Biomolecular Markers of Recurrent Implantation Failure—A Review
Abstract
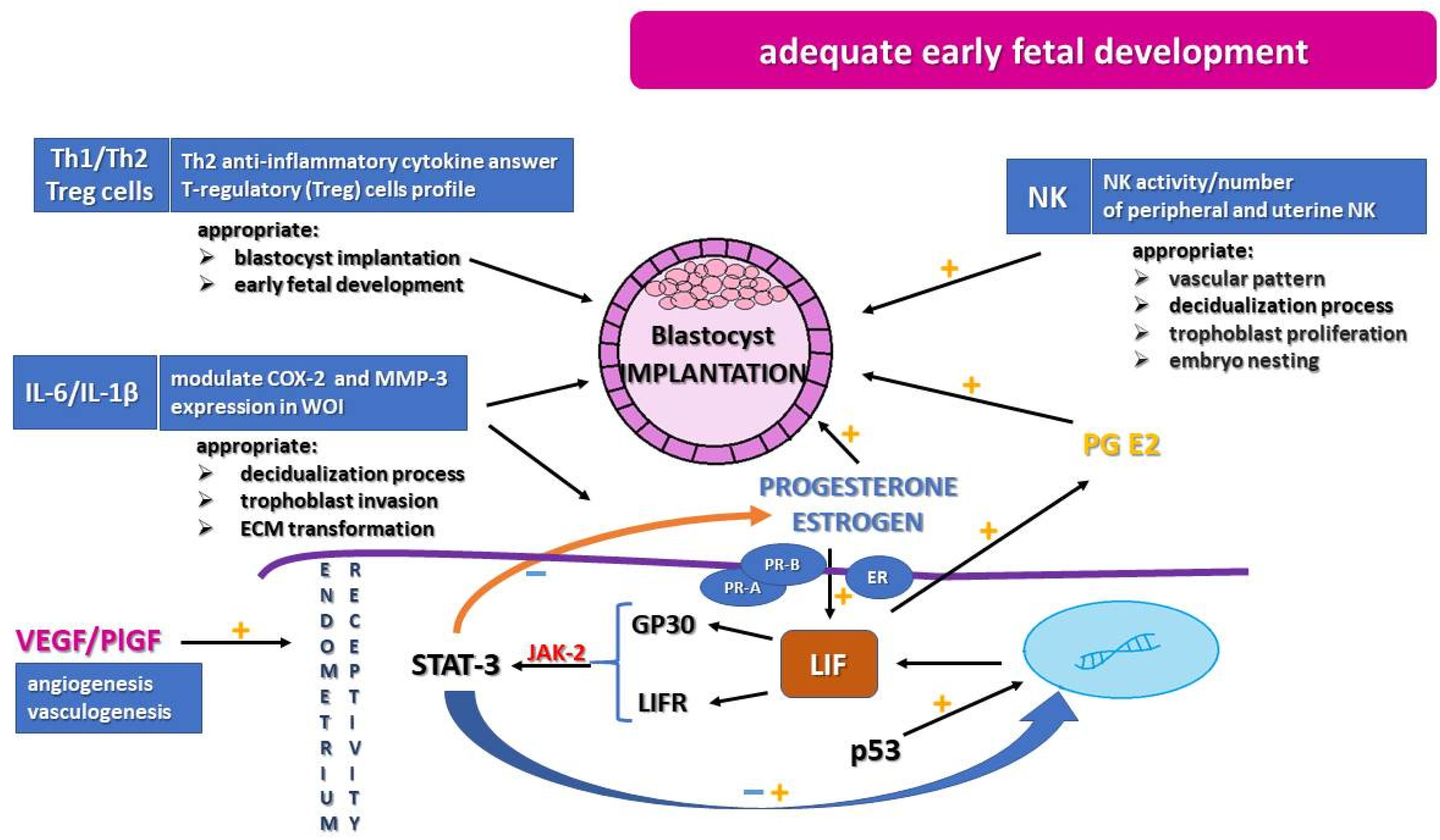
:1. Introduction
2. Immunological Profile
3. Leukemia Inhibitor Factor
4. Glycodelin-A
5. Progesterone, Estrogen, and Their Hormonal Receptors
6. Angiogenic Factors
7. Genetic Factors
8. Vaginal and Endometrial Microbiome (Microbiota) Disturbances
9. Treatment of RIF
10. Materials and Methods
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Zhang, Y.; Ma, X.; Jia, W.; Su, Y. Determining diagnostic criteria of unexplained recurrent implantation failure: A ret-rospective study of two vs. three or more implantation failure. Front. Endocrinol. 2021, 12, 619437. [Google Scholar] [CrossRef]
- Coughlan, C. What to do when good-quality embryos repeatedly fail to implant. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 53, 48–59. [Google Scholar] [CrossRef]
- Kim, J.O.; Ahn, E.H.; Sakong, J.H.; An, H.J.; Park, H.S.; Kim, Y.R.; Lee, J.R.; Lee, W.S.; Kim, N.K. Association of miR-27aA>G, miR-423C>a, miR-449bA>G, and miR-604A>G Polymorphisms with Risk of Recurrent Implantation Failure. Reprod. Sci. 2020, 27, 29–38. [Google Scholar] [CrossRef]
- Busnelli, A.; Reschini, M.; Cardellicchio, L.; Vegetti, W.; Somigliana, E.; Vercelllini, P. How common is real repeated implantation failure? An indirect estimate of the prevalence. Reprod. Biomed. Online 2020, 40, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Cimadomo, D.; Craciunas, L.; Vermeulen, N.; Vomstein, K.; Toth, B. Definition, diagnostic and therapeutic options in recurrent implantation failure: An international survey of clinicians and embryologists. Hum. Reprod. 2021, 36, 305–317. [Google Scholar] [CrossRef]
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T. Recurrent implantation failure: Definition and management. Reprod. Biomed. Online 2014, 28, 14–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef] [Green Version]
- Sanders, B. Uterine factors and infertility. J. Reprod. Med. 2006, 51, 169–176. [Google Scholar]
- Kwak-Kim, J.; Bao, S.; Lee, S.K.; Kim, J.W.; Gilman-Sachs, A. Immunological Modes of Pregnancy Loss: Inflammation, Immune Effectors, and Stress. Am. J. Reprod. Immunol. 2014, 72, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Santillán, I.; Lozano, I.; Illán, J.; Verdú, V.; Coca, S.; Bajo-Arenas, J.M.; Martínez-Pastor, F. Where and when should natural killer cells be tested in women with repeated implantation failure? J. Reprod. Immunol. 2015, 108, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lim, H.; Das, S.K.; Paria, B.C.; Dey, S.K. Dysregulation of EGF Family of Growth Factors and COX-2 in the Uterus during the Preattachment and Attachment Reactions of the Blastocyst with the Luminal Epithelium Correlates with Implantation Failure in LIF- Deficient Mice. Mol. Endocrinol. 2000, 14, 1147–1161. [Google Scholar] [CrossRef]
- Chen, J.R.; Cheng, J.-G.; Shatzer, T.; Sewell, L.; Hernandez, L.; Stewart, C.L. Leukemia Inhibitory Factor Can Substitute for Nidatory Estrogen and Is Essential to Inducing a Receptive Uterus for Implantation But Is Not Essential for Subsequent Embryogenesis1. Endocrinology 2000, 141, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Salleh, N. Diverse Roles of Prostaglandins in Blastocyst Implantation. Sci. World J. 2014, 2014, 968141. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-J.; Rosenwaks, Z. p53 and reproduction. Fertil. Steril. 2018, 109, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Focarelli, R.; Luddi, A.; De Leo, V.; Capaldo, A.; Stendardi, A.; Pavone, V.; Benincasa, L.; Belmonte, G.; Petraglia, F.; Piomboni, P. Dysregulation of GdA Expression in Endometrium of Women With Endometriosis: Implication for Endometrial Receptivity. Reprod. Sci. 2017, 25, 579–586. [Google Scholar] [CrossRef]
- Uchida, H.; Maruyama, T.; Nishikawa-Uchida, S.; Miyazaki, K.; Masuda, H.; Yoshimura, Y. Glycodelin in reproduction. Reprod. Med. Biol. 2013, 12, 79–84. [Google Scholar] [CrossRef]
- Lee, C.-L.; Lam, E.Y.F.; Lam, K.K.W.; Koistinen, H.; Seppälä, M.; Ng, E.H.Y.; Yeung, W.S.B.; Chiu, P.C.N. Glycodelin-A Stimulates Interleukin-6 Secretion by Human Monocytes and Macrophages through L-selectin and the Extracellular Signal-regulated Kinase Pathway. J. Biol. Chem. 2012, 287, 36999–37009. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Zhang, X.; Liang, Y.; Lin, S.; Qian, W.; Fan, S. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. MBio 2020, 11, e03242-19. [Google Scholar] [CrossRef]
- Moreno, I.; Simon, C. Relevance of assessing the uterine microbiota in infertility. Fertil. Steril. 2018, 110, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Liu, Z.; Chen, T. Role of Vaginal Microbiota Dysbiosis in Gynecological Diseases and the Potential Interventions. Front. Microbiol. 2021, 12, 643422. [Google Scholar] [CrossRef]
- Raziel, A.; Friedler, S.; Schachter, M.; Kasterstein, E.; Strassburger, D.; Ron-El, R. Increased frequency of female partner chromosomal abnormalities in patients with high-order implantation failure after in vitro fertilization. Fertil. Steril. 2002, 78, 515–519. [Google Scholar] [CrossRef]
- Voullaire, L.; Collins, V.; Callaghan, T.; McBain, J.; Williamson, R.; Wilton, L. High incidence of complex chromosome abnormality in cleavage embryos from patients with repeated implantation failure. Fertil. Steril. 2007, 87, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Agarwal, A.; Nelson, D.R.; Nada, E.; El-Tonsy, M.H.; Alvarez, J.G.; Thomas, A.J.; Sharma, R. Increased sperm nuclear DNA damage in normozoospermic infertile men: A prospective study. Fertil. Steril. 2002, 78, 313–318. [Google Scholar] [CrossRef]
- Nikolaeva, M.; Arefieva, A.; Babayan, A.; Chagovets, V.; Kitsilovskaya, N.; Starodubtseva, N.; Frankevich, V.; Kalinina, E.; Krechetova, L.; Sukhikh, G. Immunoendocrine Markers of Stress in Seminal Plasma at IVF/ICSI Failure: A Preliminary Study. Reprod. Sci. 2021, 28, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Azem, F.; Many, A.; Yovel, I.; Amit, A.; Lessing, J.B.; Kupferminc, M.J. Increased rates of thrombophilia in women with repeated IVF failures. Hum. Reprod. 2004, 19, 368–370. [Google Scholar] [CrossRef] [Green Version]
- Grandone, E.; Colaizzo, D.; Bue, A.L.; Checola, M.G.; Cittadini, E.; Margaglione, M. Inherited thrombophilia and in vitro fertilization implantation failure. Fertil. Steril. 2001, 76, 201–202. [Google Scholar] [CrossRef]
- Safdarian, L.; Najmi, Z.; Aleyasin, A.; Aghahosseini, M.; Rashidi, M.; Asadollah, S. Recurrent IVF failure and hereditary thrombophilia. Iran. J. Reprod. Med. 2014, 12, 467–470. [Google Scholar]
- Chen, C.H.; Lu, F.; Yang, W.J.; Yang, P.E.; Chen, W.M.; Kang, S.T.; Huang, Y.S.; Kao, Y.C.; Feng, C.T.; Chang, P.C.; et al. A novel platform for discovery of differentially expressed microRNAs in patients with repeated implantation failure. Fertil. Steril. 2021, 116, 181–188. [Google Scholar] [CrossRef]
- Zeng, H.; Hu, L.; Xie, H.; Ma, W.; Quan, S. Polymorphisms of vascular endothelial growth factor and recurrent implantation failure: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021, 304, 297–307. [Google Scholar] [CrossRef]
- Dumont, P.; Leu, J.I.-J.; Iii, A.C.D.P.; George, D.L.; Murphy, M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 2003, 33, 357–365. [Google Scholar] [CrossRef]
- Nowak, I.; Wilczyńska, K.; Radwan, P.; Wiśniewski, A.; Krasiński, R.; Radwan, M.; Wilczyński, J.R.; Malinowski, A.; Kuśnierczyk, P. Association of Soluble HLA-G Plasma Level and HLA-G Genetic Polymorphism With Pregnancy Outcome of Patients Undergoing in vitro Fertilization Embryo Transfer. Front. Immunol. 2020, 10, 2982. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Diao, L.; Lian, R.; Qi, L.; Yu, S.; Liu, S.; Lin, S.; Xue, Z.; Zeng, Y. Potential impact of maternal vitamin D status on peripheral blood and endometrium cellular immunity in women with recurrent implantation failure. Am. J. Reprod. Immunol. 2020, 84, e13243. [Google Scholar] [CrossRef]
- Schröder-Heurich, B.; Springer, C.J.P.; Von Versen-Höynck, F. Vitamin D Effects on the Immune System from Periconception through Pregnancy. Nutrients 2020, 12, 1432. [Google Scholar] [CrossRef]
- Makrigiannakis, A.; Makrygiannakis, F.; Vrekoussis, T. Approaches to Improve Endometrial Receptivity in Case of Repeated Implantation Failures. Front. Cell Dev. Biol. 2021, 9, 613277. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zheng, L.; Zhao, D.; Xu, Y.; Wang, Y. The Role of Immune Cells in Recurrent Spontaneous Abortion. Reprod. Sci. 2021, 28, 1–13. [Google Scholar] [CrossRef]
- Ticconi, C.; Pietropolli, A.; Di Simone, N.; Piccione, E.; Fazleabas, A. Endometrial Immune Dysfunction in Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2019, 20, 5332. [Google Scholar] [CrossRef] [Green Version]
- Tsao, F.-Y.; Wu, M.-Y.; Chang, Y.-L.; Wu, C.-T.; Ho, H.-N. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J. Formos. Med. Assoc. 2018, 117, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Nagamatsu, T.; Schust, D.J. The Contribution of Macrophages to Normal and Pathological Pregnancies. Am. J. Reprod. Immunol. 2010, 63, 460–471. [Google Scholar] [CrossRef]
- Mori, A.; Nishi, H.; Sasaki, T.; Nagamitsu, Y.; Kawaguchi, R.; Okamoto, A.; Kuroda, M.; Isaka, K. HLA-G expression is regulated by miR-365 in trophoblasts under hypoxic conditions. Placenta 2016, 45, 37–41. [Google Scholar] [CrossRef]
- Donoghue, J.; Paiva, P.; Teh, W.T.; Cann, L.M.; Nowell, C.; Rees, H.; Bittinger, S.; Obers, V.; Bulmer, J.N.; Stern, C.; et al. Endometrial uNK cell counts do not predict successful implantation in an IVF population. Hum. Reprod. 2019, 34, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.B.; Jeelall, Y.; Pennell, C.; Hart, R.; McLean-Tooke, A.; Lucas, M. The role of immunological testing and intervention in reproductive medicine: A fertile collaboration? Am. J. Reprod. Immunol. 2018, 79, e12784. [Google Scholar] [CrossRef]
- Perrier d’Hauterive, S.; Charlet-Renard, C.; Berndt, S.; Dubois, M.; Munaut, C.; Gon, F.; Hagelstein, M.T.; Noel, A.; Hazout, A.; Foidart, J.M.; et al. Human chorionic gonadotropin and growth factors at the embryonic-endometrial interface control leukemia inhibitory factor (LIF) and interleukin 6 (IL-6) secretion by human endometrial epithelium. Hum. Reprod. 2004, 19, 2633–2643. [Google Scholar] [CrossRef] [Green Version]
- Achache, H.; Revel, A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Updat. 2006, 12, 731–746. [Google Scholar] [CrossRef] [Green Version]
- Strakova, Z.; Mavrogianis, P.; Meng, X.; Hastings, J.M.; Jackson, K.S.; Cameo, P.; Brudney, A.; Knight, O.; Fazleabas, A.T. In vivo infusion of interleukin-1beta and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology 2005, 146, 4097–4104. [Google Scholar] [CrossRef] [Green Version]
- Qiong, Z.; Jie, H.; Yonggang, W.; Bin, X.; Jing, Z.; Yanping, L. Clinical validation of pinopode as a marker of endometrial receptivity: A randomized controlled trial. Fertil. Steril. 2017, 108, 513–517.e2. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Wu, L.; Hu, W. The Regulation of Leukemia Inhibitory Factor. Cancer Cell Microenviron. 2015, 2, 877. [Google Scholar] [CrossRef]
- Stewart, C.L.; Kaspar, P.; Brunet, L.J.; Bhatt, H.; Gadi, I.; Köntgen, F.; Abbondanzo, S.J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992, 359, 76–79. [Google Scholar] [CrossRef]
- Salleh, N.; Giribabu, N. Leukemia Inhibitory Factor: Roles in Embryo Implantation and in Nonhormonal Contraception. Sci. World J. 2014, 2014, 201514. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Feng, Z.; Teresky, A.K.; Levine, A.J. p53 regulates maternal reproduction through LIF. Nat. Cell Biol. 2007, 450, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Hirota, Y.; Fukui, Y.; Fujita, H.; Saito-Fujita, T.; Kaku, T.; Gebril, M.; Hirata, T.; Akaeda, S.; Hiraoka, T.; et al. Levonorgestrel Inhibits Embryo Attachment by Eliminating Uterine Induction of Leukemia Inhibitory Factor. Endocrinology 2019, 161, bqz005. [Google Scholar] [CrossRef]
- Cheng, J.-G.; Chen, J.R.; Hernandez, L.; Alvord, W.G.; Stewart, C.L. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc. Natl. Acad. Sci. USA 2001, 98, 8680–8685. [Google Scholar] [CrossRef] [Green Version]
- Hiraoka, T.; Hirota, Y.; Fukui, Y.; Gebril, M.; Kaku, T.; Aikawa, S.; Hirata, T.; Akaeda, S.; Matsuo, M.; Haraguchi, H.; et al. Differential roles of uterine epithelial and stromal STAT3 coordinate uterine receptivity and embryo attachment. Sci. Rep. 2020, 10, 15523. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, H.-R.; Lim, E.J.; Park, M.; Yoon, J.A.; Kim, Y.S.; Kim, E.-K.; Shin, J.-E.; Kim, J.H.; Kwon, H.; et al. Integrative Analyses of Uterine Transcriptome and MicroRNAome Reveal Compromised LIF-STAT3 Signaling and Progesterone Response in the Endometrium of Patients with Recurrent/Repeated Implantation Failure (RIF). PLoS ONE 2016, 11, e0157696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Bartos, A.; Whitsett, J.A.; Dey, S.K. Uterine Deletion ofGp130orStat3Shows Implantation Failure with Increased Estrogenic Responses. Mol. Endocrinol. 2013, 27, 1492–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Ahn, E.; Park, H.; Kim, J.; Kim, Y.; Lee, W.; Kim, N. Association between HOX Transcript Antisense RNA Single-Nucleotide Variants and Recurrent Implantation Failure. Int. J. Mol. Sci. 2021, 22, 3021. [Google Scholar] [CrossRef]
- So, K.; Lee, C.-L.; Yeung, W.S.; Lee, K.-F. Glycodelin suppresses endometrial cell migration and invasion but stimulates spheroid attachment. Reprod. Biomed. Online 2012, 24, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.L.; Chiu, P.C.; Lam, K.K.; Chan, R.W.; Chu, I.K.; Koistinen, R.; Koistinen, H.; Seppälä, M.; Lee, K.F.; Yeung, W.S. Glycodelin-A modulates cytokine production of peripheral blood natural killer cells. Fertil. Steril. 2010, 94, 769–771. [Google Scholar] [CrossRef]
- Lee, C.-L.; Lam, K.K.; Koistinen, H.; Seppala, M.; Kurpisz, M.; Fernandez, N.; Pang, R.T.; Yeung, W.S.; Chiu, C.N. Glycodelin-A as a paracrine regulator in early pregnancy. J. Reprod. Immunol. 2011, 90, 29–34. [Google Scholar] [CrossRef]
- Dixit, A.; Karande, A.A. Glycodelin regulates the numbers and function of peripheral natural killer cells. J. Reprod. Immunol. 2020, 137, 102625. [Google Scholar] [CrossRef]
- Lee, C.-L.; Lam, K.K.W.; Vijayan, M.; Koistinen, H.; Seppala, M.; Ng, E.; Yeung, S.B.W.; Chiu, C.N. The Pleiotropic Effect of Glycodelin-A in Early Pregnancy. Am. J. Reprod. Immunol. 2016, 75, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Scholz, C.; Toth, B.; Brunnhuber, R.; Rampf, E.; Weissenbacher, T.; Santoso, L.; Friese, K.; Jeschke, U. Glycodelin-A induces a tolerogenic phenotype in monocyte-derived dendritic cells in vitro. Am. J. Reprod. Immunol. 2008, 60, 501–512. [Google Scholar] [CrossRef]
- Bastu, E.; Mutlu, M.F.; Yasa, C.; Dural, O.; Aytan, A.N.; Celik, C.; Buyru, F.; Yeh, J. Role of Mucin 1 and Glycodelin A in recurrent implantation failure. Fertil. Steril. 2015, 103, 1059–1064.e2. [Google Scholar] [CrossRef]
- Prapas, Y.; Prapas, N.; Jones, E.E.; Duleba, A.J.; Olive, D.L.; Chatziparasidou, A.; Vlassis, G. The window for embryo transfer in oocyte donation cycles depends on the duration of progesterone therapy. Hum. Reprod. 1998, 13, 720–723. [Google Scholar] [CrossRef] [Green Version]
- Klonos, E.; Katopodis, P.; Karteris, E.; Papanikolaou, E.; Tarlatzis, B.; Pados, G. Endometrial changes in estrogen and progesterone receptor expression during implantation in an oocyte donation program. Exp. Ther. Med. 2020, 20, 178. [Google Scholar] [CrossRef] [PubMed]
- Mulac-Jericevic, B.; Lydon, J.P.; DeMayo, F.; Conneely, O.M. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. USA 2003, 100, 9744–9749. [Google Scholar] [CrossRef] [Green Version]
- Long, N.; Liu, N.; Liu, X.; Li, J.; Cai, B.; Cai, X. Endometrial expression of telomerase, progesterone, and estrogen receptors during the implantation window in patients with recurrent implantation failure. Genet. Mol. Res. 2016, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Vagnini, L.D.; Renzi, A.; Petersen, B.; Canas, M.D.C.T.; Petersen, C.G.; Mauri, A.L.; Mattila, M.C.; Ricci, J.; Dieamant, F.; Oliveira, J.B.A.; et al. Association between estrogen receptor 1 (ESR1) and leukemia inhibitory factor (LIF) polymorphisms can help in the prediction of recurrent implantation failure. Fertil. Steril. 2019, 111, 527–534. [Google Scholar] [CrossRef]
- Suh, E.-K.; Yang, A.; Kettenbach, A.; Bamberger, T.C.; Michaelis, A.H.; Zhu, Z.; Elvin, J.A.; Bronson, R.T.; Crum, C.P.; McKeon, F. p63 protects the female germ line during meiotic arrest. Nat. Cell Biol. 2006, 444, 624–628. [Google Scholar] [CrossRef]
- Ravi, R.; Mookerjee, B.; Bhujwalla, Z.M.; Sutter, C.H.; Artemov, D.; Zeng, Q.; Dillehay, L.E.; Madan, A.; Semenza, G.L.; Bedi, A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genome Res. 2000, 14, 34–44. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC316350/ (accessed on 15 August 2021).
- Guo, X.; Yi, H.; Li, T.; Wang, Y.; Wang, H.; Chen, X. Role of Vascular Endothelial Growth Factor (VEGF) in Human Embryo Implantation: Clinical Implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Okada, H.; Okamoto, R.; Tsuzuki, T.; Tsuji, S.; Yasuda, K.; Kanzaki, H. Progestins inhibit estradiol-induced vascular endothelial growth factor and stromal cell–derived factor 1 in human endometrial stromal cells. Fertil. Steril. 2011, 96, 786–791. [Google Scholar] [CrossRef]
- Selvaraj, S.K.; Giri, R.K.; Perelman, N.; Johnson, C.; Malik, P.; Kalra, V.K. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood 2003, 102, 1515–1524. [Google Scholar] [CrossRef] [Green Version]
- Tayade, C.; Hilchie, D.; He, H.; Fang, Y.; Moons, L.; Carmeliet, P.; Foster, R.A.; Croy, B.A. Genetic Deletion of Placenta Growth Factor in Mice Alters Uterine NK Cells. J. Immunol. 2007, 178, 4267–4275. [Google Scholar] [CrossRef]
- Lash, G.E.; Innes, B.A.; Drury, J.A.; Robson, S.C.; Quenby, S.; Bulmer, J.N. Localization of angiogenic growth factors and their receptors in the human endometrium throughout the menstrual cycle and in recurrent miscarriage. Hum. Reprod. 2012, 27, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Sadekova, O.N.; Nikitina, L.A.; Rashidov, T.N.; Voloschuk, I.N.; Samokhodskaya, L.M.; Demidova, E.M.; Tkachuk, V.A. Luteal phase defect is associated with impaired VEGF mRNA expression in the secretory phase endometrium. Reprod. Biol. 2015, 15, 65–68. [Google Scholar] [CrossRef]
- Benkhalifa, M.; Zidi, W.; Bahri, H.; Mahjoub, S.; Boudhraa, K.; Sanhaji, H.; Khorsi-Cauet, H.; Feki, M.; Benkhalifa, M.; Allal-Elasmi, M. Circulating MMP-7 and VEGF as potential predictive biomarkers for recurrent implantation failures. Zygote 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Chen, X.; Man, G.C.W.; Liu, Y.; Wu, F.; Huang, J.; Li, T.C.; Wang, C.C. Physiological and pathological angiogenesis in endometrium at the time of embryo implantation. Am. J. Reprod. Immunol. 2017, 78, e12693. [Google Scholar] [CrossRef]
- Bansal, R.; Ford, B.; Bhaskaran, S.; Thum, M.; Bansal, A. Elevated Levels of Serum Vascular Endothelial Growth Factor-A Are Not Related to NK Cell Parameters in Recurrent IVF Failure. J. Reprod. Infertil. 2017, 18, 280–287. [Google Scholar]
- Van Bergen, T.; Etienne, I.; Cunningham, F.; Moons, L.; Schlingemann, R.O.; Feyen, J.H.; Stitt, A.W. The role of placental growth factor (PlGF) and its receptor system in retinal vascular diseases. Prog. Retin. Eye Res. 2019, 69, 116–136. [Google Scholar] [CrossRef]
- Albonici, L.; Benvenuto, M.; Focaccetti, C.; Cifaldi, L.; Miele, M.T.; Limana, F.; Manzari, V.; Bei, R. PlGF Immunological Impact during Pregnancy. Int. J. Mol. Sci. 2020, 21, 8714. [Google Scholar] [CrossRef]
- Ukah, U.V.; Payne, B.A.; Hutcheon, J.A.; Chappell, L.; Seed, P.T.; Conti-Ramsden, F.I.; Ansermino, J.M.; Magee, L.A.; Von Dadelszen, P. Placental growth factor for the prognosis of women with preeclampsia (fullPIERS model extension): Context matters. BMC Pregnancy Childbirth 2020, 20, 668. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Charnock-Jones, D.S.; Zhang, E.; Hiby, S.; Malik, S.; Day, K.; Licence, D.; Bowen, J.M.; Gardner, L.; King, A.; et al. Angiogenic Growth Factor Messenger Ribonucleic Acids in Uterine Natural Killer Cells1. J. Clin. Endocrinol. Metab. 2001, 86, 1823–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rah, H.; Chung, K.W.; Ko, K.H.; Kim, E.S.; Kim, J.O.; Sakong, J.H.; Kim, J.H.; Lee, W.S.; Kim, N.K. miR-27a and miR-449b polymorphisms associated with a risk of idiopathic recurrent pregnancy loss. PLoS ONE 2017, 12, e0177160. [Google Scholar] [CrossRef] [PubMed]
- Qublan, H.S.; Eid, S.S.; Ababneh, H.A.; Amarin, Z.O.; Smadi, A.Z.; Al-Khafaji, F.F.; Khader, Y. Acquired and inherited thrombophilia: Implication in recurrent IVF and embryo transfer failure. Hum. Reprod. 2006, 21, 2694–2698. [Google Scholar] [CrossRef] [Green Version]
- Simur, A.; Ozdemir, S.; Acar, H.; Colakoğlu, M.C.; Görkemli, H.; Balci, O.; Nergis, S. Repeated in vitro Fertilization Failure and Its Relation with Thrombophilia. Gynecol. Obstet. Investig. 2009, 67, 109–112. [Google Scholar] [CrossRef]
- Zeng, H.; He, D.; Zhao, Y.; Liu, N.G.; Xie, H. Association between MTHFR polymorphisms (MTHFR C677T, MTHFR A1298C) and recurrent implantation failure: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021, 303, 1089–1098. [Google Scholar] [CrossRef]
- Bautch, V.L. VEGF-Directed Blood Vessel Patterning: From Cells to Organism. Cold Spring Harb. Perspect. Med. 2012, 2, a006452. [Google Scholar] [CrossRef] [Green Version]
- Bond, G.L.; Hu, W.; Bond, E.E.; Robins, H.; Lutzker, S.G.; Arva, N.C.; Bargonetti, J.; Bartel, F.; Taubert, H.; Wuerl, P.; et al. A Single Nucleotide Polymorphism in the MDM2 Promoter Attenuates the p53 Tumor Suppressor Pathway and Accelerates Tumor Formation in Humans. Cell 2004, 119, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-J.; Feng, Z.; Sun, Y.; Atwal, G.; Murphy, M.; Rebbeck, T.R.; Rosenwaks, Z.; Levine, A.J.; Hu, W. Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 9761–9766. [Google Scholar] [CrossRef] [Green Version]
- Lledó, B.; Turienzo, A.; Ortiz, J.A.; Morales, R.; Ten, J.; Llacer, J.; Bernabeu, R. Negative effect of P72 polymorphism on p53 gene in IVF outcome in patients with repeated implantation failure and pregnancy loss. J. Assist. Reprod. Genet. 2013, 31, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadeh, M.; Ghorbian, S.; Nouri, M. Evaluation of clinical utility of P53 gene variations in repeated implantation failure. Mol. Biol. Rep. 2019, 46, 2885–2891. [Google Scholar] [CrossRef]
- Turienzo, A.; Lledó, B.; Ortiz, J.A.; Morales, R.; Sanz, J.; Llácer, J.; Bernabeu, R. Prevalence of candidate single nucleotide polymorphisms on p53, IL-11, IL-10, VEGF and APOE in patients with repeated implantation failure (RIF) and pregnancy loss (RPL). Hum. Fertil. 2018, 23, 117–122. [Google Scholar] [CrossRef]
- Fan, W.; Huang, Z.; Li, S.; Xiao, Z. The HLA-G 14-bp polymorphism and recurrent implantation failure: A meta-analysis. J. Assist. Reprod. Genet. 2017, 34, 1559–1565. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [Green Version]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenmakers, S.; Laven, J. The vaginal microbiome as a tool to predict IVF success. Curr. Opin. Obstet. Gynecol. 2020, 32, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadieux, P.A.; Burton, J.; Devillard, E.; Reid, G. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J. Physiol. Pharmacol. 2009, 60, 13–18. [Google Scholar]
- Alakomi, H.-L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- Aldunate, M.; Tyssen, D.; Johnson, A.; Zakir, T.; Sonza, S.; Moench, T.; Cone, R.; Tachedjian, G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013, 68, 2015–2025. [Google Scholar] [CrossRef]
- Brotman, R.M. Vaginal microbiome and sexually transmitted infections: An epidemiologic perspective. J. Clin. Investig. 2011, 121, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Chase, D.; Goulder, A.; Zenhausern, F.; Monk, B.; Herbst-Kralovetz, M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: A review of applications in etiology, symptoms and treatment. Gynecol. Oncol. 2015, 138, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Hyman, R.W.; Herndon, C.N.; Jiang, H.; Palm, C.; Fukushima, M.; Bernstein, D.; Vo, K.C.; Zelenko, Z.; Davis, R.W.; Giudice, L.C. The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J. Assist. Reprod. Genet. 2012, 29, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [Green Version]
- Punzón-Jiménez, P.; Labarta, E. The impact of the female genital tract microbiome in women health and reproduction: A review. J. Assist. Reprod. Genet. 2021, 38, 1–23. [Google Scholar] [CrossRef]
- Ahmadi, M.; Abdolmohammadi-Vahid, S.; Ghaebi, M.; Aghebati-Maleki, L.; Dolati, S.; Farzadi, L.; Ghasemzadeh, A.; Hamdi, K.; Younesi, V.; Nouri, M.; et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst. Biol. Reprod. Med. 2017, 63, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Sung, N.; Han, A.R.; Park, C.W.; Park, D.W.; Park, J.C.; Kim, N.Y.; Lim, K.S.; Shin, J.E.; Joo, C.W.; Lee, S.E.; et al. Intravenous immunoglobulin G in women with reproductive failure: The Korean Society for Reproductive Immunology practice guidelines. Clin. Exp. Reprod. Med. 2017, 44, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kolanska, K.; Bendifallah, S.; Cohen, J.; Placais, L.; Selleret, L.; Johanet, C.; Suner, L.; Delhommeau, F.; Chabbert-Buffet, N.; Darai, E.; et al. Unexplained recurrent implantation failures: Predictive factors of pregnancy and therapeutic management from a French multicentre study. J. Reprod. Immunol. 2021, 145, 103313. [Google Scholar] [CrossRef] [PubMed]
- Sadeghpour, S.; Berenji, M.G.; Nazarian, H.; Ghasemnejad, T.; Nematollahi, M.H.; Abroon, S.; Paktinat, S.; Khoei, H.H.; Berenji, H.G.; Novin, M.G. Effects of treatment with hydroxychloroquine on the modulation of Th17/Treg ratio and pregnancy outcomes in women with recurrent implantation failure: Clinical trial. Immunopharmacol. Immunotoxicol. 2020, 42, 632–642. [Google Scholar] [CrossRef]
- Shapiro, B.S.; Daneshmand, S.T.; Desai, J.; Garner, F.C.; Aguirre, M.; Hudson, C. The risk of embryo-endometrium asynchrony increases with maternal age after ovarian stimulation and IVF. Reprod. Biomed. Online 2016, 33, 50–55. [Google Scholar] [CrossRef] [Green Version]
- National Centers for Disease Control and Prevention and Health Promotion of the Centers for Disease Control and Prevention in Consultation with the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Assisted Reproductive Technology National Summary Report; US Departmentof Health and Human Services: Atlanta, GA, USA, 2017.
- Orvieto, R.; Meltcer, S.; Nahum, R.; Rabinson, J.; Anteby, E.Y.; Ashkenazi, J. The influence of body mass index on in vitro fertilization outcome. Int. J. Gynecol. Obstet. 2008, 104, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Moragianni, V.A.; Jones, S.-M.L.; Ryley, D.A. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil. Steril. 2012, 98, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Waylen, A.; Metwally, M.; Jones, G.; Wilkinson, A.; Ledger, W. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: A meta-analysis. Hum. Reprod. Updat. 2008, 15, 31–44. [Google Scholar] [CrossRef]
- Anblagan, D.; Jones, N.W.; Costigan, C.; Parker, A.J.J.; Allcock, K.; Aleong, R.; Coyne, L.H.; Deshpande, R.; Raine-Fenning, N.; Bugg, G.; et al. Maternal Smoking during Pregnancy and Fetal Organ Growth: A Magnetic Resonance Imaging Study. PLoS ONE 2013, 8, e67223. [Google Scholar] [CrossRef]
- Künzle, R.; Mueller, M.; Hänggi, W.; Birkhäuser, M.H.; Drescher, H.; Bersinger, N.A. Semen quality of male smokers and nonsmokers in infertile couples. Fertil. Steril. 2003, 79, 287–291. [Google Scholar] [CrossRef]
- Nepomnaschy, P.A.; Welch, K.B.; McConnell, D.S.; Low, B.S.; Strassmann, B.I.; England, B.G. Cortisol levels and very early pregnancy loss in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 3938–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paskulin, D.D.; Paixão-Côrtes, V.R.; Hainaut, P.; Bortolini, M.C.; Ashton-Prolla, P. The TP53 fertility network. Genet. Mol. Biol. 2012, 35, 939–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Risk Factors of RIF | References | |
|---|---|---|
| Uterine | anatomical abnormalities (bicornal uterus, uterine septa, myomas, endometrial polyps, intrauterine adhesions) | [8] |
| immunological (HLA, NK cells, TK cells) | [9,10] | |
| biomolecular (p53, LIF) | [11,12,13,14] | |
| glycodelin-A | [15,16,17] | |
| infection | [18,19,20] | |
| Embryo | genetic abnormalities | [21,22] |
| Male | sperm quality | [23,24] |
| Female | thrombophilia, inherited and acquired | [25,26,27] |
| other genetic polymorphisms (miRNA, HLA-G, p53, VEGF) | [28,29,30,31] | |
| vitamin D deficiency | [32,33] | |
| Risk Factor | Pathophysiological Processes in Relation to RIF Development | References |
|---|---|---|
| Environmental Risk Factor | ||
| Maternal age | chromosomal abnormalities—aneuploidy, polyploidy, mosaicism, translocations, inversions/deletions | [111,112] |
| BMI (>25 kg/m2) | disturbances in gonadotropin secretion | [113,114] |
| Tobacco intake | lower estradiol level during ovarian stimulation disturbances of corpus luteum formation and embryo implantation reduction in oxygen supply to the fetus (carbon monoxide) vasoconstriction and decreased delivery of nutrients to the fetus (nicotine) significantly decreased sperm count | [115,116,117] |
| Stress | elevated cortisol level (stress hormone) | [118] |
| Pathophysiological Risk Factors | ||
| Th1/Th2 imbalance | elevated number and activity of peripheral and uterine NK cells elevated activity of Th1 cells and elevated levels of cytokines produced by Th1 cells (TNF-α)—inflammatory promoting, trophoblastic growth suppression, thrombotic responses in maternal uterine blood vessels, embryo rejection | [9,41,80] |
| Infection | chronic endometritis—Escherichia coli, Enterococcus faecalis, group B Streptococcus, Mycoplasma, Chlamydia | [18,19,77,105] |
| Inherited thrombophilia | carrying of factor V Leiden mutation, deficiency of MTHFR, PTM and ATIII | [27,84,85,86] |
| Molecules expression | LIF—decreased level leads to influence the reproductive capacity VEGF—reduced expression in peri-implantation period in RIF PlGF—decreasing concentration in RIF | [46,50,70,74,76] |
| Genetics factors | microRNAs—function modulators, control the expression of genes involved in peri-implantation period | [28,83] |
| factor V Leiden mutation, MTHFR mutation—the role in IVF–embryo transfer and implantation failure | [27,84] | |
| p53—genetic polymorphisms modulate the biological activity of protein p53, leading to lower rate of implantation success | [14,30,87,88,89,90,91,92,119] | |
| HLA-G—genetic polymorphisms modulate immune system influencing fertilization process | [14,31] | |
| VEGF—genetic polymorphisms involved in angiogenesis process, support the successful implantation | [29,92] | |
| ESR1—genetic polymorphisms associated with the implantation process | [67] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrozikiewicz, A.E.; Ożarowski, M.; Jędrzejczak, P. Biomolecular Markers of Recurrent Implantation Failure—A Review. Int. J. Mol. Sci. 2021, 22, 10082. https://doi.org/10.3390/ijms221810082
Mrozikiewicz AE, Ożarowski M, Jędrzejczak P. Biomolecular Markers of Recurrent Implantation Failure—A Review. International Journal of Molecular Sciences. 2021; 22(18):10082. https://doi.org/10.3390/ijms221810082
Chicago/Turabian StyleMrozikiewicz, Aleksandra E., Marcin Ożarowski, and Piotr Jędrzejczak. 2021. "Biomolecular Markers of Recurrent Implantation Failure—A Review" International Journal of Molecular Sciences 22, no. 18: 10082. https://doi.org/10.3390/ijms221810082
APA StyleMrozikiewicz, A. E., Ożarowski, M., & Jędrzejczak, P. (2021). Biomolecular Markers of Recurrent Implantation Failure—A Review. International Journal of Molecular Sciences, 22(18), 10082. https://doi.org/10.3390/ijms221810082







