NGF Eye Administration Recovers the TrkB and Glutamate/GABA Marker Deficit in the Adult Visual Cortex Following Optic Nerve Crush
Abstract
:1. Introduction
2. Results
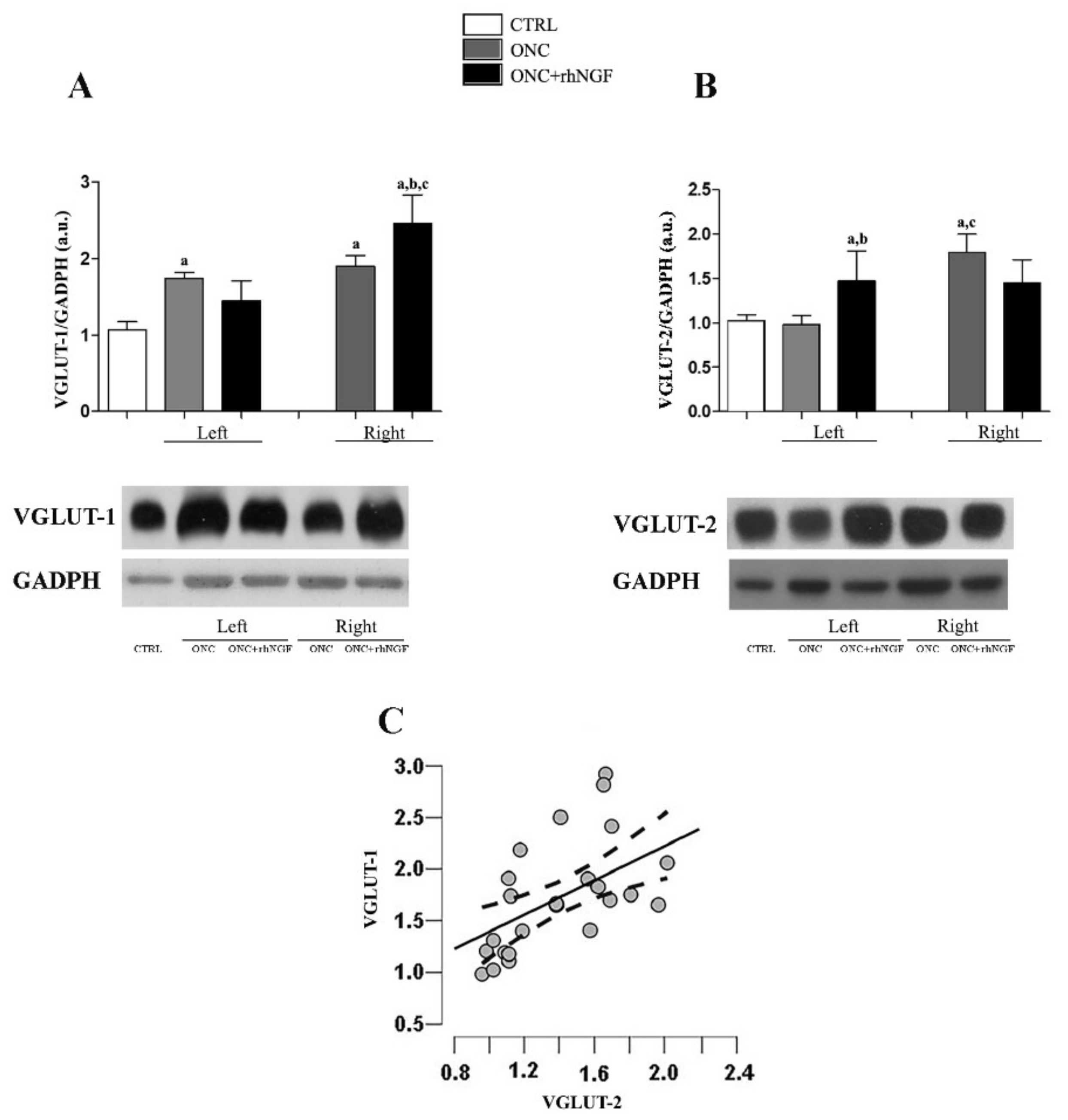
2.1. Effect of ed-rhNGF Treatment on VGLUT-1 and VGLUT-2 in Visual Cortex of ONC Rats
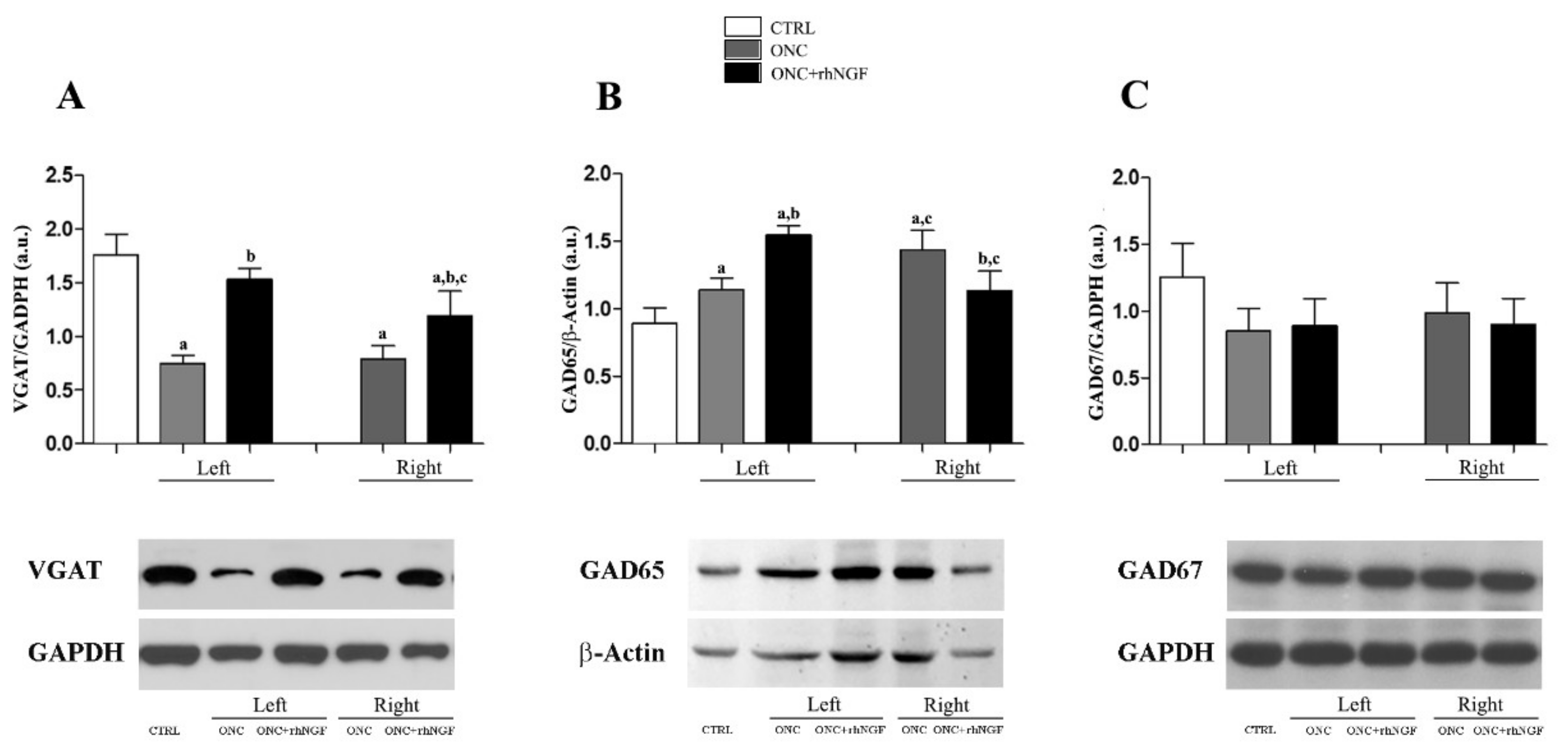
2.2. Expression of Cortical GABA Markers Following ONC and rhNGF Administration
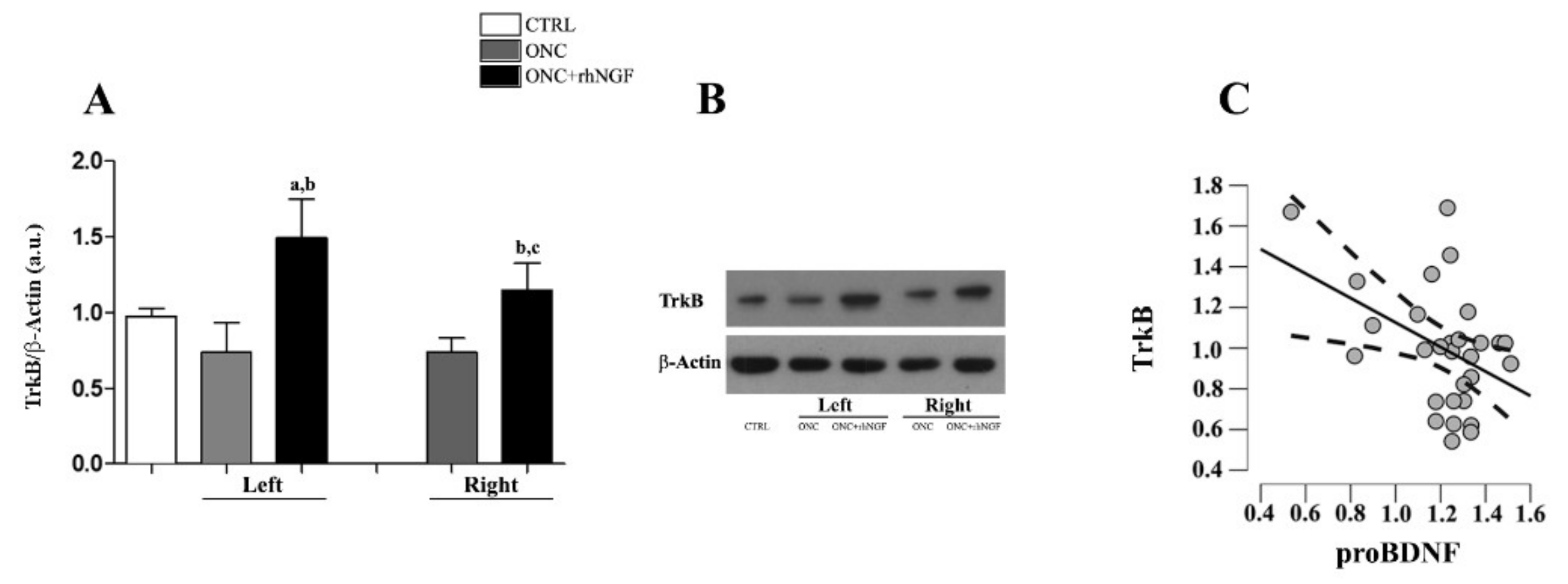
2.3. Effects of ONC and rhNGF Treatment on BDNF
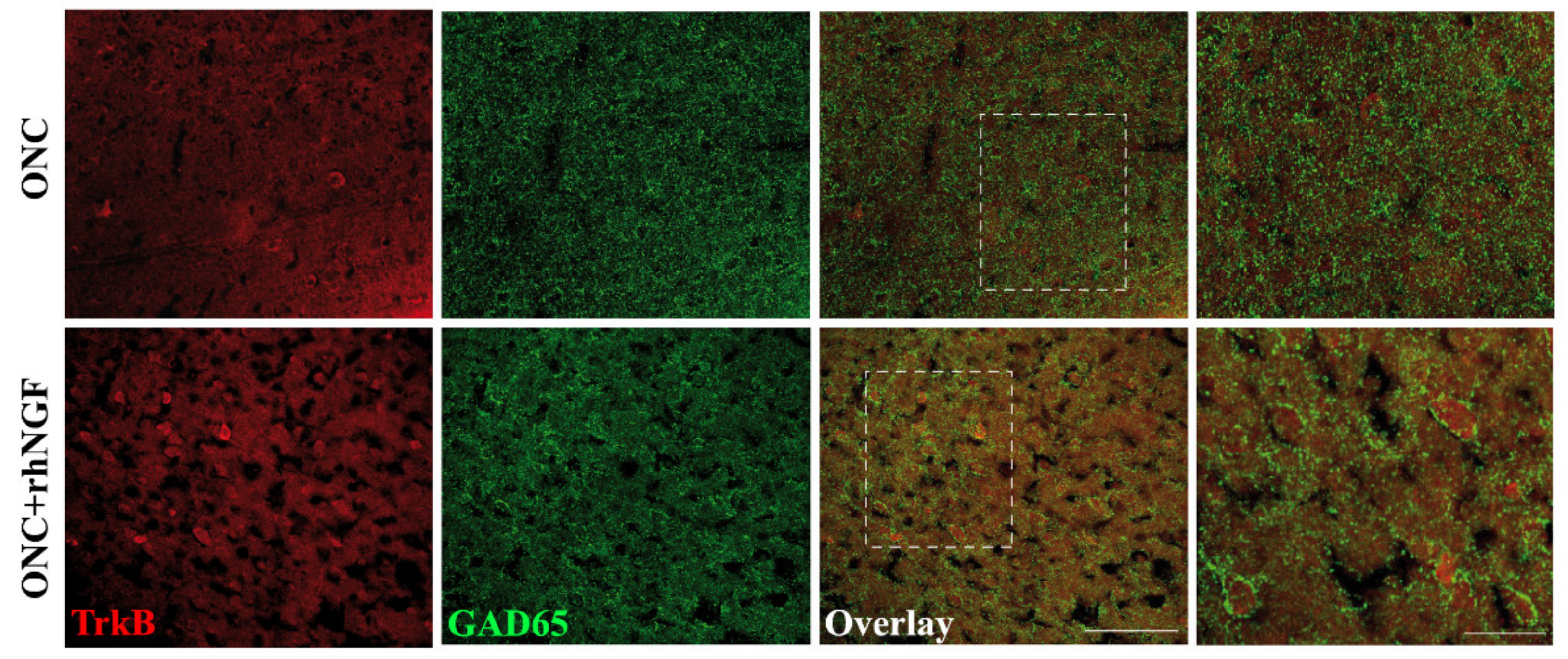
2.4. Expression of TrkB in VCx
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Unilateral Optic Nerve Crush
4.3. NGF Treatment
4.4. Brain Dissection and Tissue Lysate Preparation
4.5. BDNF ELISA
4.6. Western Blot Analysis
4.7. Confocal Microscopy Studies
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Rosso, P.; De Nicolò, S.; Carito, V.; Fiore, M.; Iannitelli, A.; Moreno, S.; Tirassa, P. Ocular Nerve Growth Factor Administration Modulates Brain-derived Neurotrophic Factor Signaling in Prefrontal Cortex of Healthy and Diabetic Rats. CNS Neurosci. Ther. 2017, 23, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Tirassa, P.; Rosso, P.; Iannitelli, A. Ocular Nerve Growth Factor (NGF) and NGF Eye Drop Application as Paradigms to Investigate NGF Neuroprotective and Reparative Actions. In Neurotrophic Factors; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1727, pp. 19–38. [Google Scholar]
- Tirassa, P.; Quartini, A.; Iannitelli, A. Nerve growth factor, brain-derived neurotrophic factor, and the chronobiology of mood: A new insight into the neurotrophic hypothesis. ChronoPhysiology Ther. 2015, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Rosso, P.; Iannitelli, A.; Pacitti, F.; Quartini, A.; Fico, E.; Fiore, M.; Greco, A.; Ralli, M.; Tirassa, P. Vagus nerve stimulation and Neurotrophins: A biological psychiatric perspective. Neurosci. Biobehav. Rev. 2020, 113, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Erskine, L.; Herreral, E. Connecting the retina to the brain. ASN Neuro 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Tirassa, P.; Maccarone, M.; Carito, V.; De Nicolò, S.; Fiore, M. Ocular nerve growth factor administration counteracts the impairment of neural precursor cell viability and differentiation in the brain subventricular area of rats with streptozotocin-induced diabetes. Eur. J. Neurosci. 2015, 41, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Carmignoto, G.; Comelli, M.C.; Candeo, P.; Cavicchioli, L.; Yan, Q.; Merighi, A.; Maffei, L. Expression of NGF receptor and NGF receptor mRNA in the developing and adult rat retina. Exp. Neurol. 1991, 111, 302–311. [Google Scholar] [CrossRef]
- Wahle, P.; Di Cristo, G.; Schwerdtfeger, G.; Engelhardt, M.; Berardi, N.; Maffei, L. Differential effects of cortical neurotrophic factors on development of lateral geniculate nucleus and superior colliculus neurons: Anterograde and retrogate actions. Development 2003, 130, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambiase, A.; Tirassa, P.; Micera, A.; Aloe, L.; Bonini, S. Pharmacokinetics of conjunctivally applied nerve growth factor in the retina and optic nerve of adult rats. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3800–3806. [Google Scholar] [CrossRef] [Green Version]
- Lambiase, A.; Aloe, L. Nerve Growth Factor delays retinal degeneration in C3H mice. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234. [Google Scholar] [CrossRef]
- Lambiase, A.; Bonini, S.; Manni, L.; Ghinelli, E.; Tirassa, P.; Rama, P.; Aloe, L. Intraocular production and release of nerve growth factor after iridectomy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2334–2340. [Google Scholar]
- Colafrancesco, V.; Coassin, M.; Rossi, S.; Aloe, L. Effect of eye NGF administration on two animal models of retinal ganglion cells degeneration. Ann. Ist. Super. Sanita 2011, 47, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.H.; Zhang, S.; Jiang, S.; Hu, N. Activation of autophagy in the retina after optic nerve crush injury in rats. Int. J. Ophthalmol. 2019, 12, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Mesentier-Louro, L.A.; De Nicolò, S.; Rosso, P.; De Vitis, L.A.; Castoldi, V.; Leocani, L.; Mendez-Otero, R.; Santiago, M.F.; Tirassa, P.; Rama, P.; et al. Time-dependent nerve growth factor signaling changes in the rat retina during optic nerve crush-induced degeneration of retinal ganglion cells. Int. J. Mol. Sci. 2017, 18, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesentier-Louro, L.A.; Rosso, P.; Carito, V.; Mendez-Otero, R.; Santiago, M.F.; Rama, P.; Lambiase, A.; Tirassa, P. Nerve Growth Factor Role on Retinal Ganglion Cell Survival and Axon Regrowth: Effects of Ocular Administration in Experimental Model of Optic Nerve Injury. Mol. Neurobiol. 2019, 56, 1056–1069. [Google Scholar] [CrossRef]
- Schmitt, U.; Cross, R.; Pazdernik, T.L.; Sabel, B.A. Loss and subsequent recovery of local cerebral glucose use in visual targets after controlled optic nerve crush in adult rats. Exp. Neurol. 1996, 139, 17–24. [Google Scholar] [CrossRef]
- Brooks, D.E.; Källberg, M.E.; Cannon, R.L.; Komàromy, A.M.; Ollivier, F.J.; Malakhova, O.E.; Dawson, W.W.; Sherwood, M.B.; Kuekuerichkina, E.E.; Lambrou, G.N. Functional and structural analysis of the visual system in the rhesus monkey model of optic nerve head ischemia. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1830–1840. [Google Scholar] [CrossRef] [Green Version]
- Kaas, J.H.; Krubitzer, L.A.; Chino, Y.M.; Langston, A.L.; Polley, E.H.; Blair, N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science 1990, 248, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, C.D.; Wiesel, T.N. Receptive field dynamics in adult primary visual cortex. Nature 1992, 356, 150–152. [Google Scholar] [CrossRef]
- Calford, M.B.; Wang, C.; Taglianetti, V.; Waleszczyk, W.J.; Burke, W.; Dreher, B. Plasticity in adult cat visual cortex (area 17) following circumscribed monocular lesions of all retinal layers. J. Physiol. 2000, 524, 587–602. [Google Scholar] [CrossRef]
- Heinen, S.J.; Skavenski, A.A. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Exp. Brain Res. 1991, 83, 670–674. [Google Scholar] [CrossRef]
- Chino, Y.M.; Kaas, J.H.; Smith, E.L.; Langston, A.L.; Cheng, H. Rapid reorganization of cortical maps in adult cats following restricted deafferentation in retina. Vis. Res. 1992, 32, 789–796. [Google Scholar] [CrossRef]
- You, Y.; Gupta, V.K.; Graham, S.L.; Klistorner, A. Anterograde Degeneration along the Visual Pathway after Optic Nerve Injury. PLoS ONE 2012, 7, e52061. [Google Scholar] [CrossRef] [Green Version]
- MacHaradze, T.; Pielot, R.; Wanger, T.; Scheich, H.; Gundelfinger, E.D.; Budinger, E.; Goldschmidt, J.; Kreutz, M.R. Altered neuronal activity patterns in the visual cortex of the adult rat after partial optic nerve crush-a single-cell resolution metabolic mapping study. Cereb. Cortex 2012, 22, 1824–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.T.; Laeremans, A.; Eysel, U.T.; Cnops, L.; Arckens, L. Analysis of c-fos and zif268 expression reveals time-dependent changes in activity inside and outside the lesion projection zone in adult cat area 17 after retinal lesions. Cereb. Cortex 2009, 19, 2982–2992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arckens, L.; Schweigart, G.; Qu, Y.; Wouters, G.; Pow, D.V.; Vandesande, F.; Eysel, U.T.; Orban, G.A. Cooperative changes in GABA, glutamate and activity levels: The missing link in cortical plasticity. Eur. J. Neurosci. 2000, 12, 4222–4232. [Google Scholar] [CrossRef]
- Lambiase, A.; Coassin, M.; Tirassa, P.; Mantelli, F.; Aloe, L. Nerve growth factor eye drops improve visual acuity and electrofunctional activity in Age-related macular degeneration: A case report. Ann. Ist. Super. Sanita 2009, 45, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Calza, A.; Florenzano, F.; Pellegrini, D.; Tirassa, P. Time-dependent activation of c-fos in limbic brain areas by ocular administration of nerve growth factor in adult rats. J. Ocul. Pharmacol. Ther. 2011, 27, 209–218. [Google Scholar] [CrossRef]
- Brigadski, T.; Leßmann, V. The physiology of regulated BDNF release. Cell Tissue Res. 2020, 382, 15–45. [Google Scholar] [CrossRef]
- Fattorini, G.; Antonucci, F.; Menna, E.; Matteoli, M.; Conti, F. Co-expression of VGLUT1 and VGAT sustains glutamate and GABA co-release and is regulated by activity in cortical neurons. J. Cell Sci. 2015, 128, 1669–1673. [Google Scholar] [CrossRef] [Green Version]
- Cherubini, E.; Conti, F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001, 24, 155–162. [Google Scholar] [CrossRef]
- Ishikawa, M. Abnormalities in Glutamate Metabolism and Excitotoxicity in the Retinal Diseases. Scientifica 2013, 2013, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Connaughton, V. Glutamate and Glutamate Receptors in the Vertebrate Retina. In Webvision: The Organization of the Retina and Visual System [Internet]; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 2007. [Google Scholar]
- Bringmann, A.; Grosche, A.; Pannicke, T.; Reichenbach, A. GABA and Glutamate Uptake and Metabolism in Retinal Glial (Müller) Cells. Front. Endocrinol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Kew, J.N.C.; Kemp, J.A. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology 2005, 179, 4–29. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [Green Version]
- BS, M. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130. [Google Scholar] [CrossRef] [Green Version]
- Nys, J.; Scheyltjens, I.; Arckens, L. Visual system plasticity in mammals: The story of monocular enucleation-induced vision loss. Front. Syst. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergeeva, E.G.; Espinosa-Garcia, C.; Atif, F.; Pardue, M.T.; Stein, D.G. Neurosteroid allopregnanolone reduces ipsilateral visual cortex potentiation following unilateral optic nerve injury HHS Public Access. Exp. Neurol. 2018, 306, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, D.; Imbrosci, B.; Mittmann, T. Modulatory effects of the novel TrkB receptor agonist 7,8-dihydroxyflavone on synaptic transmission and intrinsic neuronal excitability in mouse visual cortex in vitro. Eur. J. Pharmacol. 2013, 709, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J.; Kirkwood, A.; Pizzorusso, T.; Porciatti, V.; Morales, B.; Bear, M.F.; Maffei, L.; Tonegawa, S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 1999, 98, 739–755. [Google Scholar] [CrossRef] [Green Version]
- Lambiase, A.; Manteli, F.; Sacheti, M.; Rosi, S.; Aloe, L.; Bonini, S. Clinical applications of NGF in ocular diseases. Arch. Ital. Biol. 2011, 149, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, F.; Hioki, H.; Tomioka, R.; Taki, K.; Tamamaki, N.; Nomura, S.; Okamoto, K.; Kaneko, T. Changes of immunocytochemical localization of vesicular glutamate transporters in the rat visual system after the retinofugal denervation. J. Comp. Neurol. 2003, 465, 234–249. [Google Scholar] [CrossRef]
- Seabrook, T.A.; El-Danaf, R.N.; Krahe, T.E.; Fox, M.A.; Guido, W. Systems/Circuits Retinal Input Regulates the Timing of Corticogeniculate Innervation. J. Neurosci. 2013, 33, 10085–10097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fattorini, G.; Ciriachi, C.; Conti, F. Few, activity-dependent, and ubiquitous VGLUT1/VGAT terminals in rat and mouse brain. Front. Cell. Neurosci. 2017, 11, 229. [Google Scholar] [CrossRef] [Green Version]
- Calford, M.B.; Wright, L.L.; Metha, A.B.; Taglianetti, V. Topographic Plasticity in Primary Visual Cortex Is Mediated by Local Corticocortical Connections. J. Neurosci. 2003, 23, 6434. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Gilbert, C.D. Long-range horizontal connections and their role in cortical reorganization revealed by optical recording of cat primary visual cortex. Nature 1995, 375, 780–784. [Google Scholar] [CrossRef]
- Palagina, G.; Eysel, U.T.; Jancke, D. Strengthening of lateral activation in adult rat visual cortex after retinal lesions captured with voltage-sensitive dye imaging in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 8743. [Google Scholar] [CrossRef] [Green Version]
- Keck, T.; Mrsic-Flogel, T.D.; Afonso, M.V.; Eysel, U.T.; Bonhoeffer, T.; Hübener, M. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat. Neurosci. 2008, 11, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, M.; Sergeeva, E.G.; Henrich-Noack, P.; Jia, S.; Grigartzik, L.; Ma, J.; You, Q.; Huppé-Gourgues, F.; Sabel, B.A.; Vaucher, E. Cholinergic Potentiation of Restoration of Visual Function after Optic Nerve Damage in Rats. Neural Plast. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, G. Architecture of the optic chiasm and the mechanisms that sculpt its development. Physiol. Rev. 2001, 81, 1393–1414. [Google Scholar] [CrossRef] [Green Version]
- Grandpré, T.; Strittmatter, S.M. Nogo: A Molecular Determinant of Axonal Growth and Regeneration. Neuroscientist 2016, 7, 377–386. [Google Scholar] [CrossRef]
- Wang, J.; Chan, C.-K.; Taylor, J.S.H.; Chan, S.-O. The growth-inhibitory protein Nogo is involved in midline routing of axons in the mouse optic chiasm. J. Neurosci. Res. 2008, 86, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Liu, H.; Chan, S.; Wang, J. Neuronal Nogo-A in New-born Retinal Ganglion Cells: Implication for the Formation of the Age-related Fiber Order in the Optic Tract. Anat. Rec. 2016, 299, 1027–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreutz, M.R.; Weise, J.; Dieterich, D.C.; Kreutz, M.; Balczarek, P.; Böckers, T.M.; Wittkowski, W.; Gundelfinger, E.D.; Sabel, B.A. Rearrangement of the retino-collicular projection after partial optic nerve crush in the adult rat. Eur. J. Neurosci. 2004, 19, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Vigneault, É.; Poirel, O.; Riad, M.; Prud’homme, J.; Dumas, S.; Turecki, G.; Fasano, C.; Mechawar, N.; El Mestikawy, S. Distribution of vesicular glutamate transporters in the human brain. Front. Neuroanat. 2015, 9, 23. [Google Scholar] [CrossRef]
- Nahmani, M.; Erisir, A. VGluT2 immunochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. J. Comp. Neurol. 2005, 484, 458–473. [Google Scholar] [CrossRef]
- Esclapez, M.; KTillakaratne, N.J.; Kaufman, D.L.; Tobin, A.J.; Houser, C.R. Comparative Localization of Two Forms of Glutamic Acid Decarboxylase and Their mRNAs in Rat Brain Supports the Concept of Functional Differences between the Forms. J. Neurosci. 1994, 14, 1834–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silver, M.A.; Stryker, M.P.; Keck, W.M. TrkB-Like Immunoreactivity Is Present on Geniculocortical Afferents in Layer IV of Kitten Primary Visual Cortex NIH Public Access. J. Comp. Neurol. 2001, 436, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Berardi, N.; Pizzorusso, T.; Ratto, G.M.; Maffei, L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003, 26, 369–378. [Google Scholar] [CrossRef]
- Hensch, T.K.; Fagiolini, M.; Mataga, N.; Stryker, M.P.; Baekkeskov, S.; Kash, S.F.; Hensch, T.K.; Fagiolini, M.; Mataga, N.; Baekkeskov, S.; et al. Local GABA Circuit Control of Experience-Dependent Plasticity in Developing Visual Cortex. Science 1998, 282, 1504–1508. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Huertas, C.; Rico, B. CREB-dependent regulation of gad65 transcription by BDNF/TrκB in cortical interneurons. Cereb. Cortex 2011, 21, 777–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-Y.; Morales, B.; Lee, H.-K.; Kirkwood, A. Absence of long-term depression in the visual cortex of glutamic Acid decarboxylase-65 knock-out mice. J. Neurosci. 2002, 22, 5271–5276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auld, D.S.; Mennicken, F.; Quirion, R. Nerve growth factor rapidly induces prolonged acetylcholine release from cultured basal forebrain neurons: Differentiation between neuromodulatory and neurotrophic influences. J. Neurosci. 2001, 21, 3375–3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biane, J.; Conner, J.M.; Tuszynski, M.H. Nerve growth factor is primarily produced by GABAergic neurons of the adult rat cortex. Front. Cell. Neurosci. 2014, 8, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Antigen | Host Species | Dilution | Producer |
|---|---|---|---|
| VGLUT-1 | Rabbit | 1:5000 | Synaptic System, Germany |
| VGLUT-2 | Mouse | 1:1000 | Millipore, Temecula, CA, USA |
| VGAT | Rabbit | 1:1000 | Chemicon International, CA, USA |
| GAD65 | Mouse | 1:1000 | Santa Cruz Biotechnology, USA |
| GAD67 | Mouse | 1:1000 | Millipore, Temecula, CA, USA |
| proBDNF | Mouse | 1:1000 | Santa Cruz Biotechnology, USA |
| TrkB | Mouse | 1:1000 | BD Biosciences, USA |
| GAPDH | Mouse | 1:1000 | Santa Cruz Biotechnology, USA |
| β-Actin+HRP | Mouse | 1:5000 | Santa Cruz Biotechnology, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosso, P.; Fico, E.; Mesentier-Louro, L.A.; Triaca, V.; Lambiase, A.; Rama, P.; Tirassa, P. NGF Eye Administration Recovers the TrkB and Glutamate/GABA Marker Deficit in the Adult Visual Cortex Following Optic Nerve Crush. Int. J. Mol. Sci. 2021, 22, 10014. https://doi.org/10.3390/ijms221810014
Rosso P, Fico E, Mesentier-Louro LA, Triaca V, Lambiase A, Rama P, Tirassa P. NGF Eye Administration Recovers the TrkB and Glutamate/GABA Marker Deficit in the Adult Visual Cortex Following Optic Nerve Crush. International Journal of Molecular Sciences. 2021; 22(18):10014. https://doi.org/10.3390/ijms221810014
Chicago/Turabian StyleRosso, Pamela, Elena Fico, Louise A. Mesentier-Louro, Viviana Triaca, Alessandro Lambiase, Paolo Rama, and Paola Tirassa. 2021. "NGF Eye Administration Recovers the TrkB and Glutamate/GABA Marker Deficit in the Adult Visual Cortex Following Optic Nerve Crush" International Journal of Molecular Sciences 22, no. 18: 10014. https://doi.org/10.3390/ijms221810014






