Silica Nanoparticles Inhibit Responses to ATP in Human Airway Epithelial 16HBE Cells
Abstract
:1. Introduction
2. Results
2.1. Intracellular Ca2+ Response of 16HBE Cells to Extracellular ATP
2.2. The Ca2+ Responses to ATP Are Mediated by Release from Intracellular Stores
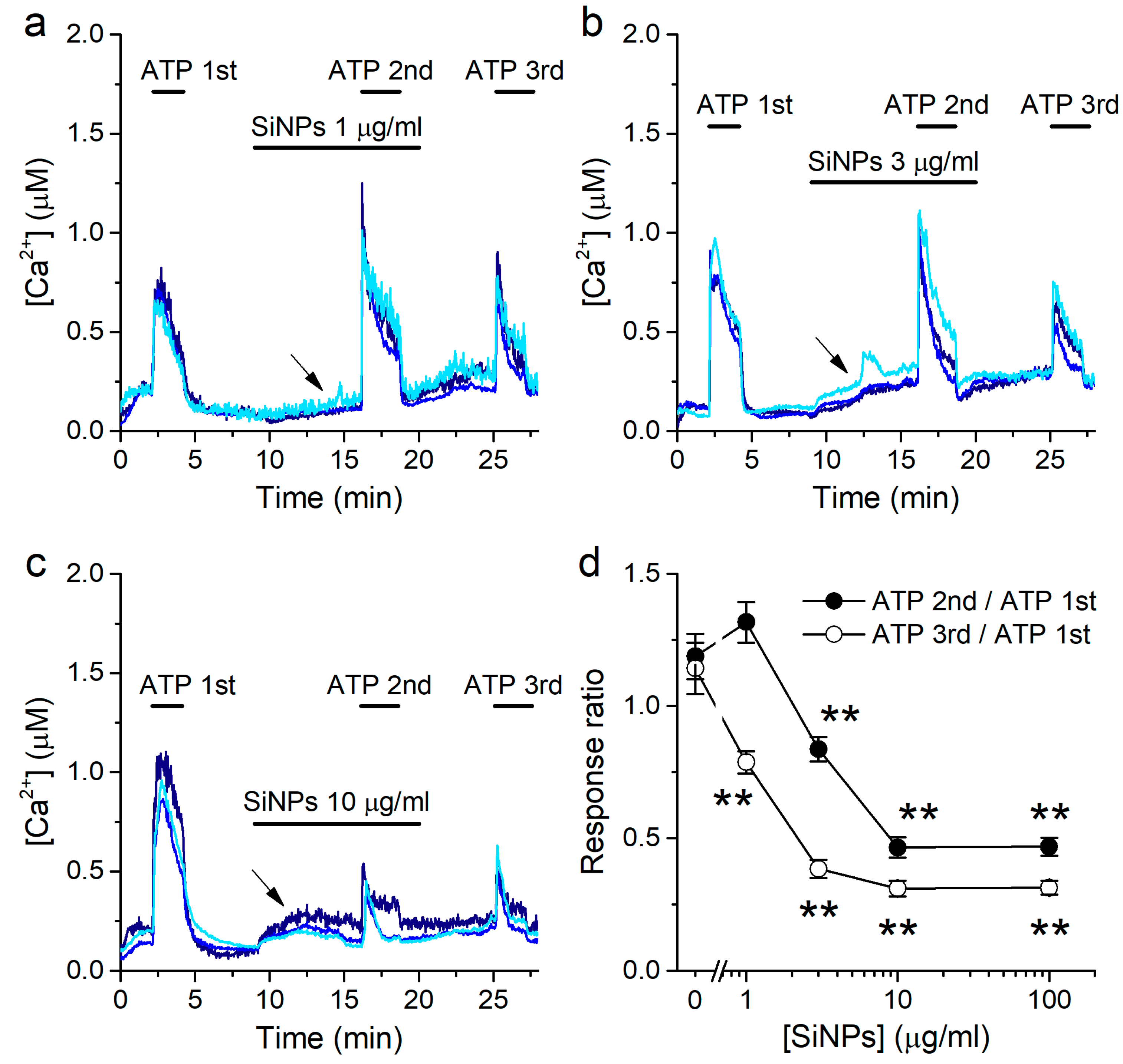
2.3. Concentration-Dependent Inhibition of Responses to ATP by SiNPs
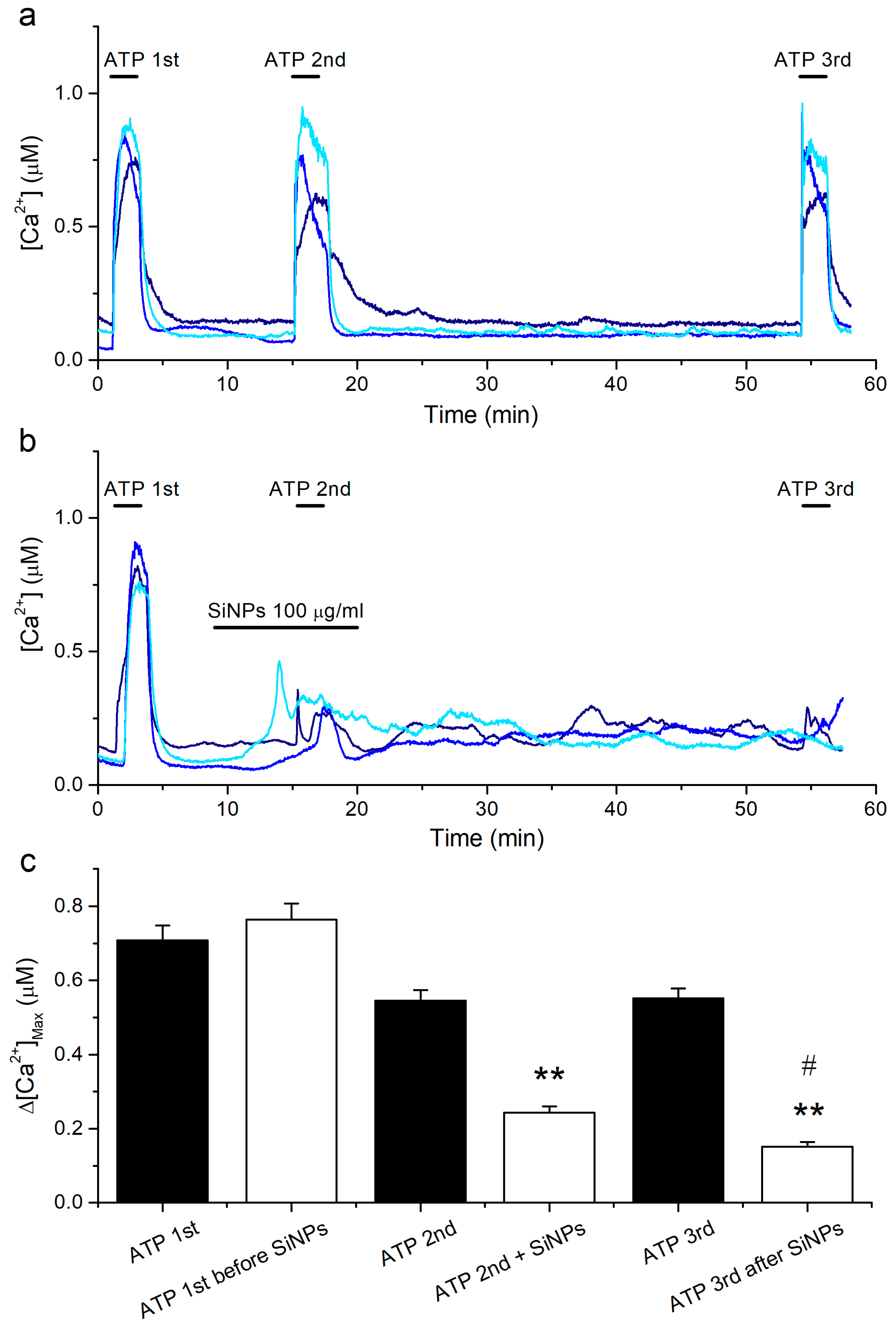
2.4. Prolonged Inhibitory Effects of SiNPs on the Responses to ATP
2.5. Effects of Intracellular Ca2+ Overload on the Responses to ATP
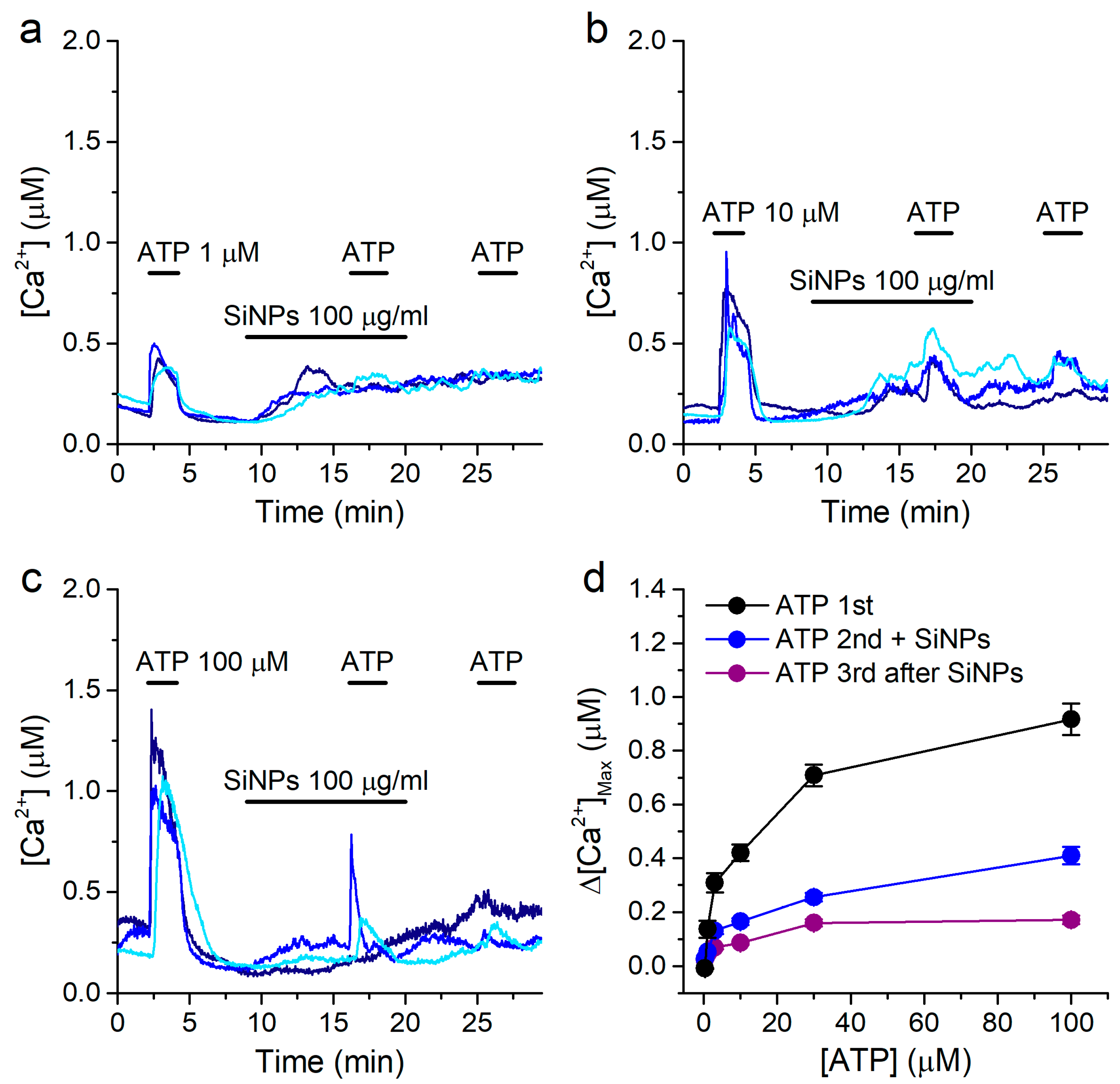
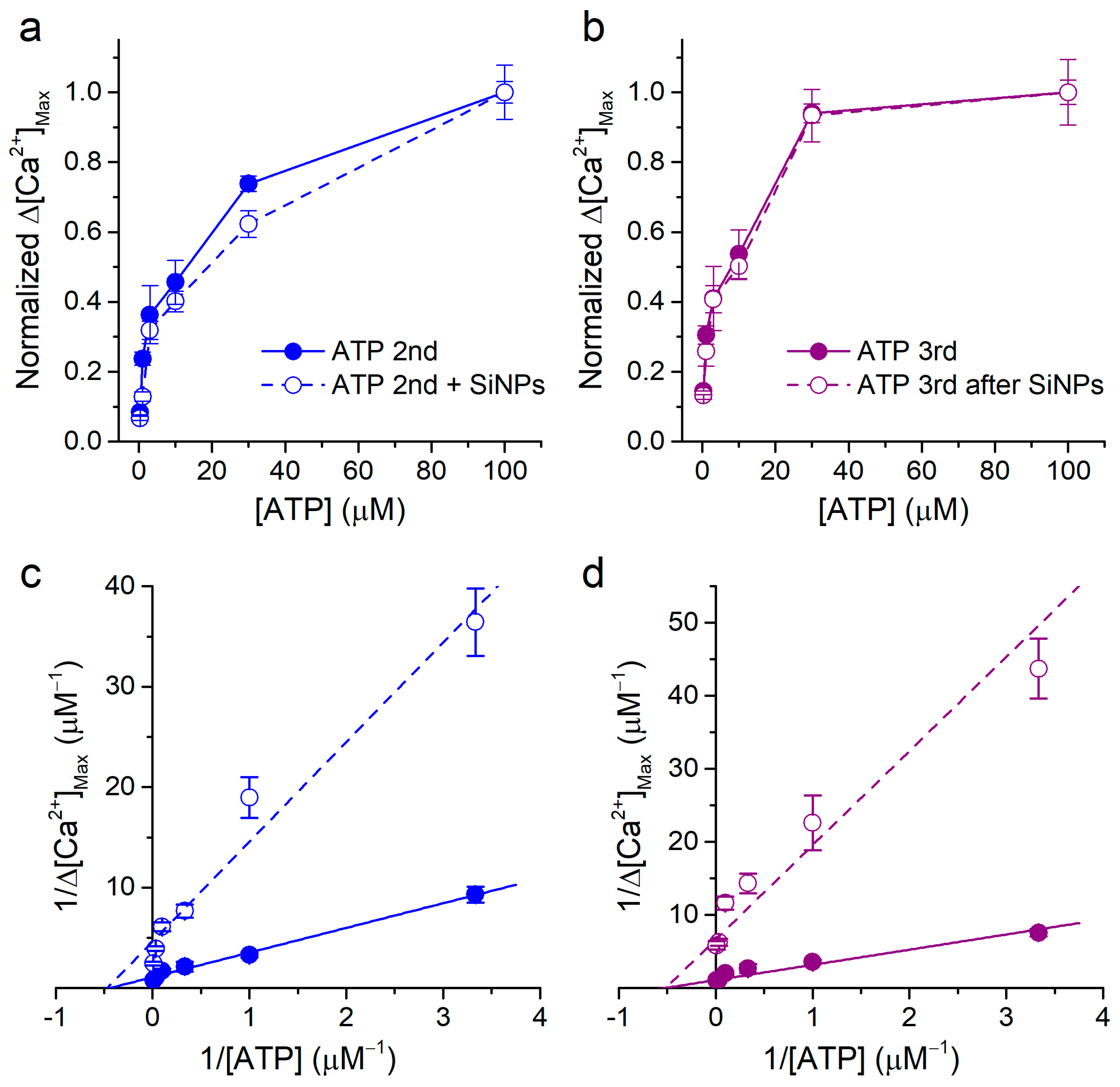
2.6. Effects of SiNPs on the Concentration Dependence of the Response to ATP
3. Discussion
4. Materials and Methods
4.1. Ludox® SiNPs
4.2. Cell Culture
4.3. Ratiometric Intracellular Ca2+ Imaging
4.4. Data and Statistical Analysis
4.5. Reagents and Solutions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreyling, W.G.; Semmler-Behnke, M.; Chaudhry, Q. A complementary definition of nanomaterial. Nano Today 2010, 5, 165–168. [Google Scholar] [CrossRef]
- Bleeker, E.A.J.; de Jong, W.H.; Geertsma, R.E.; Groenewold, M.; Heugens, E.H.W.; Koers-Jacquemijns, M.; van de Meent, D.; Popma, J.R.; Rietveld, A.G.; Wijnhoven, S.W.P.; et al. Considerations on the EU definition of a nanomaterial: Science to support policy making. Regul. Toxicol. Pharmacol. 2013, 65, 119–125. [Google Scholar] [CrossRef]
- Naito, M.; Yokoyama, T.; Hosokawa, K.; Nogi, K. Nanoparticle Technology Handbook; Elsevier: Hoboken, NJ, USA, 2018. [Google Scholar]
- Liu, D.; Gu, N. Nanomaterials for Fresh-Keeping and Sterilization in Food Preservation. Recent Pat. Food Nutr. Agric. 2009, 1, 149–154. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, L.; Di, W.; Li, K.; Li, G.; Sun, D. Methyl-grafted silica nanoparticle stabilized water-in-oil Pickering emulsions with low-temperature stability. J. Colloid Interface Sci. 2021, 588, 501–509. [Google Scholar] [CrossRef]
- Qhobosheane, M.; Santra, S.; Zhang, P.; Tan, W. Biochemically functionalized silica nanoparticles. Analyst 2001, 126, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Asbach, C.; Fissan, H.; Hülser, T.; Kuhlbusch, T.A.J.; Thompson, D.; Pui, D.Y.H. How can nanobiotechnology oversight advance science and industry: Examples from environmental, health, and safety studies of nanoparticles (nano-EHS). J. Nanopart. Res. 2011, 13, 1373–1387. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Samal, S.S.; Manoharan, N. Safety and Risk Associated with Nanoparticles-A Review. J. Miner. Mater. Charact. Eng. 2010, 9, 455–459. [Google Scholar] [CrossRef]
- Dubes, V.; Parpaite, T.; Ducret, T.; Quignard, J.F.; Mornet, S.; Reinhardt, N.; Baudrimont, I.; Dubois, M.; Freund-Michel, V.; Marthan, R.; et al. Calcium signalling induced by in vitro exposure to silicium dioxide nanoparticles in rat pulmonary artery smooth muscle cells. Toxicology 2017, 375, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J. Control Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, D.; Jing, L.; Cui, G.; Jin, M.; Li, Y.; Liu, X.; Liu, Y.; Du, H.; Guo, C.; et al. Cardiovascular toxicity of different sizes amorphous silica nanoparticles in rats after intratracheal instillation. Cardiovasc. Toxicol. 2013, 13, 194–207. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M.; Samadian, H.; Barabadi, H.; Azarnezhad, A.; Ahmadi, A. Redox interactions and genotoxicity of metal-based nanoparticles: A comprehensive review. Chem. Biol. Interact. 2019, 312, 108814. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Liu, X.; Jin, M.; Zhang, L.; Du, Z.; Guo, C.; Huang, P.; Sun, Z. Cytotoxicity and mitochondrial damage caused by silica nanoparticles. Toxicol. Vitr. 2011, 25, 1619–1629. [Google Scholar] [CrossRef]
- Messerschmidt, C.; Hofmann, D.; Kroeger, A.; Landfester, K.; Mailänder, V.; Lieberwirth, I. On the pathway of cellular uptake: New insight into the interaction between the cell membrane and very small nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1296–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiser, M.; Rothen-Rutishauser, B.; Kapp, N.; Schürch, S.; Kreyling, W.; Schulz, H.; Semmler, M.; Im Hof, V.; Heyder, J.; Gehr, P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005, 113, 1555–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 2014, 122, 122. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Corbalan, J.J.; Medina, C.; Jacoby, A.; Malinski, T.; Radomski, M.W. Amorphous silica nanoparticles trigger nitric oxide/peroxynitrite imbalance in human endothelial cells: Inflammatory and cytotoxic effects. Int. J. Nanomed. 2011, 6, 2821–2835. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yoo, E.; Han, C.; Mahler, G.J.; Doiron, A.L. Endothelial barrier dysfunction induced by nanoparticle exposure through actin remodeling via caveolae/raft-regulated calcium signalling. NanoImpact 2018, 11, 82–91. [Google Scholar] [CrossRef]
- Chen, E.Y.; Garnica, M.; Wang, Y.C.; Mintz, A.J.; Chen, C.S.; Chin, W.C. A mixture of anatase and rutile TiO2nanoparticles induces histamine secretion in mast cells. Part. Fibre Toxicol. 2012, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Selvan, S.T.; Yang Tan, T.T.; Kee Yi, D.; Jana, N.R. Functional and multifunctional nanoparticles for bioimaging and biosensing. Langmuir 2010, 26, 11631–11641. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Su, C.C.; Fang, K.M.; Wu, C.C.; Wu, C.T.; Chen, Y.W. Ultrafine silicon dioxide nanoparticles cause lung epithelial cells apoptosis via oxidative stress-activated PI3K/Akt-mediated mitochondria- and endoplasmic reticulum stress-dependent signaling pathways. Sci. Rep. 2020, 10, 9928. [Google Scholar] [CrossRef] [PubMed]
- Marchiando, A.M.; Graham, W.V.; Turner, J.R. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 119–144. [Google Scholar] [CrossRef]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier function of airway tract epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef]
- Legendre, M.; Zaragosi, L.E.; Mitchison, H.M. Motile cilia and airway disease. Semin. Cell Dev. Biol. 2021, 110, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef]
- Fariss, M.W.; Gilmour, M.I.; Reilly, C.A.; Liedtke, W.; Ghio, A.J. Emerging mechanistic targets in lung injury induced by combustion-generated particles. Toxicol. Sci. 2013, 132, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, A.; Alvarez, J.L.; Demydenko, K.; Jung, C.; Alpizar, Y.A.; Alvarez-Collazo, J.; Cokic, S.M.; Valverde, M.A.; Hoet, P.H.; Talavera, K. Silica nanoparticles inhibit the cation channel TRPV4 in airway epithelial cells. Part. Fibre Toxicol. 2017, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Li, Q.; Kolosov, V.P.; Perelman, J.M.; Zhou, X. Regulation of particulate matter-induced mucin secretion by transient receptor potential vanilloid 1 receptors. Inflammation 2012, 35, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Memon, T.A.; Nguyen, N.D.; Burrell, K.L.; Scott, A.F.; Almestica-Roberts, M.; Rapp, E.; Deering-Rice, C.E.; Reilly, C.A. Wood Smoke Particles Stimulate MUC5AC Overproduction by Human Bronchial Epithelial Cells Through TRPA1 and EGFR Signaling. Toxicol. Sci. 2020, 174, 278–290. [Google Scholar] [CrossRef]
- Kim, B.G.; Park, M.K.; Lee, P.H.; Lee, S.H.; Hong, J.; Aung, M.M.M.; Moe, K.T.; Han, N.Y.; Jang, A.S. Effects of nanoparticles on neuroinflammation in a mouse model of asthma. Respir. Physiol. Neurobiol. 2020, 271, 103292. [Google Scholar] [CrossRef]
- Shapiro, D.; Deering-Rice, C.E.; Romero, E.G.; Hughen, R.W.; Light, A.R.; Veranth, J.M.; Reilly, C.A. Activation of transient receptor potential ankyrin-1 (TRPA1) in lung cells by wood smoke particulate material. Chem. Res. Toxicol. 2013, 26, 750–758. [Google Scholar] [CrossRef] [Green Version]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef] [Green Version]
- Breysse, P.N. Toxicological Profile for Silica; ATSDR: Atlanta, GA, USA, 2019. [Google Scholar]
- Gilardino, A.; Catalano, F.; Ruffinatti, F.A.; Alberto, G.; Nilius, B.; Antoniotti, S.; Martra, G.; Lovisolo, D. Interaction of SiO2 nanoparticles with neuronal cells: Ionic mechanisms involved in the perturbation of calcium homeostasis. Int. J. Biochem. Cell Biol. 2015, 66, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Jiang, W.; Yu, J.; Ding, L.; Hu, J.; Jiang, G. Effects of SiO2 nanoparticles on phospholipid membrane integrity and fluidity. J. Hazard. Mater. 2015, 287, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ariano, P.; Zamburlin, P.; Gilardino, A.; Mortera, R.; Onida, B.; Tomatis, M.; Ghiazza, M.; Fubini, B.; Lovisolo, D. Interaction of spherical silica nanoparticles with neuronal cells: Size-dependent toxicity and perturbation of calcium homeostasis. Small 2011, 7, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.; Dommershausen, N.; Schulz, M.; Landsiedel, R.; Reichardt, P.; Krause, B.C.; Tentschert, J.; Luch, A. Genotoxicity testing of different surface-functionalized SiO2, ZrO2 and silver nanomaterials in 3D human bronchial models. Arch. Toxicol. 2017, 91, 3991–4007. [Google Scholar] [CrossRef]
- Skuland, T.; Låg, M.; Gutleb, A.C.; Brinchmann, B.C.; Serchi, T.; Øvrevik, J.; Holme, J.A.; Refsnes, M. Pro-inflammatory effects of crystalline- And nano-sized non-crystalline silica particles in a 3D alveolar model. Part. Fibre Toxicol. 2020, 17, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Jha, S. Amorphous nanosilica induced toxicity, inflammation and innate immune responses: A critical review. Toxicology 2020, 441, 152519. [Google Scholar] [CrossRef]
- Distasi, C.; Dionisi, M.; Ruffinatti, F.A.; Gilardino, A.; Bardini, R.; Antoniotti, S.; Catalano, F.; Bassino, E.; Munaron, L.; Martra, G.; et al. The interaction of SiO2 nanoparticles with the neuronal cell membrane: Activation of ionic channels and calcium influx. Nanomedicine 2019, 8, 575–594. [Google Scholar] [CrossRef]
- Milici, A.; Talavera, K. Trp channels as cellular targets of particulate matter. Int. J. Mol. Sci. 2021, 22, 2783. [Google Scholar] [CrossRef] [PubMed]
- Dekali, S.; Divetain, A.; Kortulewski, T.; Vanbaelinghem, J.; Gamez, C.; Rogerieux, F.; Lacroix, G.; Rat, P. Cell cooperation and role of the P2X7 receptor in pulmonary inflammation induced by nanoparticles. Nanotoxicology 2013, 7, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Negishi, Y.; Tsukimoto, M.; Takenouchi, T.; Kitani, H.; Takeda, K. Purinergic signaling via P2X7 receptor mediates IL-1β production in Kupffer cells exposed to silica nanoparticle. Toxicology 2014, 321, 13–20. [Google Scholar] [CrossRef]
- Nagakura, C.; Negishi, Y.; Tsukimoto, M.; Itou, S.; Kondo, T.; Takeda, K.; Kojima, S. Involvement of P2Y11 receptor in silica nanoparticles 30-induced IL-6 production by human keratinocytes. Toxicology 2014, 322, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Bläsche, R.; Kasper, M.; Barth, K. A co-culture system with an organotypic lung slice and an immortal alveolar macrophage cell line to quantify silica-induced inflammation. PLoS ONE 2015, 10, e0117056. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tsukimoto, M.; Tanuma, S.I.; Takeda, K.; Kojima, S. Silica nanoparticles activate purinergic signaling via P2X7 receptor in dendritic cells, leading to production of pro-inflammatory cytokines. Toxicol. Vitr. 2016, 35, 202–211. [Google Scholar] [CrossRef]
- Braun, K.; Stürzel, C.M.; Biskupek, J.; Kaiser, U.; Kirchhoff, F.; Lindén, M. Comparison of different cytotoxicity assays for in vitro evaluation of mesoporous silica nanoparticles. Toxicol. Vitr. 2018, 52, 214–221. [Google Scholar] [CrossRef]
- Qi, Z.; Murase, K.; Obata, S.; Sokabe, M. Extracellular ATP-dependent activation of plasma membrane Ca2+ pump in HEK-293 cells. Br. J. Pharmacol. 2000, 131, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Dubyak, G.R.; el-Moatassim, C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 1993, 265, C577–C606. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G. Historical review: ATP as a neurotransmitter. Trends Pharmacol. Sci. 2006, 27, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 119–244. [Google Scholar] [CrossRef]
- Sherwood, C.L.; Clark Lantz, R.; Boitano, S. Chronic arsenic exposure in nanomolar concentrations compromises wound response and intercellular signaling in airway epithelial cells. Toxicol. Sci. 2013, 132, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Van der Vliet, A.; Bove, P.F. Purinergic signaling in wound healing and airway remodeling. Subcell. Biochem. 2015, 55, 139–157. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Boucher, R.C. Purinergic receptors in airway epithelia. Curr. Opin. Pharmacol. 2009, 9, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnstock, G. P2X receptors in sensory neurones. Br. J. Anaesth. 2000, 84, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Strittmatter, S.M. P2Y1 purinergic receptors in sensory neurons: Contribution to touch-induced impulse generation. Proc. Natl. Acad. Sci. USA 1996, 93, 10465–10470. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G.; Brouns, I.; Adriaensen, D.; Timmermans, J.P. Purinergic signaling in the airways. Pharmacol. Rev. 2012, 64, 834–868. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G. Purinergic Signalling and Neurological Diseases: An Update. CNS Neurol. Disord.-Drug Targets 2017, 16, 257–265. [Google Scholar] [CrossRef]
- Burnstock, G. Purines and sensory nerves. Handb. Exp. Pharmacol. 2009, 194, 333–392. [Google Scholar] [CrossRef]
- Fischer, W.; Wirkner, K.; Weber, M.; Eberts, C.; Köles, L.; Reinhardt, R.; Franke, H.; Allgaier, C.; Gillen, C.; Illes, P. Characterization of P2X3, P2Y1 and P2Y4 receptors in cultured HEK293-hP2X3 cells and their inhibition by ethanol and trichloroethanol. J. Neurochem. 2003, 85, 779–790. [Google Scholar] [CrossRef]
- Vilotti, S.; Marchenkova, A.; Ntamati, N.; Nistri, A. B-type natriuretic peptide-induced delayed modulation of TRPV1 and P2X3 Receptors of mouse trigeminal sensory neurons. PLoS ONE 2013, 8, e81138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droguett, K.; Rios, M.; Carreño, D.V.; Navarrete, C.; Fuentes, C.; Villalón, M.; Barrera, N.P. An autocrine ATP release mechanism regulates basal ciliary activity in airway epithelium. J. Physiol. 2017, 595, 4755–4767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirsching, E.; Fauler, M.; Fois, G.; Frick, M. P2 purinergic signaling in the distal lung in health and disease. Int. J. Mol. Sci. 2020, 21, 4973. [Google Scholar] [CrossRef]
- Carew, M.A.; Wu, M.L.; Law, G.J.; Tseng, Y.Z.; Mason, W.T. Extracellular ATP activates calcium entry and mobilization via P2U-purinoceptors in rat lactotrophs. Cell Calcium 1994, 16, 227–235. [Google Scholar] [CrossRef]
- Riteau, N.; Baron, L.; Villeret, B.; Guillou, N.; Savigny, F.; Ryffel, B.; Rassendren, F.; Le Bert, M.; Gombault, A.; Couillin, I. ATP release and purinergic signaling: A common pathway for particle-mediated inflammasome activation. Cell Death Dis. 2012, 3, e403. [Google Scholar] [CrossRef] [Green Version]
- Baron, L.; Gombault, A.; Fanny, M.; Villeret, B.; Savigny, F.; Guillou, N.; Panek, C.; Le Bert, M.; Lagente, V.; Rassendren, F.; et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015, 6, e1629. [Google Scholar] [CrossRef] [Green Version]
- Sienaert, I.; Huyghe, S.; Parys, J.B.; Malfait, M.; Kunzelman, K.; De Smedt, H.; Verleden, G.M.; Missiaen, L. ATP-induced Ca2+ signals in bronchial epithelial cells. Pflug. Arch. Eur. J. Physiol. 1998, 436, 40–48. [Google Scholar] [CrossRef]
- Communi, D.; Paindavoine, P.; Place, G.A.; Parmentier, M.; Boeynaems, J.M. Expression of P2Y receptors in cell lines derived from the human lung. Br. J. Pharmacol. 1999, 127, 562–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasper, J.; Hermanns, M.I.; Bantz, C.; Maskos, M.; Stauber, R.; Pohl, C.; Unger, R.E.; Kirkpatrick, J.C. Inflammatory and cytotoxic responses of an alveolar-capillary coculture model to silica nanoparticles: Comparison with conventional monocultures. Part. Fibre Toxicol. 2011, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabolli, V.; Badissi, A.A.; Devosse, R.; Uwambayinema, F.; Yakoub, Y.; Palmai-Pallag, M.; Lebrun, A.; De Gussem, V.; Couillin, I.; Ryffel, B.; et al. The alarmin IL-1α is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Part. Fibre Toxicol. 2014, 11, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skuland, T.; Øvrevik, J.; Låg, M.; Schwarze, P.; Refsnes, M. Silica nanoparticles induce cytokine responses in lung epithelial cells through activation of a p38/TACE/TGF-α/EGFR-pathway and NF-κΒ signalling. Toxicol. Appl. Pharmacol. 2014, 279, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]




| Property | Value |
|---|---|
| Size | 10.2 nm (P10 = 8.1 nm and P90 = 11.8 nm) |
| Shape | Spherical |
| Solid structure | Amorphous |
| Zeta potential | −20 ± 3 mV |
| Dispersion | Monodispersed |
| Endotoxin content | <0.05 EU/mL |
| Krebs | 150 mM NaCl |
| 6 mM KCl | |
| 1.5 mM CaCl2 × 2H2O | |
| 1 mM MgCl × 6H2O | |
| 10 mM glucose | |
| 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) | |
| Ca2+-Free Krebs | 150 mM NaCl |
| 6 mM KCl | |
| 1 mM MgCl × 6H2O | |
| 10 mM glucose | |
| 10 mM HEPES | |
| 10 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milici, A.; Sanchez, A.; Talavera, K. Silica Nanoparticles Inhibit Responses to ATP in Human Airway Epithelial 16HBE Cells. Int. J. Mol. Sci. 2021, 22, 10173. https://doi.org/10.3390/ijms221810173
Milici A, Sanchez A, Talavera K. Silica Nanoparticles Inhibit Responses to ATP in Human Airway Epithelial 16HBE Cells. International Journal of Molecular Sciences. 2021; 22(18):10173. https://doi.org/10.3390/ijms221810173
Chicago/Turabian StyleMilici, Alina, Alicia Sanchez, and Karel Talavera. 2021. "Silica Nanoparticles Inhibit Responses to ATP in Human Airway Epithelial 16HBE Cells" International Journal of Molecular Sciences 22, no. 18: 10173. https://doi.org/10.3390/ijms221810173
APA StyleMilici, A., Sanchez, A., & Talavera, K. (2021). Silica Nanoparticles Inhibit Responses to ATP in Human Airway Epithelial 16HBE Cells. International Journal of Molecular Sciences, 22(18), 10173. https://doi.org/10.3390/ijms221810173






