Enhanced Thermostability of D-Psicose 3-Epimerase from Clostridium bolteae through Rational Design and Engineering of New Disulfide Bridges
Abstract
:1. Introduction
2. Results
2.1. Computational Design and Screening of Disulfide Bonds
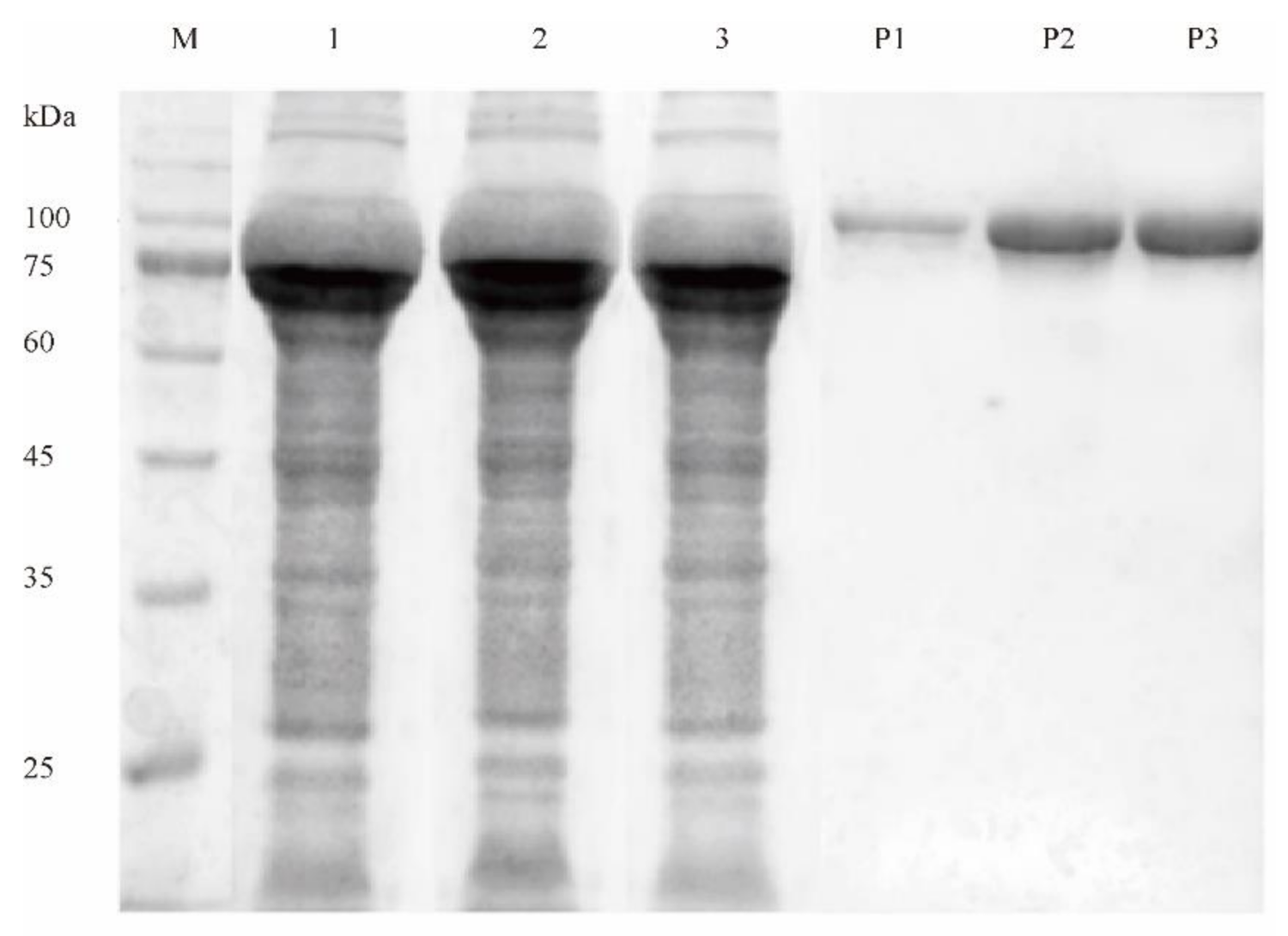
2.2. Determination of Disulfide Bridge Content in Purified Mutant Enzymes
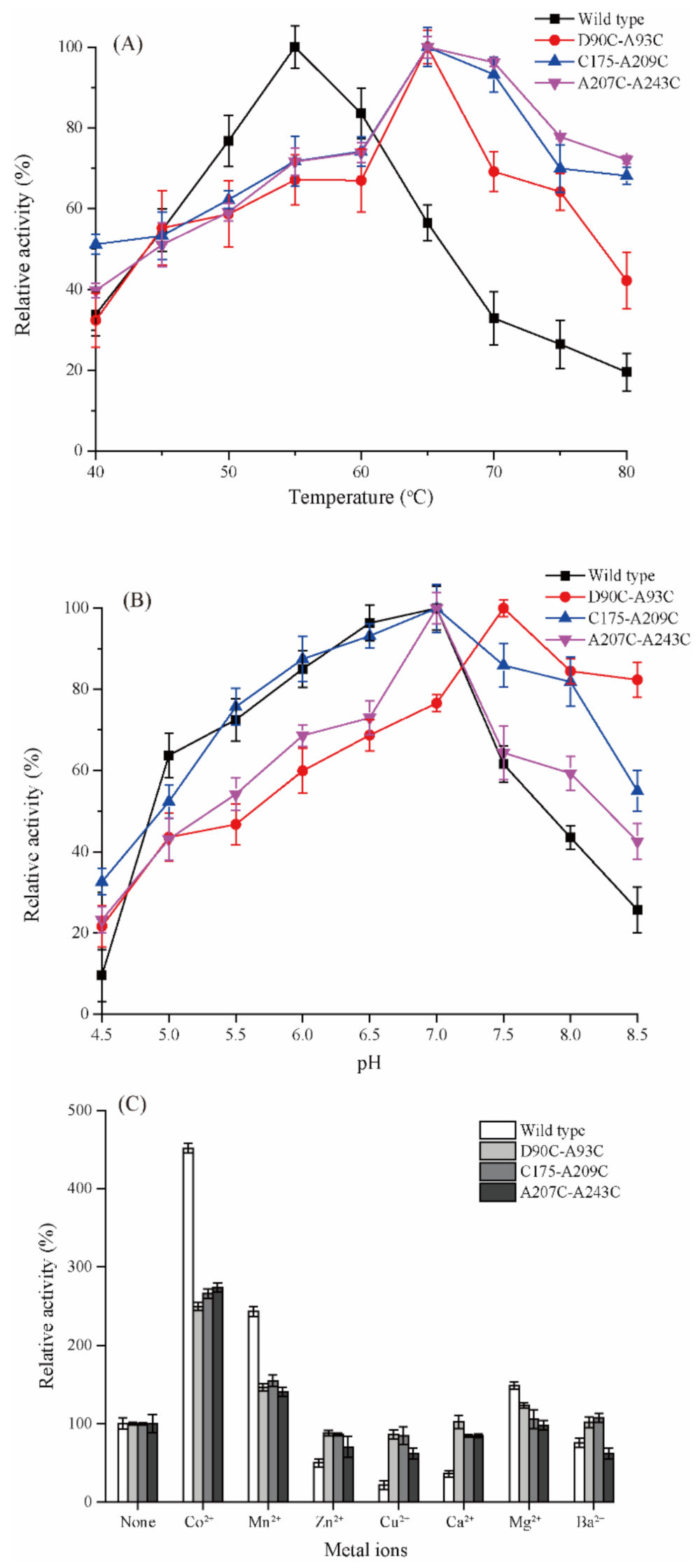
2.3. Enzymatic Characterizations of Wild-Type Cb-DPEase and the Mutants
2.4. Effect of Introduced Disulfide Bonds on Thermostability
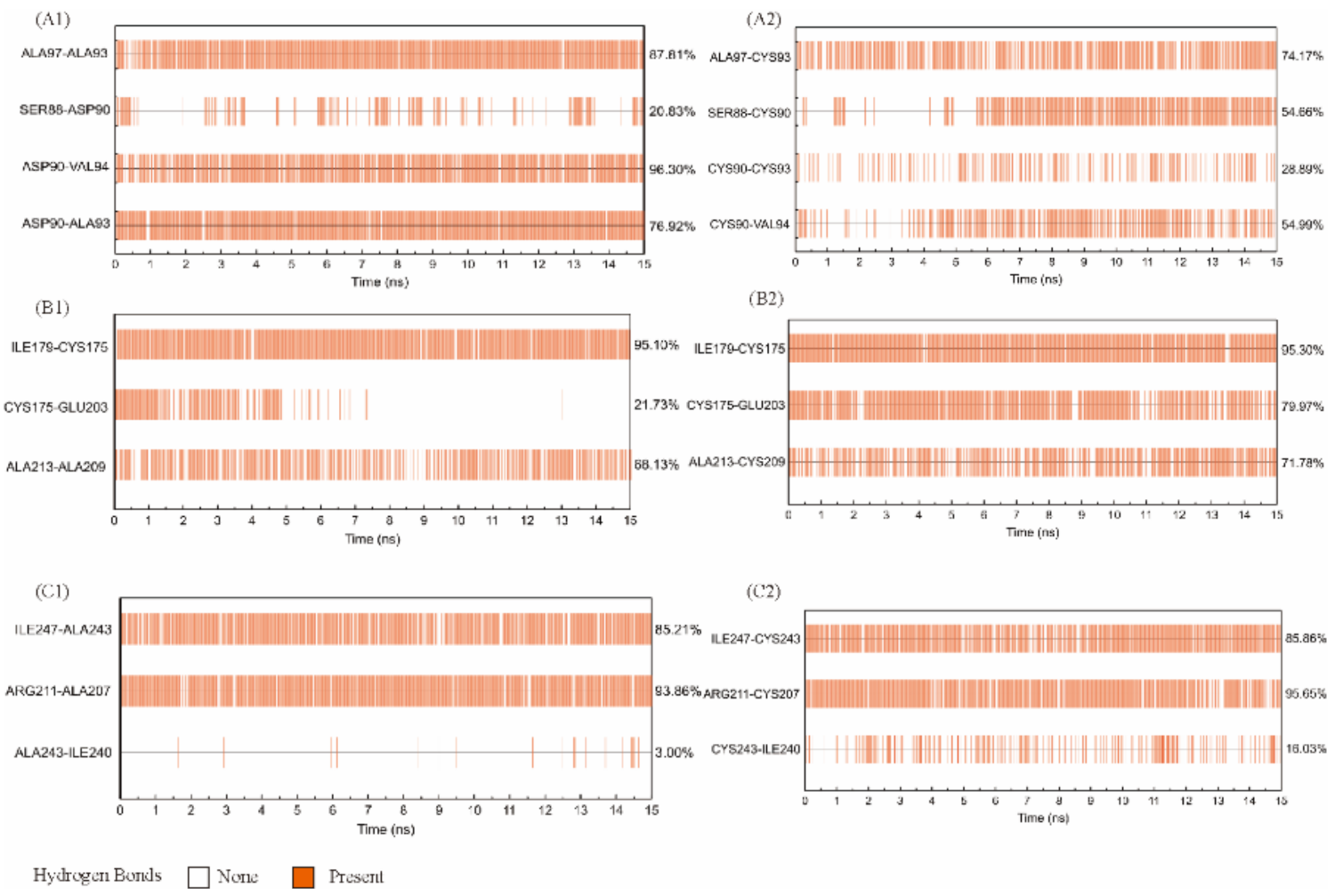
2.5. MD Simulation of Wild-Type Cb-DPEase and the Mutants
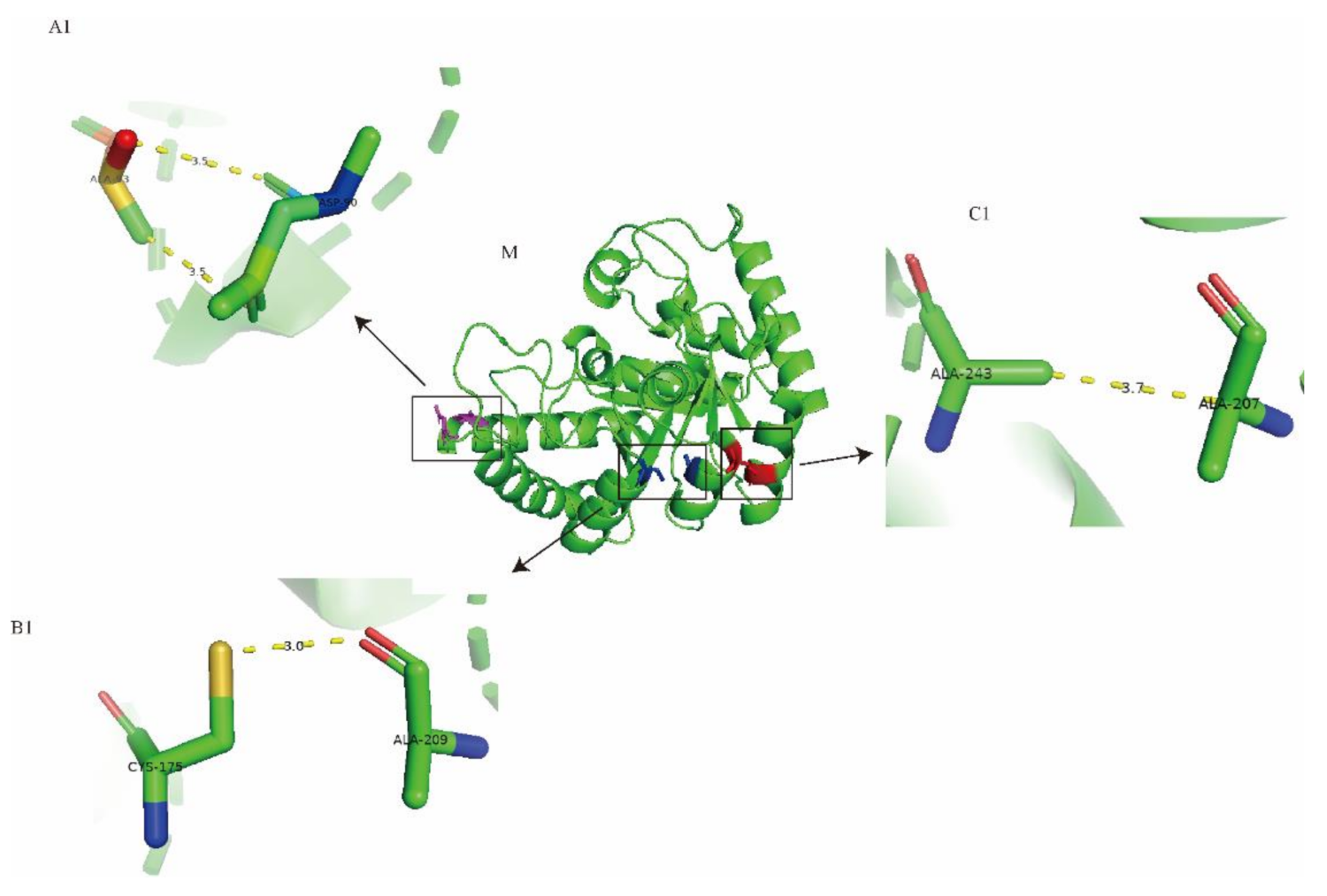
2.6. Molecular Modeling of Wild-Type Cb-DPEase and the Mutants
3. Discussion
4. Materials and Methods
4.1. Gene, Plasmids and Strains
4.2. Mutagenesis and DNA Manipulations
4.3. Production and Purification of DPEase and Its Mutants
4.4. Enzyme Activity Assay
4.5. Characterization of DPEase and Its Mutants
4.6. Structural Modeling
4.7. Verification of Disulfide Bond Formation
4.8. Molecular Dynamics (MD) Simulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene Name | Sequence |
| Cb-dpe | ATGCGTTACTTCAAAGAAGAAGTTGCTGGTATGAAATACGGTATCTA CTTCGCTTACTGGACTAAAGAATGGTTCGCTGATTACAAAAAATACA TGGATAAAGTTTCAGCTCTTGGTTTCGACGTATTAGAAATCTCTTGTG CTGCTTTACGTGATGTTTACACTACTAAAGAACAATTAATCGAATTAC GTGAATACGCTAAAGAAAAAGGTTTAGTTTTAACAGCAGGTTACGGT CCAACTAAAGCTGAAAACCTTTGTTCTGAAGATCCTGAAGCTGTTCG TCGTGCAATGACTTTCTTCAAAGACTTATTACCAAAATTACAATTAAT GGATATCCACATCCTTGGTGGTGGTTTATACTCTTACTGGCCAGTTGA TTTCACAATTAACAACGATAAACAAGGTGATCGTGCTCGTGCAGTAC GTAACCTTCGTGAACTTTCTAAAACTGCTGAAGAATGTGATGTTGTAC TTGGAATGGAAGTTCTTAACCGTTACGAAGGATACATCCTTAACACT TGTGAAGAAGCTATCGATTTCGTTGACGAAATCGGTTCTTCTCATGTA AAAATCATGTTAGACACTTTTCATATGAACATCGAAGAAACAAACAT GGCTGACGCTATCCGTAAAGCTGGTGATAGATTAGGTCATCTTCATTT AGGTGAACAAAATAGATTAGTTCCTGGTAAAGGTAGTTTACCGTGGG CTGAAATAGGTCAAGCATTACGTGATATTAACTACCAAGGTGCTGCT GTAATGGAACCATTCGTAATGCAAGGTGGTACAATCGGTAGTGAGAT TAAAGTATGGCGTGATATGGTTCCAGATTTATCTGAAGAAGCATTAG ATCGTGATGCTAAAGGTGCTCTTGAGTTCTGTAGGCACGTTTTCGGTA TCTAA |
| Res1 Seq # | Res1 AA | Res2 Seq # | Res2 AA | Sum B-Factors |
|---|---|---|---|---|
| 12 | LYS | 41 | ASP | 1.73 |
| 17 | PHE | 31 | TYR | 1.7 |
| 21 | THR | 26 | ALA | 1.51 |
| 36 | SER | 70 | LYS | 1.73 |
| 36 | SER | 72 | LEU | 1.73 |
| 80 | PRO | 85 | ASN | 1.54 |
| 85 | ASN | 123 | TYR | 1.6 |
| 86 | LEU | 144 | ASN | 1.74 |
| 87 | CYS | 137 | ASP | 1.72 |
| 88 | SER | 93 | ALA | 1.74 |
| 90 | ASP | 93 | ALA | 1.76 |
| 109 | GLN | 155 | CYS | 1.64 |
| 121 | TYR | 141 | ALA | 1.75 |
| 149 | SER | 187 | SER | 1.74 |
| 152 | ALA | 157 | VAL | 1.71 |
| 160 | GLY | 219 | HIS | 1.74 |
| 164 | LEU | 169 | GLY | 1.68 |
| 165 | ASN | 201 | ILE | 1.69 |
| 166 | ARG | 270 | LYS | 1.66 |
| 167 | TYR | 167 | TYR | 1.65 |
| 175 | CYS | 209 | ALA | 1.76 |
| 187 | SER | 190 | VAL | 1.75 |
| 205 | ASN | 208 | ASP | 1.74 |
| 207 | ALA | 243 | ALA | 1.76 |
| 210 | ILE | 243 | ALA | 1.75 |
| 213 | ALA | 217 | LEU | 1.74 |
| 224 | GLU | 228 | LEU | 1.72 |
| 227 | ARG | 258 | PHE | 1.71 |
| 228 | LEU | 232 | LYS | 1.65 |
| 229 | VAL | 291 | ALA | 1.75 |
| 244 | LEU | 249 | TYR | 1.72 |
| 270 | LYS | 166 | ARG | 1.64 |
| 279 | LEU | 283 | ALA | 1.68 |
References
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar D-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef]
- Fu, G.; Zhang, S.; Dong, H.; Chen, J.; Tu, R.; Zhang, D. Enhanced production of d-psicose 3-epimerase in Bacillus subtilis by regulation of segmented fermentation. Biotechnol. Appl. Biochem. 2020, 67, 812–818. [Google Scholar] [CrossRef]
- Jia, M.; Mu, W.; Chu, F.; Zhang, X.; Jiang, B.; Zhou, L.L.; Zhang, T. A D-psicose 3-epimerase with neutral pH optimum from Clostridium bolteae for D-psicose production: Cloning, expression, purification, and characterization. Appl. Microbiol. Biotechnol. 2014, 98, 717–725. [Google Scholar] [CrossRef]
- Sanchez, M.I.; Ting, A.Y. Directed evolution improves the catalytic efficiency of TEV protease. Nat. Methods 2020, 17, 167–174. [Google Scholar] [CrossRef]
- Guan, L.; Gao, Y.; Li, J.; Wang, K.; Zhang, Z.; Yan, S.; Ji, N.; Zhou, Y.; Lu, S. Directed Evolution of Pseudomonas fluorescens Lipase Variants with Improved Thermostability Using Error-Prone PCR. Front. Bioeng. Biotechnol. 2020, 8, 1034. [Google Scholar] [CrossRef]
- Su, H.H.; Peng, F.; Xu, P.; Wu, X.L.; Zong, M.H.; Yang, J.G.; Lou, W.Y. Enhancing the thermostability and activity of uronate dehydrogenase from Agrobacterium tumefaciens LBA4404 by semi-rational engineering. Bioresour. Bioprocess. 2019, 6, 9. [Google Scholar] [CrossRef]
- Li, J.; Jiang, L.; Cao, X.; Wu, Y.; Lu, F.; Liu, F.; Li, Y.; Liu, Y. Improving the activity and stability of Bacillus clausii alkaline protease using directed evolution and molecular dynamics simulation. Enzyme Microb. Technol. 2021, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, X.; Zhao, H. Biosystems design by directed evolution. AICHE J. 2020, 66, e16716. [Google Scholar] [CrossRef]
- Li, G.; Fang, X.; Su, F.; Chen, Y.; Xu, L.; Yan, Y. Enhancing the Thermostability of Rhizomucor miehei Lipase with a Limited Screening Library by Rational-Design Point Mutations and Disulfide Bonds. Appl. Environ. Microbiol. 2018, 84, e02129-17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Jia, M.; Yu, S.; Zhang, T.; Zhou, L.; Jiang, B.; Mu, W. Improving the Thermostability and Catalytic Efficiency of the D-Psicose 3-Epimerase from Clostridium bolteae ATCC BAA-613 Using Site-Directed Mutagenesis. J. Agric. Food Chem. 2016, 64, 3386–3393. [Google Scholar] [CrossRef]
- Ban, X.; Wu, J.; Kaustubh, B.; Lahiri, P.; Dhoble, A.S.; Gu, Z.; Li, C.; Cheng, L.; Hong, Y.; Tong, Y.; et al. Additional salt bridges improve the thermostability of 1,4-alpha-glucan branching enzyme. Food Chem. 2020, 316, e02129-17. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Wang, H.-J.; Hwang, J.-K.; Tseng, C.-P. Protein Thermal Stability Enhancement by Designing Salt Bridges: A Combined Computational and Experimental Study. PLoS ONE 2014, 9, e112751. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wu, Y.; Su, L.; Wu, J. Contribution of disulfide bond to the stability of Thermobifida fusca cutinase. Food Biosci. 2019, 27, 6–10. [Google Scholar] [CrossRef]
- Quang Anh Tuan, L.; Joo, J.C.; Yoo, Y.J.; Kim, Y.H. Development of thermostable Candida antarctica lipase B through novel in silico design of disulfide bridge. Biotechnol. Bioeng. 2012, 109, 867–876. [Google Scholar] [CrossRef]
- Teng, C.; Jiang, Y.; Xu, Y.; Li, Q.; Li, X.; Fan, G.; Xiong, K.; Yang, R.; Zhang, C.; Ma, R.; et al. Improving the thermostability and catalytic efficiency of GH11 xylanase PjxA by adding disulfide bridges. Int. J. Biol. Macromol. 2019, 128, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.; Wu, W.; Guan, W.; Deng, Z.; Qiao, H. Enhancing thermostability of Yarrowia lipolytica lipase 2 through engineering multiple disulfide bonds and mitigating reduced lipase production associated with disulfide bonds. Enzym. Microb. Technol. 2019, 126, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dehnavi, E.; Fathi-Roudsari, M.; Mirzaie, S.; Arab, S.S.; Siadat, S.O.R.; Khajeh, K. Engineering disulfide bonds in Selenomonas ruminantium beta-xylosidase by experimental and computational methods. Int. J. Biol. Macromol. 2017, 95, 248–255. [Google Scholar] [CrossRef]
- Li, C.; Ban, X.; Zhang, Y.; Gu, Z.; Hong, Y.; Cheng, L.; Tang, X.; Li, Z. Rational Design of Disulfide Bonds for Enhancing the Thermostability of the 1,4-alpha-Glucan Branching Enzyme from Geobacillus thermoglucosidans STB02. J. Agric. Food Chem. 2020, 68, 13791–13797. [Google Scholar] [CrossRef]
- Nozach, H.; Fruchart-Gaillard, C.; Fenaille, F.; Beau, F.; Ramos, O.H.P.; Douzi, B.; Saez, N.J.; Moutiez, M.; Servent, D.; Gondry, M.; et al. High throughput screening identifies disulfide isomerase DsbC as a very efficient partner for recombinant expression of small disulfide-rich proteins in E. coli. Microb. Cell Fact. 2013, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Miao, L.; Li, J.-M.; Li, Y.-Y.; Zhu, Q.-Y.; Zhou, C.-L.; Fang, H.-Q.; Chen, H.-P. Receptor-binding domain of SARS-Cov spike protein: Soluble expression in E.coli, purification and functional characterization. World J. Gastroenterol. 2005, 11, 6159–6164. [Google Scholar] [CrossRef]
- Hu, X.; Fan, G.; Liao, H.; Fu, Z.; Ma, C.; Ni, H.; Li, X. Optimized soluble expression of a novel endoglucanase from Burkholderia pyrrocinia in Escherichia coli. 3 Biotech. 2020, 10, 387. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.N.; Bai, R.; Chen, H.Y.; Wu, Y.Y.; Shang, J.J.; Bao, D.P. Identification of a Novel Anti-cancer Protein, FIP-bbo, from Botryobasidium botryosum and Protein Structure Analysis using Molecular Dynamic Simulation. Sci. Rep. 2019, 9, 5818. [Google Scholar] [CrossRef]
- Kim, H.J.; Hyun, E.K.; Kim, Y.S.; Lee, Y.J.; Oh, D.K. Characterization of an Agrobacterium tumefaciens D-psicose 3-epimerase that converts D-fructose to D-psicose. Appl. Environ. Microbiol. 2006, 72, 981–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, W.; Zhang, W.; Fang, D.; Zhou, L.; Jiang, B.; Zhang, T. Characterization of a D-psicose-producing enzyme, D-psicose 3-epimerase, from Clostridium sp. Biotechnol. Lett. 2013, 35, 1481–1486. [Google Scholar] [CrossRef]
- Mu, W.M.; Chu, F.F.; Xing, Q.C.; Yu, S.H.; Zhou, L.; Jiang, B. Cloning, expression, and characterization of a D-psicose 3-epimerase from Clostridium cellulolyticum H10. J. Agric. Food Chem. 2011, 59, 7785–7792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fang, D.; Xing, Q.; Zhou, L.; Jiang, B.; Mu, W. Characterization of a novel metal-dependent D-psicose 3-epimerase from Clostridium scindens 35704. PLoS ONE 2013, 8, e62987. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, D.; Zhang, T.; Zhou, L.; Jiang, B.; Mu, W. Characterization of a metal-dependent D-psicose 3-epimerase from a novel strain, Desmospora sp. 8437. J. Agric. Food Chem. 2013, 61, 11468–11476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tao, Z.; Bo, J.; Mu, W. Biochemical characterization of a d-psicose 3-epimerase from Treponema primitia ZAS-1 and its application on enzymatic production of d-psicose. J. Sci. Food Agric. 2016, 96, 49–56. [Google Scholar] [CrossRef]
- Zhang, W.L.; Li, H.; Zhang, T.; Jiang, B.; Zhou, L.; Mu, W.M. Characterization of a D-psicose 3-epimerase from Dorea sp CAG317 with an acidic pH optimum and a high specific activity. J. Mol. Catal. B-Enzym. 2015, 120, 68–74. [Google Scholar] [CrossRef]
- Oh, D.K.; Kim, N.H.; Kim, H.J.; Park, C.S.; Kim, S.W.; Ko, M.; Park, B.W.; Jung, M.H.; Yoon, K.H. D-Psicose production from D-fructose using an isolated strain, Sinorhizobium sp. World J. Microbiol. Biotechnol. 2007, 23, 559–563. [Google Scholar] [CrossRef]
- Yoshihara, A.; Kozakai, T.; Shintani, T.; Matsutani, R.; Gullapalli, P.K. Purification and characterization of d-allulose 3-epimerase derived from Arthrobacter globiformis M30, a GRAS microorganism. J. Biosci. Bioeng. 2017, 123, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Z.; Zhang, W.; Guang, C.; Mu, W. Characterization of a d-tagatose 3-epimerase from Caballeronia fortuita and its application in rare sugar production. Int. J. Biol. Macromol. 2019, 138, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Badieyan, S.; Bevan, D.R.; Zhang, C. Study and design of stability in GH5 cellulases. Biotechnol. Bioeng. 2012, 109, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Luo, H.; Bai, Y.; Wang, K.; Niu, C.; Huang, H.; Shi, P.; Wang, C.; Yang, P.; Yao, B. A Thermostable Glucoamylase from Bispora sp MEY-1 with Stability over a Broad pH Range and Significant Starch Hydrolysis Capacity. PLoS ONE 2014, 9, e113581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navone, L.; Vogl, T.; Luangthongkam, P.; Blinco, J.-A.; Luna-Flores, C.H.; Chen, X.; von Hellens, J.; Mahler, S.; Speight, R. Disulfide bond engineering of AppA phytase for increased thermostability requires co-expression of protein disulfide isomerase in Pichia pastoris. Biotechnol. Biofuels 2021, 14, 80. [Google Scholar] [CrossRef]
- Ladero, M.; Ruiz, G.; Pessela, B.C.C.; Vian, A.; Santos, A.; Garcia-Ochoa, F. Thermal and pH inactivation of an immobilized thermostable beta-galactosidase from Thermus sp strain T2: Comparison to the free enzyme. Biochem. Eng. J. 2006, 31, 14–24. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Srinivasan, S.P.; Salem, S.M.; Matthews, S.J.; Colon, W.; Zaki, M.; Bystroff, C. GeoFold: Topology-based protein unfolding pathways capture the effects of engineered disulfides on kinetic stability. Proteins-Struct. Funct. Bioinform. 2012, 80, 920–934. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-h.; Lu, F.-p.; Li, Y.; Wang, J.-l.; Gao, C. Acid stabilization of Bacillus licheniformis alpha amylase through introduction of mutations. Appl. Microbiol. Biotechnol. 2008, 80, 795–803. [Google Scholar] [CrossRef]
- Niu, C.; Zhu, L.; Xu, X.; Li, Q. Rational Design of Disulfide Bonds Increases Thermostability of a Mesophilic 1,3-1,4-beta-Glucanase from Bacillus terquilensis. PLoS ONE 2016, 11, e0154036. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.X.; Wang, Y.B.; Pan, Y.J.; Li, W.F. Differences in amino acids composition and coupling patterns between mesophilic and thermophilic proteins. Amino Acids 2008, 34, 25–33. [Google Scholar] [CrossRef]
- Yu, H.; Huang, H. Engineering proteins for thermostability through rigidifying flexible sites. Biotechnol. Adv. 2014, 32, 308–315. [Google Scholar] [CrossRef]
- Vieira, D.S.; Degreve, L. An insight into the thermostability of a pair of xylanases: The role of hydrogen bonds. Mol. Phys. 2009, 107, 59–69. [Google Scholar] [CrossRef]
- Matsuno, T.; Fujita, M.; Fukunaga, K.; Sato, S.; Isobe, H. Concyclic CH-pi arrays for single-axis rotations of a bowl in a tube. Nat. Commun. 2018, 9, 3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Yang, S.-X.; Liu, Z.-M.; Li, N.-N.; Li, L.; Mou, H.-J. Rational design of alginate lyase from Microbulbifer sp. Q7 to improve thermal stability. Mar. Drugs 2019, 17, 378. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.B.; Jiang, J.; Bai, Y.J.; Fan, T.P.; Zhao, Y.; Zheng, X.H.; Cai, Y.J. Mimicking a New 2-Phenylethanol Production Pathway from Proteus mirabilis JN458 in Escherichia coli. J. Agric. Food Chem. 2018, 66, 3498–3504. [Google Scholar] [CrossRef]
- Liu, J.; Lu, S.-Y.; Orfe, L.H.; Ren, C.-H.; Hu, C.-Q.; Call, D.R.; Avillan, J.J.; Zhao, Z. ExsE Is a Negative Regulator for T3SS Gene Expression in Vibrio alginolyticus. Front. Cell. Infect. Microbiol. 2016, 6, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettinati, I.; Grzechnik, P.; de Almeida, C.R.; Brem, J.; McDonough, M.A.; Dhir, S.; Proudfoot, N.J.; Schofield, C.J. Biosynthesis of histone messenger RNA employs a specific 3’ end endonuclease. Elife 2018, 7. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Wei, X.; Li, K.; Liu, J. Food-Grade Expression and Characterization of a Dextranase from Chaetomium gracile Suitable for Sugarcane Juice Clarification. Chem. Biodivers. 2021, 18, e2000797. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hubert, H.; Berg, I.A. Enzymes Catalyzing Crotonyl-CoA Conversion to Acetoacetyl-CoA During the Autotrophic CO2 Fixation in Metallosphaera sedula. Front. Microbiol. 2020, 11, 354. [Google Scholar] [CrossRef] [Green Version]
- Natalia Gonzalez, S.; Mariela Valsecchi, W.; Maugeri, D.; Maria Delfino, J.; Jose Cazzulo, J. Structure, kinetic characterization and subcellular localization of the two ribulose 5-phosphate epimerase isoenzymes from Trypanosoma cruzi. PLoS ONE 2017, 12, e0172405. [Google Scholar] [CrossRef]
- Qiao, Z.; Xu, M.; Shao, M.; Zhao, Y.; Long, M.; Yang, T.; Zhang, X.; Yang, S.; Nakanishi, H.; Rao, Z. Engineered disulfide bonds improve thermostability and activity of L-isoleucine hydroxylase for efficient 4-HIL production in Bacillus subtilis 168. Eng. Life Sci. 2020, 20, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.-F.; Fang, H.; Mei, J.-Q.; Gong, J.-Y.; Wang, H.-P.; Shen, X.-Y.; Huang, J.; Mei, L.-H. Improving thermostability of (R)-selective amine transaminase from Aspergillus terreus through introduction of disulfide bonds. Biotechnol. Appl. Biochem. 2018, 65, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, R.; Valentin-Hansen, L.; Mokrosinski, J.; Frimurer, T.M.; Schwartz, T.W. Conserved water-mediated hydrogen bond network between TM-I, -II, -VI, and -VII in 7TM receptor activation. J. Biol. Chem. 2010, 285, 19625–19636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, J.; Kovacic, F.; Antonia, Y.D.; Krauss, U.; Fersini, F.; Schmeisser, C.; Lauinger, B.; Bongen, P.; Pietruszka, J.; Schmidt, M.; et al. The Metagenome-Derived Enzymes LipS and LipT Increase the Diversity of Known Lipases. PLoS ONE 2012, 7, e47665. [Google Scholar] [CrossRef] [Green Version]

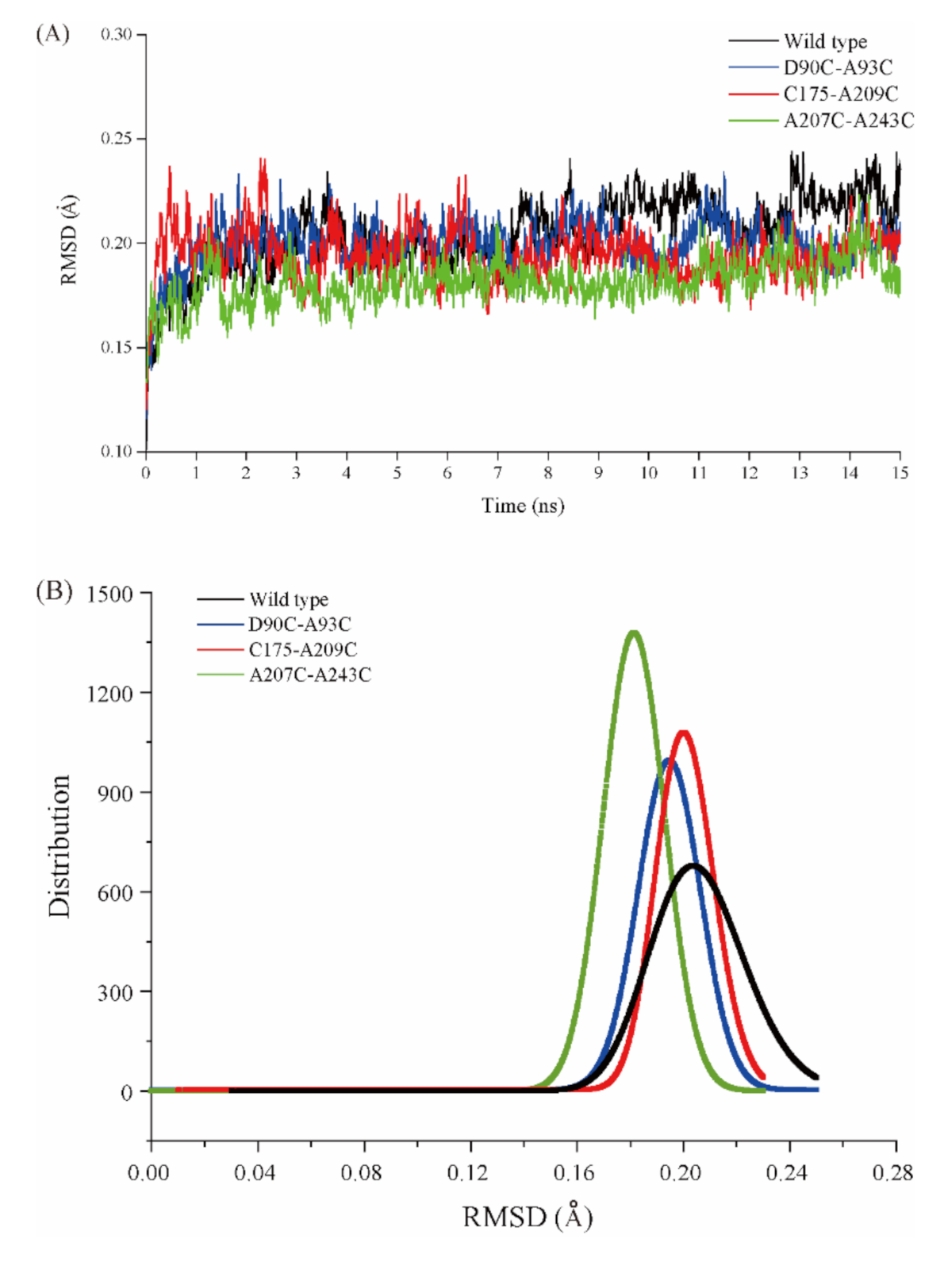


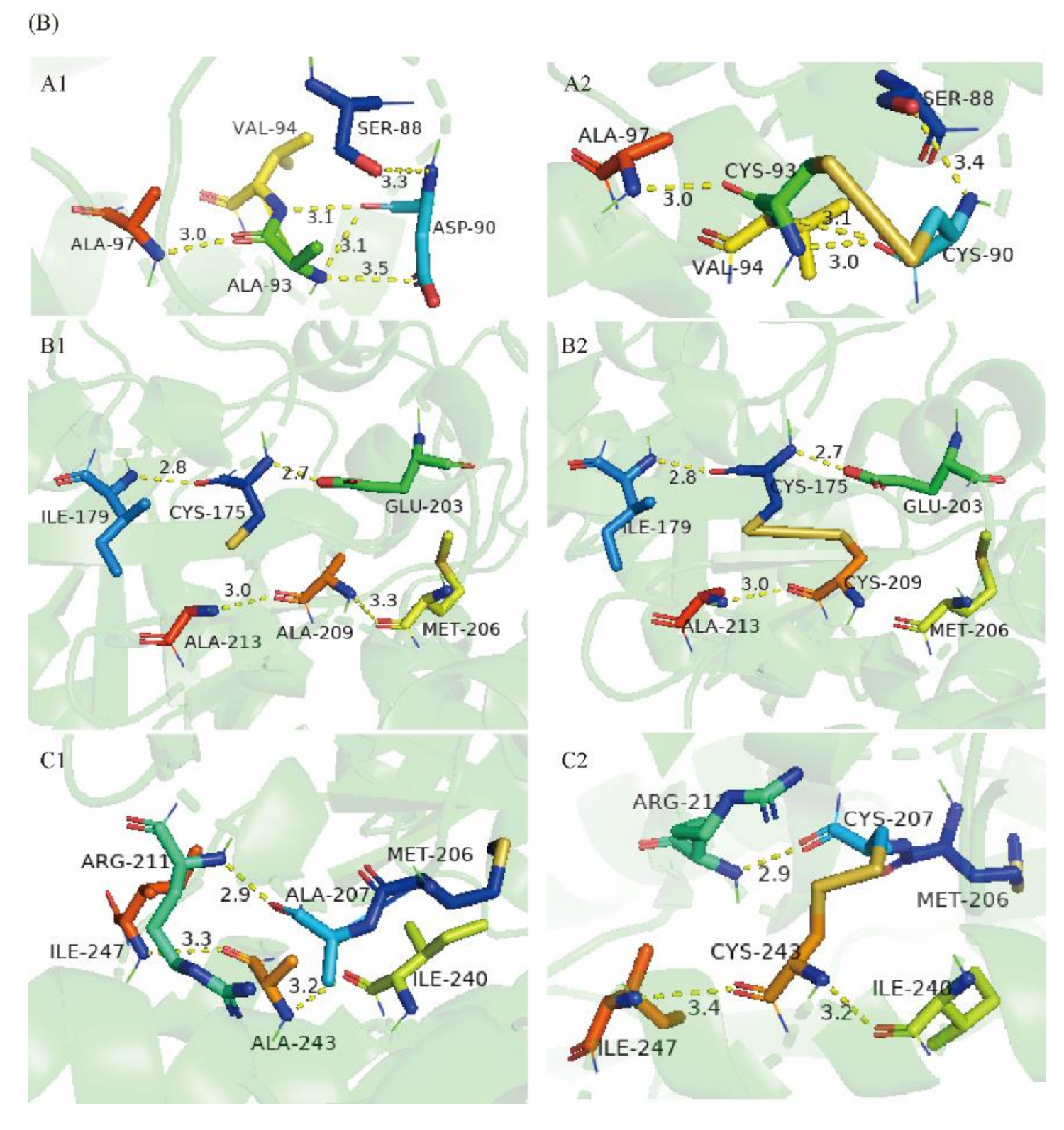
| Enzyme | Free Cysteine Concentration (mmol/L) | Free Cysteine Number |
|---|---|---|
| Wild type | 0.0903 ± 0.02 | 5 |
| D90C-A93C | 0.0874 ± 0.03 | 5 |
| C175-A209C | 0.0720 ± 0.02 | 4 |
| A207C-A243C | 0.0882 ± 0.02 | 5 |
| Sources | Half-Life (t1/2) | Optimal Metal Ions | Optimal pH | Optimal Temperature | References |
|---|---|---|---|---|---|
| A. tumefaciens | 63.5 min (60 °C) | Mn2+ | 8.0 | 50 °C | [23] |
| Clostridium sp. BNL1100 | 15 min (60 °C) | Co2+ | 8.0 | 65 °C | [24] |
| Clostridium cellulolyticum H10 | 9.5 h (55 °C) | Co2+ | 8.0 | 65 °C | [25] |
| C. scindens ATCC 35704 | 1.8 h (50 °C) | Mn2+ | 7.5 | 60 °C | [26] |
| Desmospora sp. 8437 | NR | Co2+ | 7.5 | 60 °C | [27] |
| T. primitia | 4 h (50 °C) | Co2+ | 8.0 | 70 °C | [28] |
| Dorea sp. | NR | Co2+ | 6.0 | 70 °C | [29] |
| Sinorhizobium sp. | NR | Mn2+ | 8.5 | 40 °C | [30] |
| Arthrobacter globiformis M30 | NR | Mg2+ | 7.5–8.0 | 70 °C | [31] |
| Caballeronia fortuita | NR | Co2+ | 7.5 | 65 °C | [32] |
| Primer | Sequence a (5′−3′) |
|---|---|
| Cb-F | CCGGAATTCATGCGTTACTTCAAAGAAGAAG |
| Cb-R | CCCAAGCTTAATGGTGATGGTGATGATGACTTGAACCGATACCGAAAACGTGCC |
| D90/93C-F | GTTCTGAATGTCCTGAATGTGT |
| D90/93C-R | ACACATTCAGGACATTCAGAACA |
| A209C-F | GCTGACTGTATCCGTAAAG |
| A209C-R | CTTTACGGATACAGTCAGC |
| A207C-F | CAAACATGTGTGACGCTATC |
| A207C-R | GATAGCGTCACACATGTTTG |
| A243C-F | GAAATAGGTCAATGTTTACGTG |
| A243C-R | CACGTAAACATTGACCTATTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Chen, J.; Wang, H.; Guo, Y.; Li, K.; Liu, J. Enhanced Thermostability of D-Psicose 3-Epimerase from Clostridium bolteae through Rational Design and Engineering of New Disulfide Bridges. Int. J. Mol. Sci. 2021, 22, 10007. https://doi.org/10.3390/ijms221810007
Zhao J, Chen J, Wang H, Guo Y, Li K, Liu J. Enhanced Thermostability of D-Psicose 3-Epimerase from Clostridium bolteae through Rational Design and Engineering of New Disulfide Bridges. International Journal of Molecular Sciences. 2021; 22(18):10007. https://doi.org/10.3390/ijms221810007
Chicago/Turabian StyleZhao, Jingyi, Jing Chen, Huiyi Wang, Yan Guo, Kai Li, and Jidong Liu. 2021. "Enhanced Thermostability of D-Psicose 3-Epimerase from Clostridium bolteae through Rational Design and Engineering of New Disulfide Bridges" International Journal of Molecular Sciences 22, no. 18: 10007. https://doi.org/10.3390/ijms221810007
APA StyleZhao, J., Chen, J., Wang, H., Guo, Y., Li, K., & Liu, J. (2021). Enhanced Thermostability of D-Psicose 3-Epimerase from Clostridium bolteae through Rational Design and Engineering of New Disulfide Bridges. International Journal of Molecular Sciences, 22(18), 10007. https://doi.org/10.3390/ijms221810007






