Advances in the Genetically Engineered KillerRed for Photodynamic Therapy Applications
Abstract
:1. Introduction
2. Basic Features of KillerRed
2.1. Structure and Property of KillerRed
2.2. Illumination Factors of KillerRed
3. Applications of KillerRed as an Endogenous Photosensitizer
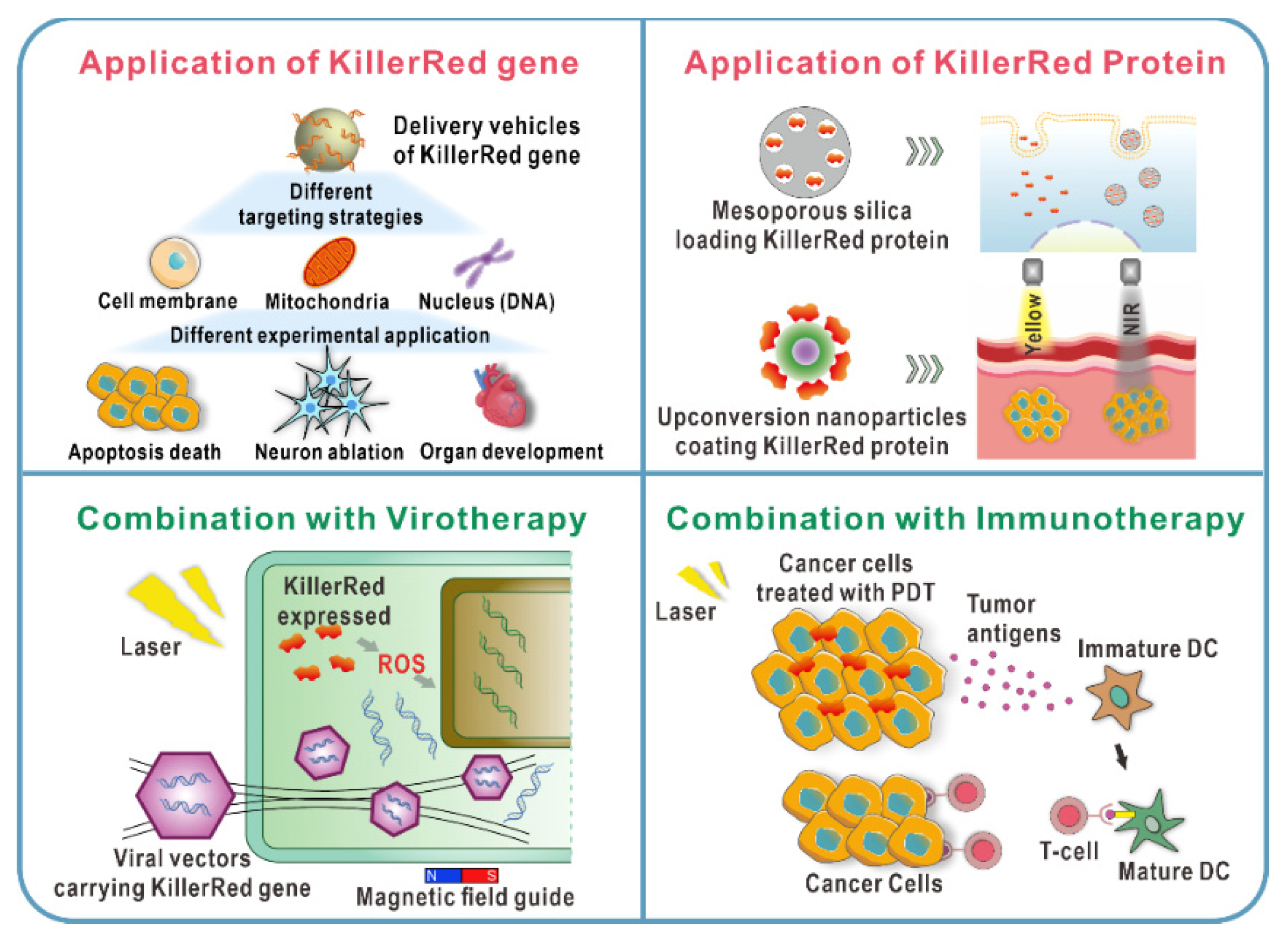
3.1. Distinct Targeting Strategies of KillerRed In Vitro
3.1.1. Membrane-Targeted KillerRed
3.1.2. Mitochondria-Targeted KillerRed
3.1.3. Nucleus-Targeted KillerRed
3.2. Diverse Delivery Strategies of KillerRed Gene In Vivo
3.2.1. KillerRed Gene Delivery Based on Viral Vectors
3.2.2. KillerRed Gene Delivery Based on Non-Viral Vectors
4. Applications of KillerRed Protein as an Exogenous Photosensitizer
4.1. KillerRed Protein Delivery Based on Inorganic Nanoparticles
4.1.1. Mesoporous Silica Nanoparticles (MSNs)
4.1.2. Upconversion Nanoparticles (UCNPs)
4.2. KillerRed Protein Delivery Based on Lipo/Membrane Nanocarriers
5. Comprehensive Therapy
5.1. KillerRed-Mediated PDT Combined with Virotherapy
5.2. KillerRed-Mediated PDT Combined with Immunotherapy
6. Conclusions
- (1)
- Systemic injection and local drug delivery are both important modes of administration. Of course, directly intertumoral injection of KillerRed vectors is also retained. Endogenous synthesis of KillerRed protein within the tumor can avoid side effects of off-target organs and maximize the efficiency of the therapy at the lesion location. Moreover, systemic administration offers unique advantages in the therapeutic process against tumor metastasis or deep tumor. Designing nanoscale drug delivery systems with tumor-targeting capability, controlled-release behavior, and responsiveness to the tumor microenvironment may be a good choice to efficiently utilize the biological function of KillerRed.
- (2)
- Poor tissue penetration limits therapeutic efficacy and applicability of conventional PDT in the clinic. Combined with UCNP, KillerRed can be excited efficiently and achieve fluorescence imaging under NIR laser irradiation (expanding the light penetration depth to ≈1 cm). Meanwhile, the potential use of UCNP in UCL optical imaging, MRI, and CT achieve multimodal imaging guidance to provide precise structural information for unknown primary or metastatic tumor location, which finally achieve effective anticancer.
- (3)
- Compared to monotherapy, photodynamic combination therapy, which is used for the majority of cancers, often yields better results. ROS generated by PDT can activate an acute inflammatory response, increase tumor immune prototype, promote drug delivery, and heighten local cytotoxicity to improve the efficacy of immunotherapy and chemotherapy. Mild photothermal therapy (mPTT) and sonodynamic therapy (SDT) will increase membrane permeability, enhance PSs uptake in tumor cells, and improve ROS aggregation to improve PDT efficiency. Meanwhile, combining with gene therapy can effectively delivery KillerRed into the specific site. PDT with other therapies, which has distinct benefits for primary cancers and peripheral metastatic tumors, can make a significant contribution to a systematic approach for cancer treatment.
Author Contributions
Funding
Conflicts of Interest
References
- Wu, H.; Minamide, T.; Yano, T. Role of photodynamic therapy in the treatment of esophageal cancer. Dig. Endosc. 2019, 31, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Morton, C.A. A synthesis of the world’s guidelines on photodynamic therapy for non-melanoma skin cancer. G. Ital. Dermatol. Venereol. 2018, 153, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Railkar, R.; Agarwal, P.K. Photodynamic Therapy in the Treatment of Bladder Cancer: Past Challenges and Current Innovations. Eur. Urol. Focus. 2018, 4, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Usuda, J.; Kato, H.; Ishizumi, T.; Ichinose, S.; Otani, K.; Honda, H.; Furukawa, K.; Okunaka, T.; Tsutsui, H. New aspects of photodynamic therapy for central type early stage lung cancer. Lasers. Surg. Med. 2011, 43, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.; Kokudo, T.; Inagaki, Y.; Hasegawa, K. Innovative treatment for hepatocellular carcinoma (HCC). Transl. Gastroenterol. Hepatol. 2018, 3, 78. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Ozog, D.M.; Rkein, A.M.; Fabi, S.G.; Gold, M.H.; Goldman, M.P.; Lowe, N.J.; Martin, G.M.; Munavalli, G.S. Photodynamic Therapy: A Clinical Consensus Guide. Dermatol. Surg. 2016, 42, 804–827. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two-cellular signaling, cell metabolism and modes of cell death. Photodiagnosis. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Hilgers, F.; Bitzenhofer, N.L.; Ackermann, Y.; Burmeister, A.; Grünberger, A.; Jaeger, K.E.; Drepper, T. Genetically Encoded Photosensitizers as Light-Triggered Antimicrobial Agents. Int. J. Mol. Sci. 2019, 20, 4608. [Google Scholar] [CrossRef] [Green Version]
- Bulina, M.E.; Chudakov, D.M.; Britanova, O.V.; Yanushevich, Y.G.; Staroverov, D.B.; Chepurnykh, T.V.; Merzlyak, E.M.; Shkrob, M.A.; Lukyanov, S.; Lukyanov, K.A. A genetically encoded photosensitizer. Nat. Biotechnol. 2006, 24, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.; Violot, S.; Blanchoin, L.; Bourgeois, D. Structural basis for the phototoxicity of the fluorescent protein KillerRed. FEBS. Lett. 2009, 583, 2839–2842. [Google Scholar] [CrossRef]
- Shu, X.; Lev-Ram, V.; Deerinck, T.J.; Qi, Y.; Ramko, E.B.; Davidson, M.W.; Jin, Y.; Ellisman, M.H.; Tsien, R.Y. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011, 9, e1001041. [Google Scholar] [CrossRef] [Green Version]
- Pletnev, S.; Gurskaya, N.G.; Pletneva, N.V.; Lukyanov, K.A.; Chudakov, D.M.; Martynov, V.I.; Popov, V.O.; Kovalchuk, M.V.; Wlodawer, A.; Dauter, Z.; et al. Structural basis for phototoxicity of the genetically encoded photosensitizer KillerRed. J. Biol. Chem. 2009, 284, 32028–32039. [Google Scholar] [CrossRef] [Green Version]
- Vegh, R.B.; Solntsev, K.M.; Kuimova, M.K.; Cho, S.; Liang, Y.; Loo, B.L.; Tolbert, L.M.; Bommarius, A.S. Reactive oxygen species in photochemistry of the red fluorescent protein “Killer Red”. Chem. Commun. 2011, 47, 4887–4889. [Google Scholar] [CrossRef] [PubMed]
- Pletneva, N.V.; Pletnev, V.Z.; Sarkisyan, K.S.; Gorbachev, D.A.; Egorov, E.S.; Mishin, A.S.; Lukyanov, K.A.; Dauter, Z.; Pletnev, S. Crystal Structure of Phototoxic Orange Fluorescent Proteins with a Tryptophan-Based Chromophore. PLoS ONE 2015, 10, e0145740. [Google Scholar] [CrossRef] [PubMed]
- Sarkisyan, K.S.; Zlobovskaya, O.A.; Gorbachev, D.A.; Bozhanova, N.G.; Sharonov, G.V.; Staroverov, D.B.; Egorov, E.S.; Ryabova, A.V.; Solntsev, K.M.; Mishin, A.S.; et al. KillerOrange, a Genetically Encoded Photosensitizer Activated by Blue and Green Light. PLoS ONE 2015, 10, e0145287. [Google Scholar]
- Takemoto, K.; Matsuda, T.; Sakai, N.; Fu, D.; Noda, M.; Uchiyama, S.; Kotera, I.; Arai, Y.; Horiuchi, M.; Fukui, K.; et al. SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci. Rep. 2013, 3, 2629. [Google Scholar] [CrossRef] [Green Version]
- Subach, O.M.; Malashkevich, V.N.; Zencheck, W.D.; Morozova, K.S.; Piatkevich, K.D.; Almo, S.C.; Verkhusha, V.V. Structural characterization of acylimine-containing blue and red chromophores in mTagBFP and TagRFP fluorescent proteins. Chem. Biol. 2010, 17, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merzlyak, E.M.; Goedhart, J.; Shcherbo, D.; Bulina, M.E.; Shcheglov, A.S.; Fradkov, A.F.; Gaintzeva, A.; Lukyanov, K.A.; Lukyanov, S.; Gadella, T.W.; et al. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat. Methods. 2007, 4, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Ragàs, X.; Cooper, L.P.; White, J.H.; Nonell, S.; Flors, C. Quantification of photosensitized singlet oxygen production by a fluorescent protein. Chemphyschem 2011, 12, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, R.; Cortajarena, A.L.; Mejias, S.H.; Agut, M.; Nonell, S.; Flors, C. Singlet oxygen generation by the genetically encoded tag miniSOG. J. Am. Chem. Soc. 2013, 135, 9564–9567. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, F.M.; Jensen, R.L.; Breitenbach, T.; Etzerodt, M.; Ogilby, P.R. Oxygen-dependent photochemistry and photophysics of “miniSOG,” a protein-encased flavin. Photochem. Photobiol. 2013, 89, 1116–1126. [Google Scholar] [CrossRef]
- Barnett, M.E.; Baran, T.M.; Foster, T.H.; Wojtovich, A.P. Quantification of light-induced miniSOG superoxide production using the selective marker, 2-hydroxyethidium. Free. Radic. Biol. Med. 2018, 116, 134–140. [Google Scholar] [CrossRef]
- Westberg, M.; Holmegaard, L.; Pimenta, F.M.; Etzerodt, M.; Ogilby, P.R. Rational design of an efficient, genetically encodable, protein-encased singlet oxygen photosensitizer. J. Am. Chem. Soc. 2015, 137, 1632–1642. [Google Scholar] [CrossRef]
- Rodríguez-Pulido, A.; Cortajarena, A.L.; Torra, J.; Ruiz-González, R.; Nonell, S.; Flors, C. Assessing the potential of photosensitizing flavoproteins as tags for correlative microscopy. Chem. Commun. 2016, 52, 8405–8408. [Google Scholar] [CrossRef] [Green Version]
- Endres, S.; Wingen, M.; Torra, J.; Ruiz-González, R.; Polen, T.; Bosio, G.; Bitzenhofer, N.L.; Hilgers, F.; Gensch, T.; Nonell, S.; et al. An optogenetic toolbox of LOV-based photosensitizers for light-driven killing of bacteria. Sci. Rep. 2018, 8, 15021. [Google Scholar] [CrossRef] [Green Version]
- Torra, J.; Burgos-Caminal, A.; Endres, S.; Wingen, M.; Drepper, T.; Gensch, T.; Ruiz-González, R.; Nonell, S. Singlet oxygen photosensitisation by the fluorescent protein Pp2FbFP L30M, a novel derivative of Pseudomonas putida flavin-binding Pp2FbFP. Photochem. Photobiol. Sci. 2015, 14, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Vegh, R.B.; Bravaya, K.B.; Bloch, D.A.; Bommarius, A.S.; Tolbert, L.M.; Verkhovsky, M.; Krylov, A.I.; Solntsev, K.M. Chromophore photoreduction in red fluorescent proteins is responsible for bleaching and phototoxicity. J. Phys. Chem. B 2014, 118, 4527–4534. [Google Scholar] [CrossRef] [PubMed]
- Shirmanova, M.; Yuzhakova, D.; Snopova, L.; Perelman, G.; Serebrovskaya, E.; Lukyanov, K.; Turchin, I.; Subochev, P.; Lukyanov, S.; Kamensky, V.; et al. Towards PDT with Genetically Encoded Photosensitizer KillerRed: A Comparison of Continuous and Pulsed Laser Regimens in an Animal Tumor Model. PLoS ONE 2015, 10, e0144617. [Google Scholar] [CrossRef]
- Serebrovskaya, E.O.; Ryumina, A.P.; Boulina, M.E.; Shirmanova, M.V.; Zagaynova, E.V.; Bogdanova, E.A.; Lukyanov, S.A.; Lukyanov, K.A. Phototoxic effects of lysosome-associated genetically encoded photosensitizer KillerRed. J. Biomed. Opt. 2014, 19, 071403. [Google Scholar] [CrossRef] [Green Version]
- Formella, I.; Svahn, A.J.; Radford, R.A.W.; Don, E.K.; Cole, N.J.; Hogan, A.; Lee, A.; Chung, R.S.; Morsch, M. Real-time visualization of oxidative stress-mediated neurodegeneration of individual spinal motor neurons in vivo. Redox. Biol. 2018, 19, 226–234. [Google Scholar] [CrossRef]
- The, C.; Korzh, V. In vivo optogenetics for light-induced oxidative stress in transgenic zebrafish expressing the KillerRed photosensitizer protein. Methods. Mol. Biol. 2014, 1148, 229–238. [Google Scholar]
- The, C.; Chudakov, D.M.; Poon, K.L.; Mamedov, I.Z.; Sek, J.Y.; Shidlovsky, K.; Lukyanov, S.; Korzh, V. Optogenetic in vivo cell manipulation in KillerRed-expressing zebrafish transgenics. BMC Dev. Biol. 2010, 10, 110. [Google Scholar]
- Jewhurst, K.; McLaughlin, K.A. Recovery of the Xenopus laevis heart from ROS-induced stress utilizes conserved pathways of cardiac regeneration. Dev. Growth. Differ. 2019, 61, 212–227. [Google Scholar] [CrossRef]
- Jewhurst, K.; Levin, M.; McLaughlin, K.A. Optogenetic Control of Apoptosis in Targeted Tissues of Xenopus laevis Embryos. J. Cell. Death. 2014, 7, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.C.; Bejjani, R.E.; Ramirez, P.M.; Coakley, S.; Kim, S.A.; Lee, H.; Wen, Q.; Samuel, A.; Lu, H.; Hilliard, M.A.; et al. Rapid and permanent neuronal inactivation in vivo via subcellular generation of reactive oxygen with the use of KillerRed. Cell. Rep. 2013, 5, 553–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, J.; Shidara, H.; Morisawa, Y.; Kawakami, M.; Tanahashi, Y.; Hotta, K.; Oka, K. A method for selective ablation of neurons in C. elegans using the phototoxic fluorescent protein, KillerRed. Neurosci. Lett. 2013, 548, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, T.; Tsujimoto, Y. Deleterious effects of mitochondrial ROS generated by KillerRed photodynamic action in human cell lines and C. elegans. J. Photochem. Photobiol. B 2012, 117, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, A.; Wang, Y.; Sheng, M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J. Neurosci. 2014, 34, 1672–1688. [Google Scholar] [CrossRef]
- Grimm, A.; Cummins, N.; Götz, J. Local Oxidative Damage in the Soma and Dendrites Quarantines Neuronal Mitochondria at the Site of Insult. Iscience 2018, 6, 114–127. [Google Scholar] [CrossRef] [Green Version]
- Petrova, N.V.; Luzhin, A.V.; Serebrovskaya, E.O.; Ryumina, A.P.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Inducing cellular senescence in vitro by using genetically encoded photosensitizers. Aging 2016, 8, 2449–2462. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Nartiss, Y.; Steipe, B.; McQuibban, G.A.; Kim, P.K. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy 2012, 8, 1462–1476. [Google Scholar] [CrossRef] [Green Version]
- Waldeck, W.; Mueller, G.; Wiessler, M.; Tóth, K.; Braun, K. Positioning effects of KillerRed inside of cells correlate with DNA strand breaks after activation with visible light. Int. J. Med. Sci. 2011, 8, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldeck, W.; Mueller, G.; Glatting, K.H.; Hotz-Wagenblatt, A.; Diessl, N.; Chotewutmonti, S.; Langowski, J.; Semmler, W.; Wiessler, M.; Braun, K. Spatial localization of genes determined by intranuclear DNA fragmentation with the fusion proteins lamin KRED and histone KRED und visible light. Int. J. Med. Sci. 2013, 10, 1136–1148. [Google Scholar] [CrossRef] [Green Version]
- Lan, L.; Nakajima, S.; Wei, L.; Sun, L.; Hsieh, C.L.; Sobol, R.W.; Bruchez, M.; Van Houten, B.; Yasui, A.; Levine, A.S. Novel method for site-specific induction of oxidative DNA damage reveals differences in recruitment of repair proteins to heterochromatin and euchromatin. Nucleic. Acids. Res. 2014, 42, 2330–2345. [Google Scholar] [CrossRef] [PubMed]
- Whitefield, D.B.; Spagnol, S.T.; Armiger, T.J.; Lan, L.; Dahl, K.N. Quantifying site-specific chromatin mechanics and DNA damage response. Sci. Rep. 2018, 8, 18084. [Google Scholar] [CrossRef] [Green Version]
- Nieborowska-Skorska, M.; Kopinski, P.K.; Ray, R.; Hoser, G.; Ngaba, D.; Flis, S.; Cramer, K.; Reddy, M.M.; Koptyra, M.; Penserga, T.; et al. Rac2-MRC-cIII-generated ROS cause genomic instability in chronic myeloid leukemia stem cells and primitive progenitors. Blood 2012, 119, 4253–4263. [Google Scholar] [CrossRef]
- Tan, R.; Lan, L. Induction of Site-Specific Oxidative Damage at Telomeres by Killerred-Fused Shelretin Proteins. Methods Mol. Biol. 2017, 1587, 139–146. [Google Scholar] [PubMed]
- Sun, L.; Tan, R.; Xu, J.; LaFace, J.; Gao, Y.; Xiao, Y.; Attar, M.; Neumann, C.; Li, G.M.; Su, B.; et al. Targeted DNA damage at individual telomeres disrupts their integrity and triggers cell death. Nucleic. Acids. Res. 2015, 43, 6334–6347. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell. Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, G.L.; Fan, J.H.; Yuan, P.; Deng, F.A.; Qiu, X.Z.; Yu, X.Y.; Li, S.Y. Epigenetics-inspired photosensitizer modification for plasma membrane-targeted photodynamic tumor therapy. Biomaterials 2019, 224, 119497. [Google Scholar] [CrossRef]
- Jia, H.R.; Zhu, Y.X.; Xu, K.F.; Liu, X.; Wu, F.G. Plasma membrane-anchorable photosensitizing nanomicelles for lipid raft-responsive and light-controllable intracellular drug delivery. J. Control. Release 2018, 286, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, W.; Kuang, X.; Hou, S.; Liu, H. Nanopreparations for mitochondria targeting drug delivery system: Current strategies and future prospective. Asian. J. Pharm. Sci. 2017, 12, 498–508. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.; Bao, Y.W.; Wu, F.G. Improving the Phototherapeutic Efficiencies of Molecular and Nanoscale Materials by Targeting Mitochondria. Molecules 2018, 23, 3016. [Google Scholar] [CrossRef] [Green Version]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Waldeck, W.; Mueller, G.; Wiessler, M.; Brom, M.; Tóth, K.; Braun, K. Autofluorescent proteins as photosensitizer in eukaryontes. Int. J. Med. Sci. 2009, 6, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.V.; da Costa, A.M.R.; Silva, G.A. Non-viral strategies for ocular gene delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1275–1289. [Google Scholar] [CrossRef]
- Chen, Y.H.; Keiser, M.S.; Davidson, B.L. Viral Vectors for Gene Transfer. Curr. Protoc. Mouse Biol. 2018, 8, e58. [Google Scholar] [CrossRef] [PubMed]
- Takehara, K.; Tazawa, H.; Okada, N.; Hashimoto, Y.; Kikuchi, S.; Kuroda, S.; Kishimoto, H.; Shirakawa, Y.; Narii, N.; Mizuguchi, H.; et al. Targeted Photodynamic Virotherapy Armed with a Genetically Encoded Photosensitizer. Mol. Cancer. Ther. 2016, 15, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takehara, K.; Yano, S.; Tazawa, H.; Kishimoto, H.; Narii, N.; Mizuguchi, H.; Urata, Y.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. Eradication of melanoma in vitro and in vivo via targeting with a Killer-Red-containing telomerase-dependent adenovirus. Cell. Cycle 2017, 16, 1502–1508. [Google Scholar] [CrossRef] [Green Version]
- Byrne, L.C.; Khalid, F.; Lee, T.; Zin, E.A.; Greenberg, K.P.; Visel, M.; Schaffer, D.V.; Flannery, J.G. AAV-mediated, optogenetic ablation of Müller Glia leads to structural and functional changes in the mouse retina. PLoS ONE 2013, 8, e76075. [Google Scholar]
- Liao, Z.X.; Peng, S.F.; Chiu, Y.L.; Hsiao, C.W.; Liu, H.Y.; Lim, W.H.; Lu, H.M.; Sung, H.W. Enhancement of efficiency of chitosan-based complexes for gene transfection with poly (γ-glutamic acid) by augmenting their cellular uptake and intracellular unpackage. J. Control. Release 2014, 193, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Planul, A.; Dalkara, D. Vectors and Gene Delivery to the Retina. Annu. Rev. Vis. Sci. 2017, 3, 121–140. [Google Scholar] [CrossRef]
- Liao, Z.X.; Li, Y.C.; Lu, H.M.; Sung, H.W. A genetically-encoded KillerRed protein as an intrinsically generated photosensitizer for photodynamic therapy. Biomaterials 2014, 35, 500–508. [Google Scholar] [CrossRef]
- Tseng, S.J.; Liao, Z.X.; Kao, S.H.; Zeng, Y.F.; Huang, K.Y.; Li, H.J.; Yang, C.L.; Deng, Y.F.; Huang, C.F.; Yang, S.C.; et al. Highly specific in vivo gene delivery for p53-mediated apoptosis and genetic photodynamic therapies of tumour. Nat. Commun. 2015, 6, 6456. [Google Scholar] [CrossRef]
- Zhou, J.; Mohamed Wali, A.R.; Ma, S.; He, Y.; Yue, D.; Tang, J.Z.; Gu, Z. Tailoring the Supramolecular Structure of Guanidinylated Pullulan toward Enhanced Genetic Photodynamic Therapy. Biomacromolecules 2018, 19, 2214–2226. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Liu, C.; Wang, S.; Li, Z.; Hu, H.; Wan, Y.; Yang, X. Hydroxyethyl Starch-Based Nanoparticles Featured with Redox-Sensitivity and Chemo-Photothermal Therapy for Synergized Tumor Eradication. Cancers 2019, 11, 207. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Zhou, Q.; Xiao, F.; Li, Y.; Hu, H.; Wan, Y.; Li, Z.; Yang, X. Enhancing Doxorubicin Delivery toward Tumor by Hydroxyethyl Starch-g-Polylactide Partner Nanocarriers. ACS. Appl. Mater. Interfaces 2017, 9, 10481–10493. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Hu, Q.; Tang, G.; Li, J. FGFR-targeted gene delivery mediated by supramolecular assembly between β-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials 2013, 34, 6482–6494. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Hu, W.; Zhang, N.; Qi, Y.; Nie, J.J.; Zhao, N.; Yu, B.; Xu, F.J. Genetically multimodal therapy mediated by one polysaccharides-based supramolecular nanosystem. Biomaterials 2020, 248, 120031. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Liu, C.; Li, J.; Ma, W.; Yu, X.; Zhang, P.; Ji, Y. The effects of photodynamic therapy on leukemia cells mediated by KillerRed, a genetically encoded fluorescent protein photosensitizer. BMC Cancer 2019, 19, 934. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Kanada, M.; Zhang, J.; Okazaki, S.; Terakawa, S. Photodynamic Treatment of Tumor with Bacteria Expressing KillerRed. PLoS ONE 2015, 10, e0131518. [Google Scholar] [CrossRef]
- Liu, X.; Wu, F.; Ji, Y.; Yin, L. Recent Advances in Anti-cancer Protein/Peptide Delivery. Bioconjug. Chem. 2019, 30, 305–324. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug. Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Master, A.; Livingston, M.; Sen Gupta, A. Photodynamic nanomedicine in the treatment of solid tumors: Perspectives and challenges. J. Control. Release 2013, 168, 88–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddi, E. Role of delivery vehicles for photosensitizers in the photodynamic therapy of tumours. J. Photochem. Photobiol. B 1997, 37, 189–195. [Google Scholar] [CrossRef]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gu, Z.; Ottewell, T.; Yu, C. Silica-based nanoparticles for therapeutic protein delivery. J. Mater. Chem. B 2017, 5, 3241–3252. [Google Scholar] [CrossRef]
- Shi, H.; Liu, S.; Cheng, J.; Yuan, S.; Yang, Y.; Fang, T.; Cao, K.; Wei, K.; Zhang, Q.; Liu, Y. Charge-Selective Delivery of Proteins Using Mesoporous Silica Nanoparticles Fused with Lipid Bilayers. ACS. Appl. Mater. Interfaces 2019, 11, 3645–3653. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Waynant, R.W.; Ilev, I.K.; Wu, X.; Barna, L.; Smith, K.; Heckert, R.; Gerst, H.; Anders, J.J. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers. Surg. Med. 2005, 36, 171–185. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Liang, L.; Lu, Y.; Zhang, R.; Care, A.; Ortega, T.A.; Deyev, S.M.; Qian, Y.; Zvyagin, A.V. Deep-penetrating photodynamic therapy with KillerRed mediated by upconversion nanoparticles. Acta. Biomater. 2017, 54, 461–470. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1327–1341. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kang, M.; Choo, Y.W.; Go, S.H.; Kwon, S.P.; Song, S.Y.; Sohn, H.S.; Hong, J.; Kim, B.S. Immunomodulatory Lipocomplex Functionalized with Photosensitizer-Embedded Cancer Cell Membrane Inhibits Tumor Growth and Metastasis. Nano Lett. 2019, 19, 5185–5193. [Google Scholar] [CrossRef]
- Khalil, A.S.; Yu, X.; Xie, A.W.; Fontana, G.; Umhoefer, J.M.; Johnson, H.J.; Hookway, T.A.; McDevitt, T.C.; Murphy, W.L. Functionalization of microparticles with mineral coatings enhances non-viral transfection of primary human cells. Sci. Rep. 2017, 7, 14211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leja, J.; Nilsson, B.; Yu, D.; Gustafson, E.; Akerström, G.; Oberg, K.; Giandomenico, V.; Essand, M. Double-detargeted oncolytic adenovirus shows replication arrest in liver cells and retains neuroendocrine cell killing ability. PLoS ONE 2010, 5, e8916. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Oh, J.S.; Ahn, I.S.; Park, K.I.; Jang, J.H. Magnetically enhanced adeno-associated viral vector delivery for human neural stem cell infection. Biomaterials 2011, 32, 8654–8662. [Google Scholar] [CrossRef] [PubMed]
- Miest, T.S.; Cattaneo, R. New viruses for cancer therapy: Meeting clinical needs. Nat. Rev. Microbiol. 2014, 12, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Kotterman, M.A.; Schaffer, D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014, 15, 445–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Z.X.; Kempson, I.M.; Fa, Y.C.; Liu, M.C.; Hsieh, L.C.; Huang, K.Y.; Wang, L.F. Magnetically Guided Viral Transduction of Gene-Based Sensitization for Localized Photodynamic Therapy to Overcome Multidrug Resistance in Breast Cancer Cells. Bioconjug. Chem. 2017, 28, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
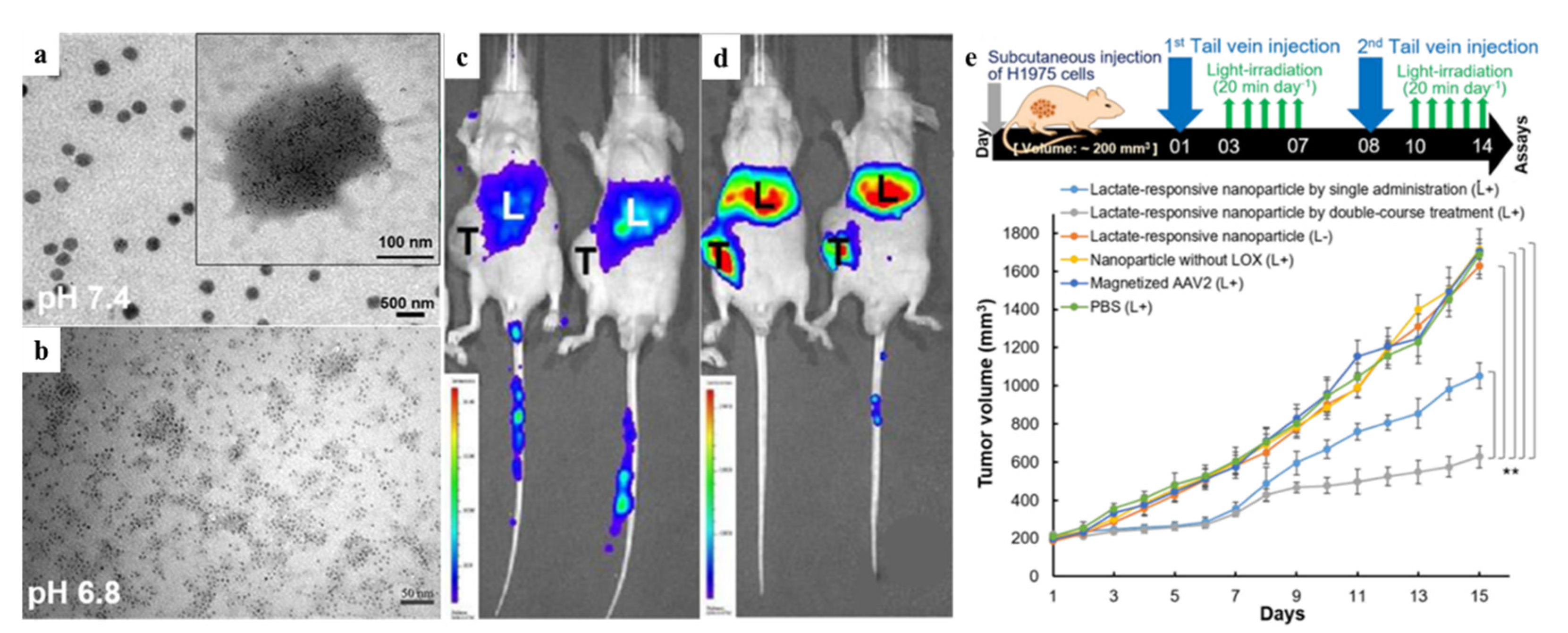
- Tseng, S.J.; Kempson, I.M.; Huang, K.Y.; Li, H.J.; Fa, Y.C.; Ho, Y.C.; Liao, Z.X.; Yang, P.C. Targeting Tumor Microenvironment by Bioreduction-Activated Nanoparticles for Light-Triggered Virotherapy. ACS Nano. 2018, 12, 9894–9902. [Google Scholar] [CrossRef]
- Tseng, S.J.; Huang, K.Y.; Kempson, I.M.; Kao, S.H.; Liu, M.C.; Yang, S.C.; Liao, Z.X.; Yang, P.C. Remote Control of Light-Triggered Virotherapy. ACS Nano. 2016, 10, 10339–10346. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer. J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug. Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Kleinovink, J.W.; van Driel, P.B.; Snoeks, T.J.; Prokopi, N.; Fransen, M.F.; Cruz, L.J.; Mezzanotte, L.; Chan, A.; Löwik, C.W.; Ossendorp, F. Combination of Photodynamic Therapy and Specific Immunotherapy Efficiently Eradicates Established Tumors. Clin. Cancer. Res. 2016, 22, 1459–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuzhakova, D.V.; Shirmanova, M.V.; Serebrovskaya, E.O.; Lukyanov, K.A.; Druzhkova, I.N.; Shakhov, B.E.; Lukyanov, S.A.; Zagaynova, E.V. CT26 murine colon carcinoma expressing the red fluorescent protein KillerRed as a highly immunogenic tumor model. J. Biomed. Opt. 2015, 20, 88002. [Google Scholar] [CrossRef] [PubMed]
- Serebrovskaya, E.O.; Yuzhakova, D.V.; Ryumina, A.P.; Druzhkova, I.N.; Sharonov, G.V.; Kotlobay, A.A.; Zagaynova, E.V.; Lukyanov, S.A.; Shirmanova, M.V. Soluble OX40L favors tumor rejection in CT26 colon carcinoma model. Cytokine 2016, 84, 10–16. [Google Scholar] [CrossRef]
- Serebrovskaya, E.O.; Edelweiss, E.F.; Stremovskiy, O.A.; Lukyanov, K.A.; Chudakov, D.M.; Deyev, S.M. Targeting cancer cells by using an antireceptor antibody-photosensitizer fusion protein. Proc. Natl. Acad. Sci. USA 2009, 106, 9221–9225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Shurin, G.V.; Peiyuan, Z.; Shurin, M.R. Dendritic cells in the cancer microenvironment. J. Cancer 2013, 4, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]



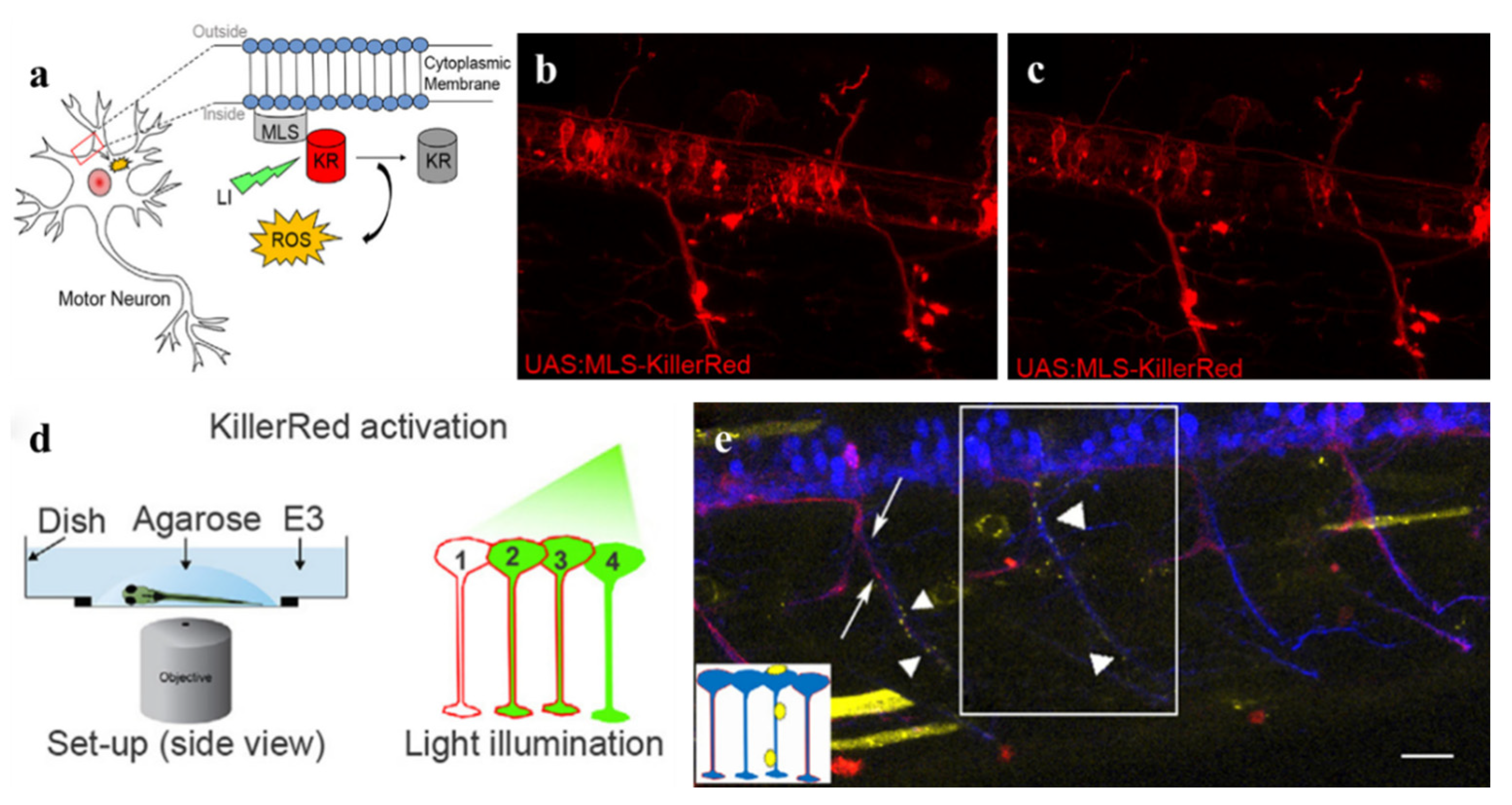

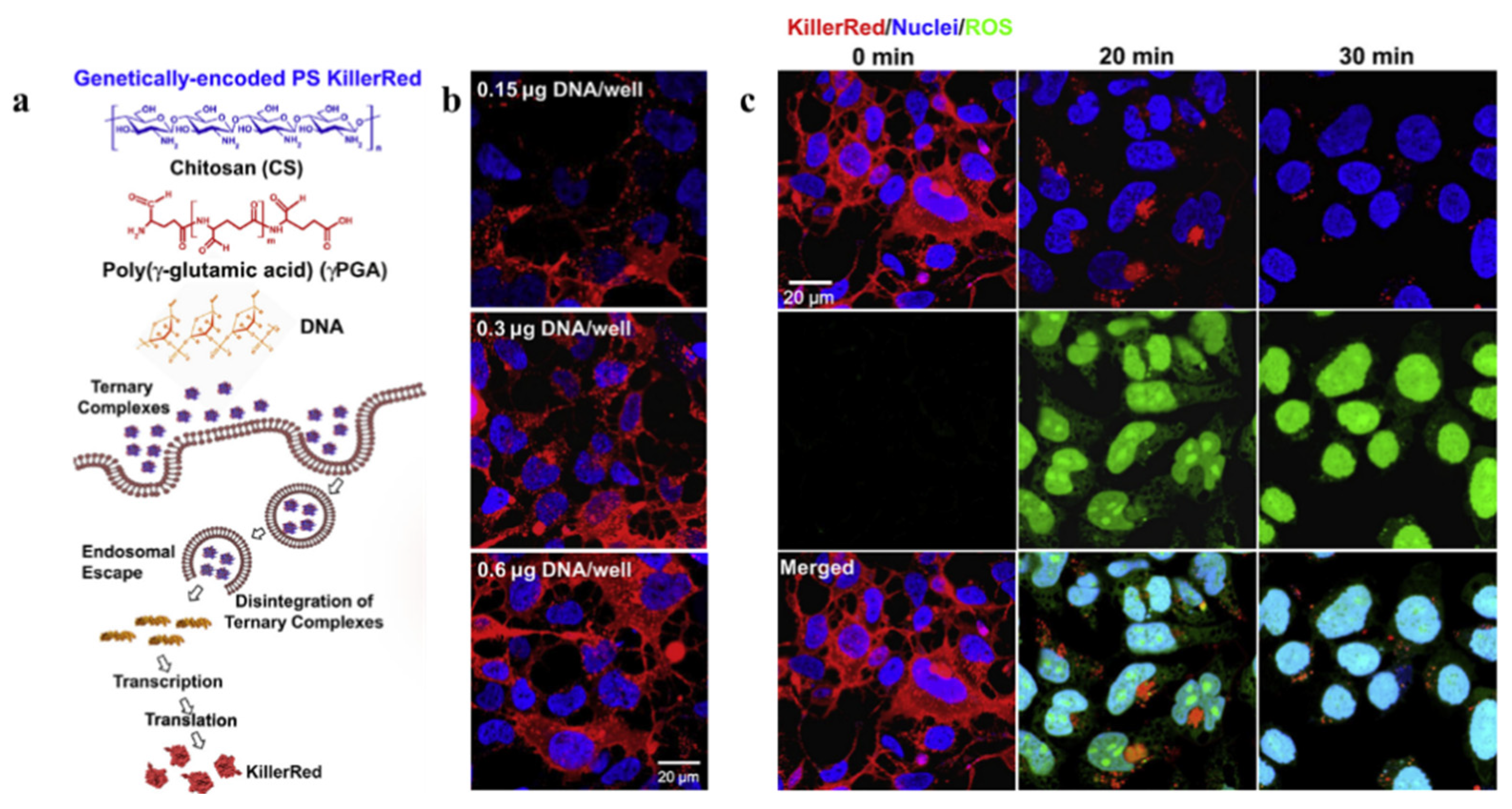

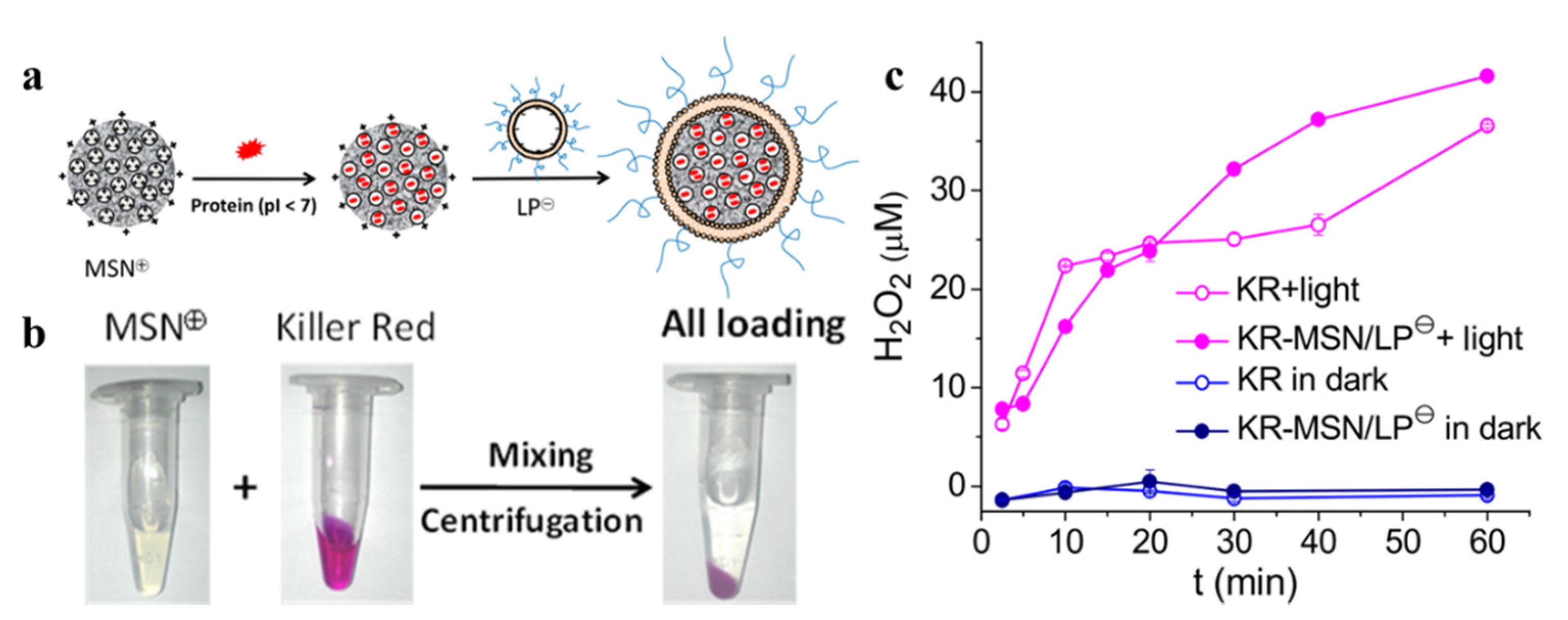
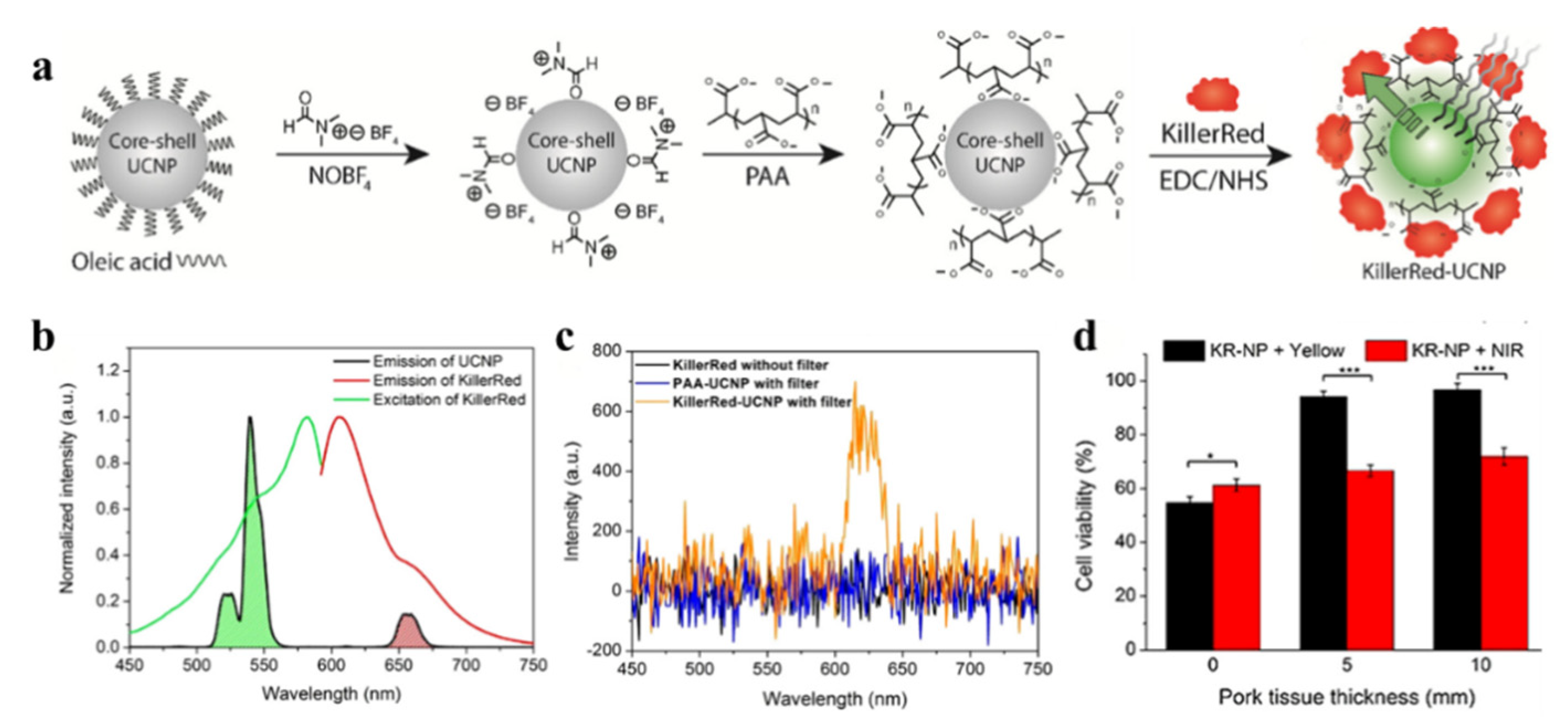


| Category | Protein Photosensitizer | No. AA | Chromophore | λex [nm] | λem [nm] | Fluorescence Quantum Yield [φF] | 1O2 Quantum Yield [φ1O2] | Photosensitized O2− Formation |
|---|---|---|---|---|---|---|---|---|
| Fluorescent protein vatiant | KillerRed | 239 | QYG [13] | 585 | 610 | 0.25 [12] | 0.000 [14] | Y [15,16] |
| KillerOrange | 248 | QWG [17] | 512 | 555 | 0.42 [18] | -1 | - | |
| SuperNova | 271 | QYG [19] | 579 | 610 | 0.30 [19] | - | Y [19] | |
| TagRFP | 237 | MYG [20] | 555 | 584 | 0.48 [21] | 0.004 [22] | N [22] | |
| Flavin-binding protein | miniSOG | 106 | - | 448 | 528 | 0.37 [14] | 0.03 [23,24] | Y [25] |
| SOPP | 106 | - | 440 | 487 | 0.43 [26] | 0.25 [26]/0.39 [27] | Y [27] | |
| Pp2FbFPL30M | 148 | - | 449 | 495 | 0.25 [28] | 0.09 [29] | Y [28] |
| Different Target Sites | Targeting Signal | Experimental Model | Parameters of Killerred Illumination | Ref. | ||
|---|---|---|---|---|---|---|
| Wavelength | Optical Powers | Duration | ||||
| Membrane | Inserting membrane localization signal (MLS) | Zebrafish | 535–575 nm | 80 mW/cm2 | 2 h | [33] |
| Zebrafish | Greenlight | - | 0.5–1 h | [34] | ||
| zebrafish | 546–558 nm | 100 W | 20 min | [35] | ||
| Xenopus laevis | 545 nm | 90 mW/cm2 | 18 h | [36] | ||
| Xenopus laevis | 545–565 nm | 200 W | - | [37] | ||
| C. elegans | 426–593 nm | 0.57–46 mW/cm2 | 0.1–2 h | [38] | ||
| C. elegans | 540–580 nm | 269 mW/cm2 | 2 h | [39] | ||
| Mitochondria | Inserting mitochondria target sequence (MTS) | C. elegans | 550–590 nm | 100 mW/cm2 | 1 h | [40] |
| C. elegans | 543–593 nm | 200–300 mW/cm2 | 1 h | [38] | ||
| Rat and mouse hippocampal neuronal | Greenlight | 120 W | 1 h | [41] | ||
| Mouse hippocampal neuronal and N2a neuroblastoma cells | 561 nm | - | 30 s | [42] | ||
| HeLa cells | Visible light | - | 1 h | [43] | ||
| HEK293T and HeLa cells | 530–610 nm | 100 mW/cm2 | 20 min | [40] | ||
| HeLa and SH-SY5Y cells | 561 nm | - | - | [44] | ||
| Nuclear | Histone 2B | HeLa and Hela Kyoto cells | Greenlight | 200 mW/cm2 | 15 min | [43] |
| Histone 2A/Lamin B1 | Hela and DU145 cells | Visible light | - | 3 h | [45,46,47] | |
| Tet-repressor/Transcription activator | U2OS TRE and 263 cells | 559 nm | 150 mW/cm2 | 10 min | [48,49] | |
| Telomere-binding protein TRF1 | U2OS, HeLa, MCF7, IMR90, and MCF7 cells | 559 nm | 15 W | 0.33–4 h | [50,51] | |
| Telomere-binding protein TRF1/2 | U2OS, 293, HeLa, and 293FT cells | Visible light | - | 0.33–1 h | [35] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, F.; Qin, Y.; Feng, X. Advances in the Genetically Engineered KillerRed for Photodynamic Therapy Applications. Int. J. Mol. Sci. 2021, 22, 10130. https://doi.org/10.3390/ijms221810130
Liu J, Wang F, Qin Y, Feng X. Advances in the Genetically Engineered KillerRed for Photodynamic Therapy Applications. International Journal of Molecular Sciences. 2021; 22(18):10130. https://doi.org/10.3390/ijms221810130
Chicago/Turabian StyleLiu, Jiexi, Fei Wang, Yang Qin, and Xiaolan Feng. 2021. "Advances in the Genetically Engineered KillerRed for Photodynamic Therapy Applications" International Journal of Molecular Sciences 22, no. 18: 10130. https://doi.org/10.3390/ijms221810130






