Junctional Modulation of Round Window Membrane Enhances Dexamethasone Uptake into the Inner Ear and Recovery after NIHL
Abstract
:1. Introduction
2. Results
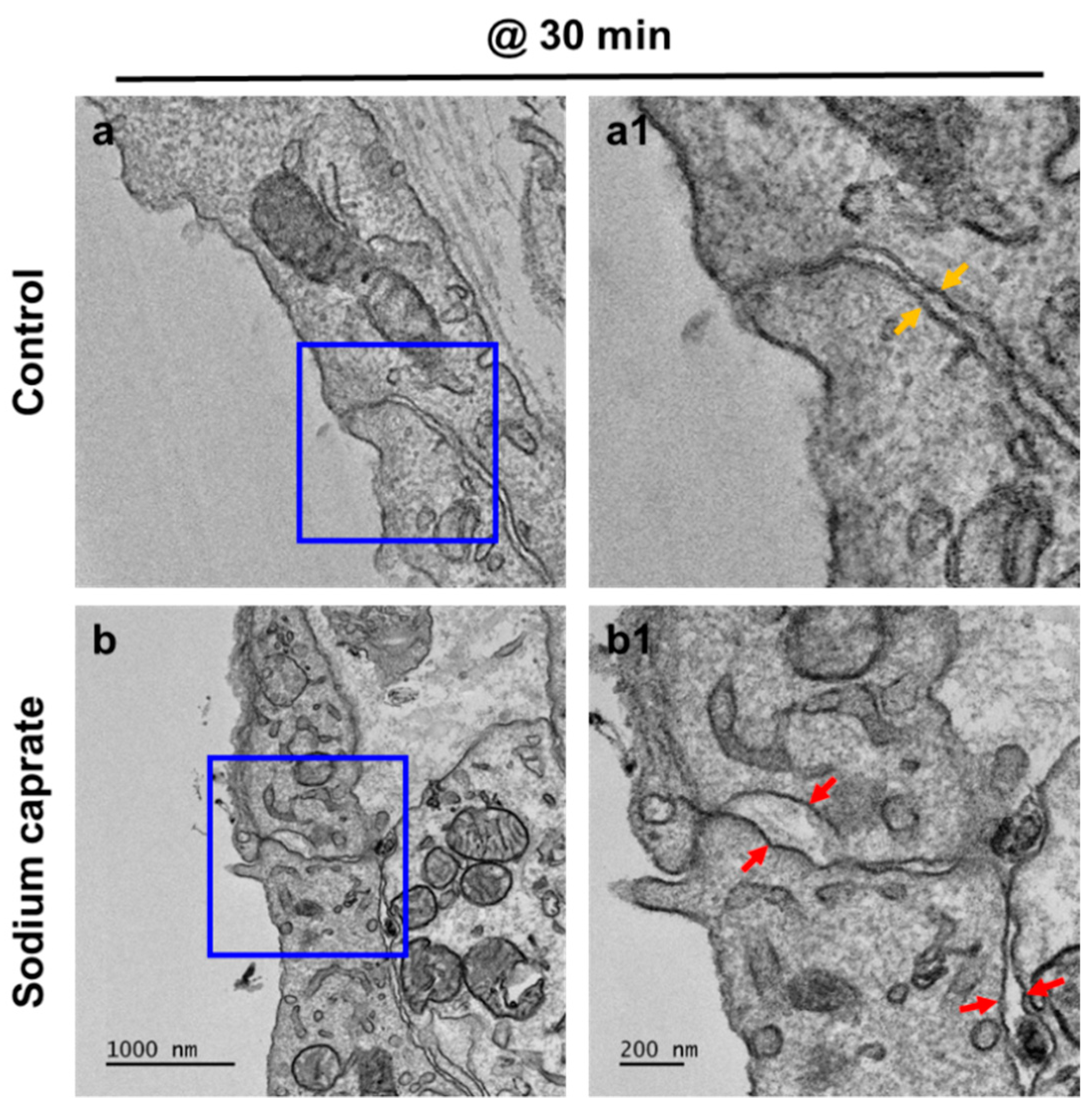
2.1. SC Modulates Cellular Junctions of Round Window Membrane
2.2. Sodium Caprate Enhances Dexamethasone Uptake into Cochlea and Perilymph
2.3. Sodium Caprate Does Not Induce Hearing Impairment Nor Hair Cell Death
2.4. Sodium Caprate Co-Treatment Hastens Recovery from Noise-Induced Hearing Loss
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Intratympanic Injection
4.3. Transmission Electronic Microscopy (TEM)
4.4. Immunostaining
4.5. Detailed Antibody Information Are as Follows
4.6. Cochlear Perilymph Collection and Dexamethasone Concentration Measurement
4.7. Noise Exposure
4.8. Auditory Brainstem Response (ABR)
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statements
Acknowledgments
Conflicts of Interest
References
- Goycoolea, M.V.; Lundman, L. Round window membrane. Structure function and permeability: A review. Microsc. Res. Tech. 1997, 36, 201–211. [Google Scholar] [CrossRef]
- Carpenter, A.M.; Muchow, D.; Goycoolea, M.V. Ultrastructural studies of the human round window membrane. Arch. Otolaryngol.-Head Neck Surg. 1989, 115, 585–590. [Google Scholar] [CrossRef]
- Duan, M.-l.; Zhi-Qiang, C. Permeability of round window membrane and its role for drug delivery: Our own findings and literature review. J. Otol. 2009, 4, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Goycoolea, M.V. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001, 121, 437–447. [Google Scholar] [CrossRef]
- Goycoolea, M.V.; Muchow, D.; Schachern, P. Experimental studies on round window structure: Function and permeability. Laryngoscope 1988, 98 Pt 2 (Suppl. S44), 1–20. [Google Scholar] [CrossRef]
- Nomura, Y. Otological significance of the round window. Adv. Oto-Rhino-Laryngol. 1984, 33, 1–162. [Google Scholar]
- Li, W.; Hartsock, J.J.; Dai, C.; Salt, A.N. Permeation Enhancers for Intratympanically-applied Drugs Studied Using Fluorescent Dexamethasone as a Marker. Otol. Neurotol. 2018, 39, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Szeto, B.; Chiang, H.; Valentini, C.; Yu, M.; Kysar, J.W.; Lalwani, A.K. Inner ear delivery: Challenges and opportunities. Laryngoscope Investig. Otolaryngol. 2020, 5, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, E.; Slinn, J.; Vinokourov, I.; Stanimirovic, D. Graded reversible opening of the rat blood–brain barrier by intracarotid infusion of sodium caprate. J. Neurosci. Methods 2008, 168, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.M.; Amasheh, M.; Dittmann, I.; Christoffel, I.; Fromm, M.; Amasheh, S. Sodium caprate as an enhancer of macromolecule permeation across tricellular tight junctions of intestinal cells. Biomater 2013, 34, 275–282. [Google Scholar] [CrossRef]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, K.L.; Stevenson, B.R.; Woo, P.L.; Firestone, G.L. Relationship of serine/threonine phosphorylation/dephosphorylation signaling to glucocorticoid regulation of tight junction permeability and ZO-1 distribution in nontransformed mammary epithelial cells. J. Biol. Chem. 1994, 269, 16108–16115. [Google Scholar] [CrossRef]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO–1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.H.; Wilson, K.F.; Ueda, Y.; Tung Wong, H.; Beyer, L.A.; Swiderski, D.L.; Dolan, D.F.; Raphael, Y. Conditioning the cochlea to facilitate survival and integration of exogenous cells into the auditory epithelium. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, R.M.; Shiffman, R.N.; Robertson, P. Clinical practice guideline development manual: A quality–driven approach for translating evidence into action. Otolaryngol.-Head Neck Surg. 2013, 148 (Suppl. S1), S1–S55. [Google Scholar] [CrossRef]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G. Clinical practice guideline: Sudden hearing loss (update). Otolaryngol.-Head Neck Surg. 2019, 161 (Suppl. S1), S1–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, A.R.; Kim, D.H.; Lee, S.H.; Shin, D.S.; Shin, S.A.; Park, Y.H. Effects of dexamethasone on intracochlear inflammation and residual hearing after cochleostomy: A comparison of administration routes. PLoS ONE 2018, 13, e0195230. [Google Scholar] [CrossRef] [PubMed]
- Paragliola, R.M.; Papi, G.; Pontecorvi, A.; Corsello, S.M. Treatment with Synthetic Glucocorticoids and the Hypothalamus–Pituitary-Adrenal Axis. Int. J. Mol. Sci. 2017, 18, 2201. [Google Scholar] [CrossRef] [PubMed]
- Meikle, A.W.; Clarke, D.H.; Tyler, F.H. Cushing syndrome from low doses of dexamethasone. A result of slow plasma clearance. JAMA 1976, 235, 1592–1593. [Google Scholar] [CrossRef]
- Fast, A.; Alon, M.; Weiss, S.; Zer–Aviv, F.R. Avascular necrosis of bone following short–term dexamethasone therapy for brain edema. Case report. J. Neurosurg. 1984, 61, 983–985. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Zweimueller-Mayer, J.; Steinbacher, P.; Lametschwandtner, A.; Bauer, H.C. The dual role of zonula occludens (ZO) proteins. J. Biomed. Biotechnol. 2010, 2010, 402–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibi, T.; Suzuki, T.; Nakashima, T. Perilymphatic concentration of gentamicin administered intratympanically in guinea pigs. Acta Oto-Laryngol. 2001, 121, 336–341. [Google Scholar]
- Chandrasekhar, S.S.; Rubinstein, R.Y.; Kwartler, J.A.; Gatz, M.; Connelly, P.E.; Huang, E.; Baredes, S. Dexamethasone pharmacokinetics in the inner ear: Comparison of route of administration and use of facilitating agents. Otolaryngol.-Head Neck Surg. 2000, 122, 521–528. [Google Scholar] [PubMed] [Green Version]
- Haynes, D.S.; O’Malley, M.; Cohen, S.; Watford, K.; Labadie, R.F. Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Laryngoscope 2007, 117, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salt, A.N.; Hartsock, J.; Plontke, S.; LeBel, C.; Piu, F. Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel–based formulation. Audiol. Neuro-Otol. 2011, 16, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Kakigi, A.; Salt, A.N.; Takeda, T. Effect of artificial endolymph injection into the cochlear duct on perilymph potassium. ORL J. Otorhinolaryngol. Relat. Spec. 2010, 71 (Suppl. S1), 16–18. [Google Scholar] [CrossRef] [Green Version]
- Salt, A.N.; Hartsock, J.J.; Gill, R.M.; Piu, F.; Plontke, S.K. Perilymph pharmacokinetics of markers and dexamethasone applied and sampled at the lateral semi–circular canal. J. Assoc. Res. Otolaryngol. 2012, 13, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.N.; Miller, J.M.; Altschuler, R.A.; Nuttall, A.L. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear. Res. 1993, 70, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Borkholder, D.A.; Zhu, X.; Hyatt, B.T.; Archilla, A.S.; Livingston, W.J., 3rd; Frisina, R.D. Murine intracochlear drug delivery: Reducing concentration gradients within the cochlea. Hear. Res. 2010, 268, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Douchement, D.; Terranti, A.; Lamblin, J.; Salleron, J.; Siepmann, F.; Siepmann, J.; Vincent, C. Dexamethasone eluting electrodes for cochlear implantation: Effect on residual hearing. Cochlear. Implant. Int. 2015, 16, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, M.; Jalessi, M.; Salehian, P.; Ghavi, F.F.; Emamjomeh, H.; Mirzadeh, H.; Imani, M.; Jolly, C. Dexamethasone eluting cochlear implant: Histological study in animal model. Cochlear. Implant. Int. 2013, 14, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, D.; Chambers, S.; Enke, Y.L.; Timbol, G.; Risi, F.; Miller, C.; Cowan, R.; Newbold, C. Development of a safe dexamethasone–eluting electrode array for cochlear implantation. Cochlear. Implant. Int. 2014, 15, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, L.; Simoni, E.; Giarbini, N.; Giordano, P.; Pannella, M.; Hatzopoulos, S.; Martini, A. Cochlear implant and inflammation reaction: Safety study of a new steroid–eluting electrode. Hear. Res. 2016, 336, 44–52. [Google Scholar] [CrossRef]
- Lyu, A.R.; Kim, T.H.; Park, S.J.; Shin, S.A.; Jeong, S.H.; Yu, Y.; Huh, Y.H.; Je, A.R.; Park, M.J.; Park, Y.H. Mitochondrial Damage and Necroptosis in Aging Cochlea. Int. J. Mol. Sci. 2020, 21, 2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-H.; Kim, Y.; Lyu, A.-R.; Shin, S.-A.; Kim, T.H.; Huh, Y.H.; Je, A.R.; Gajibhiye, A.; Yu, Y.; Jin, Y.; et al. Junctional Modulation of Round Window Membrane Enhances Dexamethasone Uptake into the Inner Ear and Recovery after NIHL. Int. J. Mol. Sci. 2021, 22, 10061. https://doi.org/10.3390/ijms221810061
Jeong S-H, Kim Y, Lyu A-R, Shin S-A, Kim TH, Huh YH, Je AR, Gajibhiye A, Yu Y, Jin Y, et al. Junctional Modulation of Round Window Membrane Enhances Dexamethasone Uptake into the Inner Ear and Recovery after NIHL. International Journal of Molecular Sciences. 2021; 22(18):10061. https://doi.org/10.3390/ijms221810061
Chicago/Turabian StyleJeong, Seong-Hun, Yoonjoong Kim, Ah-Ra Lyu, Sun-Ae Shin, Tae Hwan Kim, Yang Hoon Huh, A Reum Je, Akanksha Gajibhiye, Yang Yu, Yongde Jin, and et al. 2021. "Junctional Modulation of Round Window Membrane Enhances Dexamethasone Uptake into the Inner Ear and Recovery after NIHL" International Journal of Molecular Sciences 22, no. 18: 10061. https://doi.org/10.3390/ijms221810061





