, 83, S78–S85. [Google Scholar] [CrossRef] [PubMed]
- Mc Fie, M.; Koneva, L.; Collins, I.; Coveney, C.R.; Clube, A.M.; Chanalaris, A.; Vincent, T.L.; Bezbradica, J.S.; Sansom, S.N.; Wann, A.K.T. Ciliary proteins specify the cell inflammatory response by tuning NFkappaB signalling, independently of primary cilia. J. Cell Sci. 2020, 133, jcs239871. [Google Scholar] [CrossRef]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011, 124, 3654–3664. [Google Scholar] [CrossRef]
- Krishnan, V.S.; Thanigaiarasu, L.P.; White, R.; Crew, R.; Larcher, T.; Le Guiner, C.; Grounds, M.D. Dystrophic Dmd(mdx) rats show early neuronal changes (increased S100beta and Tau5) at 8 months, supporting severe dystropathology in this rodent model of Duchenne muscular dystrophy. Mol. Cell Neurosci. 2020, 108, 103549. [Google Scholar] [CrossRef]
- Tytell, M.; Lasek, R.J.; Gainer, H. Axonal maintenance, glia, exosomes, and heat shock proteins. F1000Research 2016, 5, F1000. [Google Scholar] [CrossRef] [PubMed]
- Drummond, C.J.; Hanna, J.A.; Garcia, M.R.; Devine, D.J.; Heyrana, A.J.; Finkelstein, D.; Rehg, J.E.; Hatley, M.E. Hedgehog pathway drives fusion-negative rhabdomyosarcoma initiated from non-myogenic endothelial progenitors. Cancer Cell 2018, 33, 108–124. [Google Scholar] [CrossRef]
- Kashi, V.P.; Hatley, M.E.; Galindo, R.L. Probing for a deeper understanding of rhabdomyosarcoma: Insights from complementary model systems. Nat. Rev. Cancer 2015, 15, 426–439. [Google Scholar] [CrossRef]
- Abmayr, S.M.; Pavlath, G.K. Myoblast fusion: Lessons from flies and mice. Development 2012, 139, 641–656. [Google Scholar] [CrossRef]
- Grounds, M.D. The proliferation and fusion of myoblasts in vivo. Adv. Exp. Med. Biol. 1990, 280, 101–104. [Google Scholar] [CrossRef]
- Sampath, S.C.; Sampath, S.C.; Millay, D.P. Myoblast fusion confusion: The resolution begins. Skelet. Muscle 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Ramirez-Martinez, A.; Li, H.; Cannavino, J.; McAnally, J.R.; Shelton, J.M.; Sánchez-Ortiz, E.; Bassel-Duby, R.; Olson, E.N. Control of muscle formation by the fusogenic micropeptide myomixer. Science 2017, 356, 323–327. [Google Scholar] [CrossRef]
- Quinn, M.E.; Goh, Q.; Kurosaka, M.; Gamage, D.G.; Petrany, M.J.; Prasad, V.; Millay, D.P. Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 2017, 8, 15665. [Google Scholar] [CrossRef] [PubMed]
- Hindi, S.M.; Tajrishi, M.M.; Kumar, A. Signaling mechanisms in mammalian myoblast fusion. Sci. Signal. 2013, 6, re2. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Berendse, M.; Lloyd, D.G.; Schevzov, G.; Grounds, M.D. A novel role for non-muscle gamma-actin in skeletal muscle sarcomere assembly. Exp. Cell Res. 2004, 297, 82–96. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.E.; Heynen-Genel, S.; Suyama, E.; Ono, K.; Lee, K.; Ideker, T.; Aza-Blanc, P.; Gleeson, J.G. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 2010, 464, 1048–1051. [Google Scholar] [CrossRef]
- Fenix, A.M.; Neininger, A.C.; Taneja, N.; Hyde, K.; Visetsouk, M.R.; Garde, R.J.; Liu, B.; Nixon, B.R.; Manalo, A.E.; Becker, J.R.; et al. Muscle-specific stress fibers give rise to sarcomeres in cardiomyocytes. Elife. 2018, 7, e42144. [Google Scholar] [CrossRef] [PubMed]
- Hoerner, C.; Stearns, T. Remembrance of cilia past. Cell 2013, 155, 271–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grounds, M.D. Complexity of extracellular matrix and skeletal muscle regeneration. In Skeletal Muscle Repair and Regeneration; Schiaffino, S., Partridge, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 269–301. [Google Scholar]
- Collins, I.; Wann, A.K.T. Regulation of the extracellular matrix by ciliary machinery. Cells 2020, 9, 278. [Google Scholar] [CrossRef]
- Seeger-Nukpezah, T.; Golemis, E.A. The extracellular matrix and ciliary signaling. Curr. Opin. Cell Biol. 2012, 24, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; DeRouen, M.C.; Chen, C.H.; Nguyen, M.; Nguyen, N.T.; Ido, H.; Harada, K.; Sekiguchi, K.; Morgan, B.A.; Miner, J.H.; et al. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 2008, 22, 2111–2124. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, W.; Liu, X.; Otkur, W.; Hayashi, T.; Yamato, M.; Fujisaki, H.; Hattori, S.; Tashiro, S.-I.; Ikejima, T. Type I collagen promotes primary cilia growth through down-regulating HDAC6-mediated autophagy in confluent mouse embryo fibroblast 3T3-L1 cells. J. Biosci. Bioeng. 2018, 125, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Serra, R.A.; Yang, S. Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Ann. N. Y. Acad. Sci. 2015, 1335, 78–99. [Google Scholar] [CrossRef]
- McDermott, K.M.; Liu, B.Y.; Tlsty, T.D.; Pazour, G.J. Primary cilia regulate branching morphogenesis during mammary gland development. Curr. Biol. 2010, 20, 731–737. [Google Scholar] [CrossRef]
- Minj, A.; Mondal, S.; Tiwari, A.K.; Sharma, B.; Varshney, V.P. Molecular characterization of follicle stimulating hormone receptor (FSHR) gene in the Indian river buffalo (Bubalus bubalis). Gen. Comp. Endocrinol. 2008, 158, 147–153. [Google Scholar] [CrossRef]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013, 4, 1964. [Google Scholar] [CrossRef] [PubMed]
- Lukjanenko, L.; Jung, M.J.; Hegde, N.; Perruisseau-Carrier, C.; Migliavacca, E.; Rozo, M.; Karaz, S.; Jacot, G.; Schmidt, M.; Li, L. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat. Med. 2016, 22, 897–905. [Google Scholar] [CrossRef]
- Grounds, M.D. The need to more precisely define aspects of skeletal muscle regeneration. Int. J. Biochem. Cell Biol. 2014, 56, 56–65. [Google Scholar] [CrossRef]
- Stearns-Reider, K.M.; D’Amore, A.; Beezhold, K.; Rothrauff, B.; Cavalli, L.; Wagner, W.R.; Vorp, D.A.; Tsamis, A.; Shinde, S.; Zhang, C.; et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 2017, 16, 518–528. [Google Scholar] [CrossRef]
- Kobayashi, T.; Soegiarto, D.W.; Yang, Y.; Lanske, B.; Schipani, E.; McMahon, A.P.; Kronenberg, H.M. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J. Clin. Investig. 2005, 115, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, S.R.; Haycraft, C.J.; Jensen, C.G.; Yoder, B.K.; Poole, C.A. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol. 2007, 26, 234–246. [Google Scholar] [CrossRef]
- Song, B.; Haycraft, C.J.; Seo, H.S.; Yoder, B.K.; Serra, R. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev. Biol. 2007, 305, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Niu, B.; Cheah, K.S.E.; Alman, B. Unique and overlapping GLI1 and GLI2 transcriptional targets in neoplastic chondrocytes. PLoS ONE 2019, 14, e0211333. [Google Scholar] [CrossRef] [PubMed]
- Monnich, M.; Borgeskov, L.; Breslin, L.; Jakobsen, L.; Rogowski, M.; Doganli, C.; Schrøder, J.M.; Mogensen, J.B.; Blinkenkjær, L.; Harder, L.M.; et al. CEP128 localizes to the subdistal appendages of the mother centriole and regulates TGF-beta/BMP signaling at the primary cilium. Cell Rep. 2018, 22, 2584–2592. [Google Scholar] [CrossRef]
- Smith, L.R.; Kok, H.J.; Zhang, B.; Chung, D.; Spradlin, R.A.; Rakoczy, K.D.; Lei, H.; Boesze-Battaglia, K.; Barton, E.R. Matrix metalloproteinase 13 from satellite cells is required for efficient muscle growth and regeneration. Cell Physiol. Biochem. 2020, 54, 333–353. [Google Scholar] [CrossRef]
- Walker, G.A.; Guerrero, I.A.; Leinwand, L.A. Myofibroblasts: Molecular crossdressers. Curr. Top Dev. Biol. 2001, 51, 91–107. [Google Scholar] [CrossRef]
- D’Urso, M.; Kurniawan, N.A. Mechanical and physical regulation of fibroblast-myofibroblast transition: From cellular mechanoresponse to tissue pathology. Front. Bioeng. Biotechnol. 2020, 8, 609653. [Google Scholar] [CrossRef]
- Fry, C.S.; Kirby, T.J.; Kosmac, K.; McCarthy, J.J.; Peterson, C.A. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem. Cell 2017, 20, 56–69. [Google Scholar] [CrossRef]
- Mangos, S.; Lam, P.Y.; Zhao, A.; Liu, Y.; Mudumana, S.; Vasilyev, A.; Liu, A.; Drummond, I.A. The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis. Model Mech. 2010, 3, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Song, C.J.; Zimmerman, K.A.; Henke, S.J.; Yoder, B.K. Inflammation and fibrosis in polycystic kidney disease. Results Probl. Cell Differ. 2017, 60, 323–344. [Google Scholar] [CrossRef]
- Waring, W.W. Cilia and cystic fibrosis. N. Engl. J. Med. 1973, 288, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Teves, M.E.; Strauss, J.F., III; Sapao, P.; Shi, B.; Varga, J. The primary cilium: Emerging role as a key player in fibrosis. Curr. Rheumatol. Rep. 2019, 21, 29. [Google Scholar] [CrossRef]
- Bolanos, A.L.; Milla, C.M.; Lira, J.C.; Ramirez, R.; Checa, M.; Barrera, L.; García-Alvarez, J.; Carbajal, V.; Becerril, C.; Gaxiola, M.; et al. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L978–L990. [Google Scholar] [CrossRef]
- Villalobos, E.; Criollo, A.; Schiattarella, G.G.; Altamirano, F.; French, K.M.; May, H.I.; Jiang, N.; Nguyen, N.U.N.; Romero, D.; Roa, J.C.; et al. Fibroblast primary cilia are required for cardiac fibrosis. Circulation 2019, 139, 2342–2357. [Google Scholar] [CrossRef] [PubMed]

| Cell Type | Targeting | Cellular Phenotype and Signalling Consequences | Reference |
|---|---|---|---|
| C2C12 and primary murine myoblasts | miRNA silencing of CEP290, IFT80 or IFT88 Ciliobrevin D | Increased proliferation. Reduced differentiation and MRF (MyoD, Myf5) expression. Reduced myoblast fusion and myogenesis. Inhibited Hedgehog signalling. | [15] |
| C2C12 myoblasts | siRNA silencing of IFT88 | Altered quiescence. Reduced self-renewal potential. Enhanced progression to G2/M. Enhanced growth factor signalling to mTOR. | [29] |
| Pax7+ve satellite cells within isolated myofibers | Nocodazole or Taxol. Forchlorfenuron (septin inhibitor) | Reduced self-renewal. Increased myogenin expression. | [10] |
| Fibro–adipogenic progenitors (in vivo) | Pdgfra-CreERT deletion of IFT88. | Decreased differentiation to adipocytes and reduced adipogenesis. Increased myofiber size in Dmdmdx and following injury. Derepressed Hh target genes (Gli1 and Ptch1). Enhanced TIMP3 expression. | [5] |
| Adipose progenitors (in vitro) | siRNA silencing of Kif3A. Ciliobrevin D | Reduced differentiation to myofibroblasts. Inhibited TGFβ signalling. | [30] |
| Myofibroblasts (in vitro) | Ciliobrevin D | Decrease in myofibroblast phenotype. | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, D.C.H.; Ho, U.Y.; Grounds, M.D. Cilia, Centrosomes and Skeletal Muscle. Int. J. Mol. Sci. 2021, 22, 9605. https://doi.org/10.3390/ijms22179605
Ng DCH, Ho UY, Grounds MD. Cilia, Centrosomes and Skeletal Muscle. International Journal of Molecular Sciences. 2021; 22(17):9605. https://doi.org/10.3390/ijms22179605
Chicago/Turabian StyleNg, Dominic C. H., Uda Y. Ho, and Miranda D. Grounds. 2021. "Cilia, Centrosomes and Skeletal Muscle" International Journal of Molecular Sciences 22, no. 17: 9605. https://doi.org/10.3390/ijms22179605
APA StyleNg, D. C. H., Ho, U. Y., & Grounds, M. D. (2021). Cilia, Centrosomes and Skeletal Muscle. International Journal of Molecular Sciences, 22(17), 9605. https://doi.org/10.3390/ijms22179605





