14,15-EET Reduced Brain Injury from Cerebral Ischemia and Reperfusion via Suppressing Neuronal Parthanatos
Abstract
:1. Introduction
2. Results
2.1. 14,15-EET Reduces Infarct Volume and Cell Death Induced by Cerebral Ischemia and Reperfusion
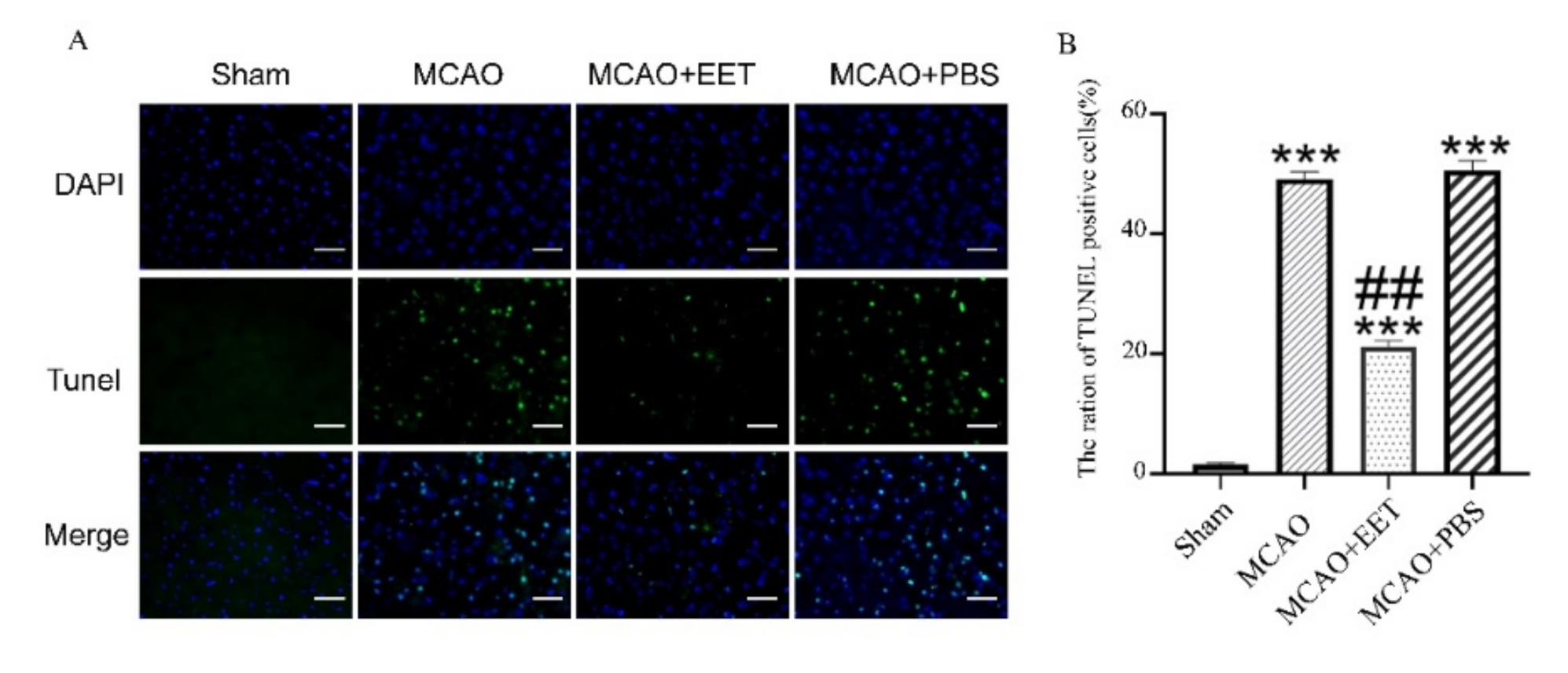
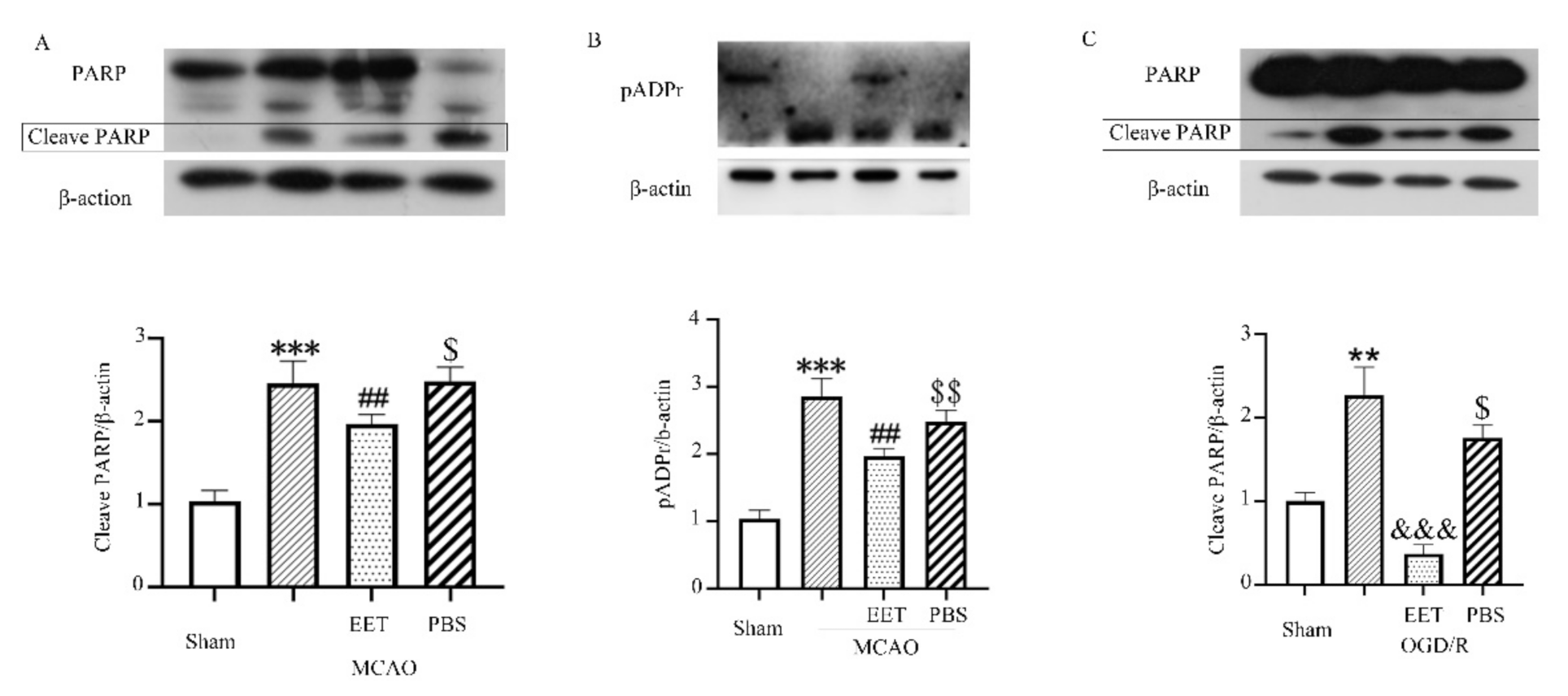
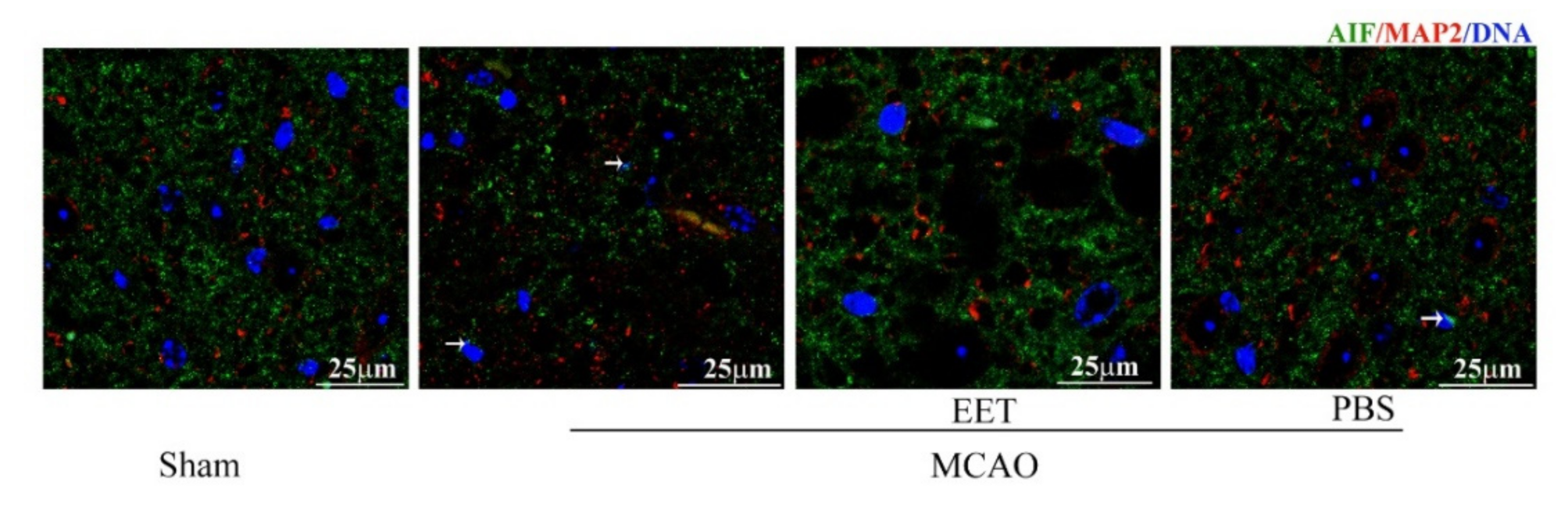
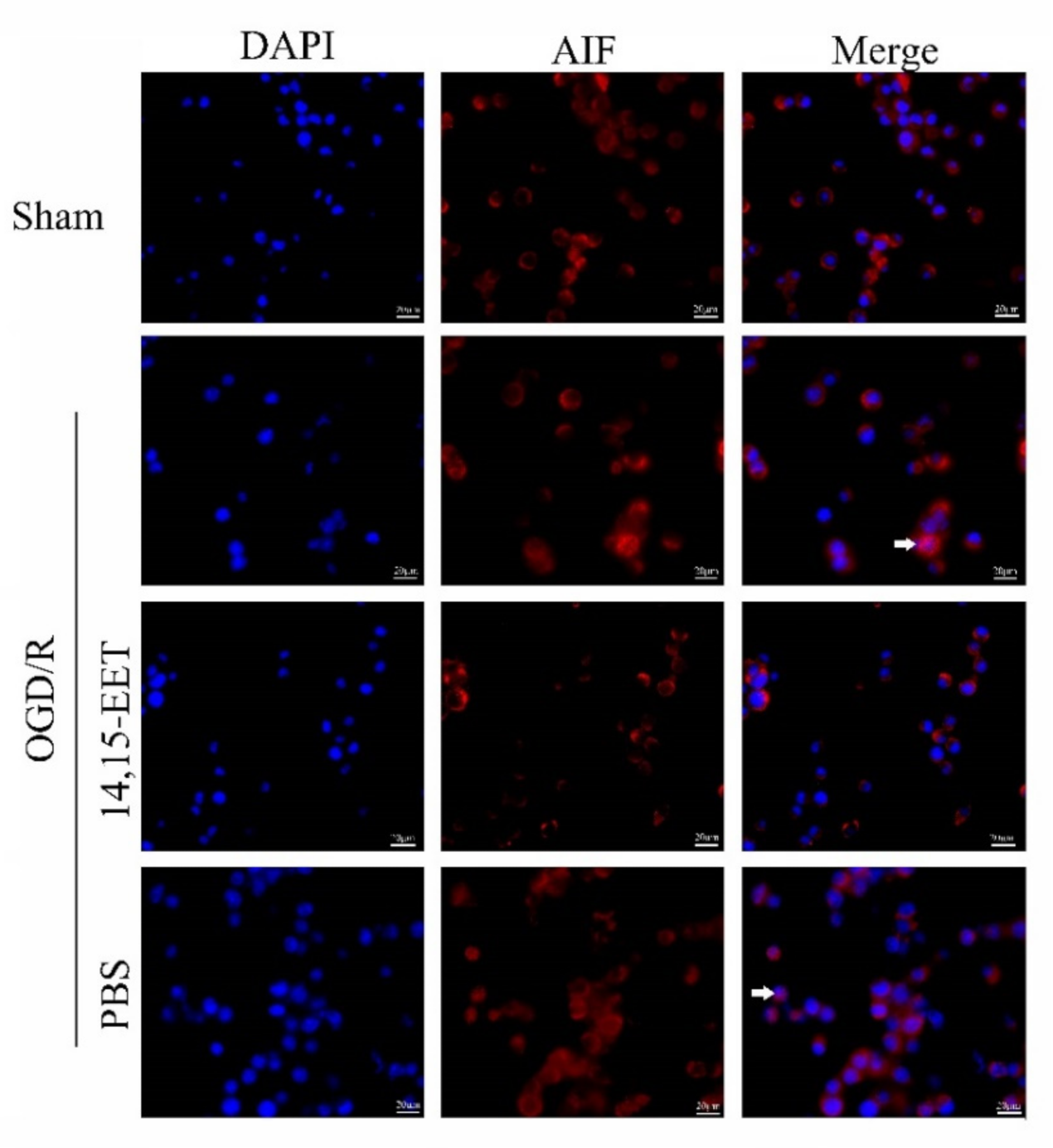
2.2. 14,15-EET Inhibits Parthanatos Induced by Cerebral Ischemia and Reperfusion
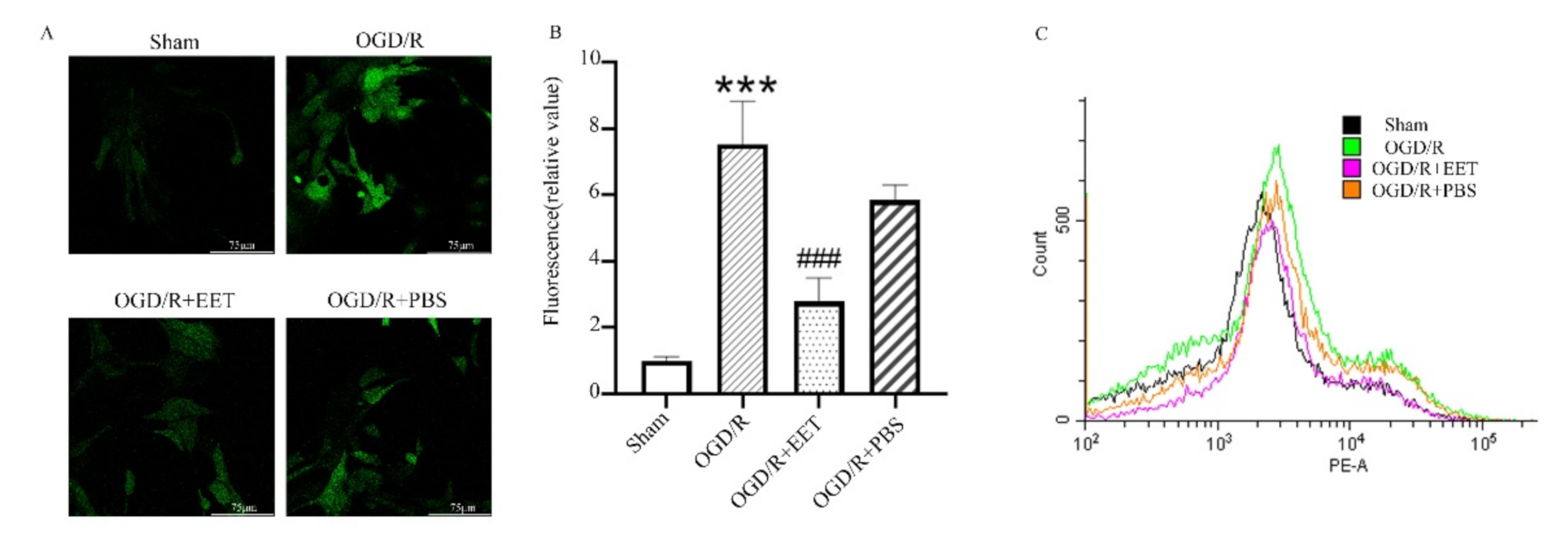
2.3. 14,15-EET Reduced the Generation of ROS Induced by OGD/R
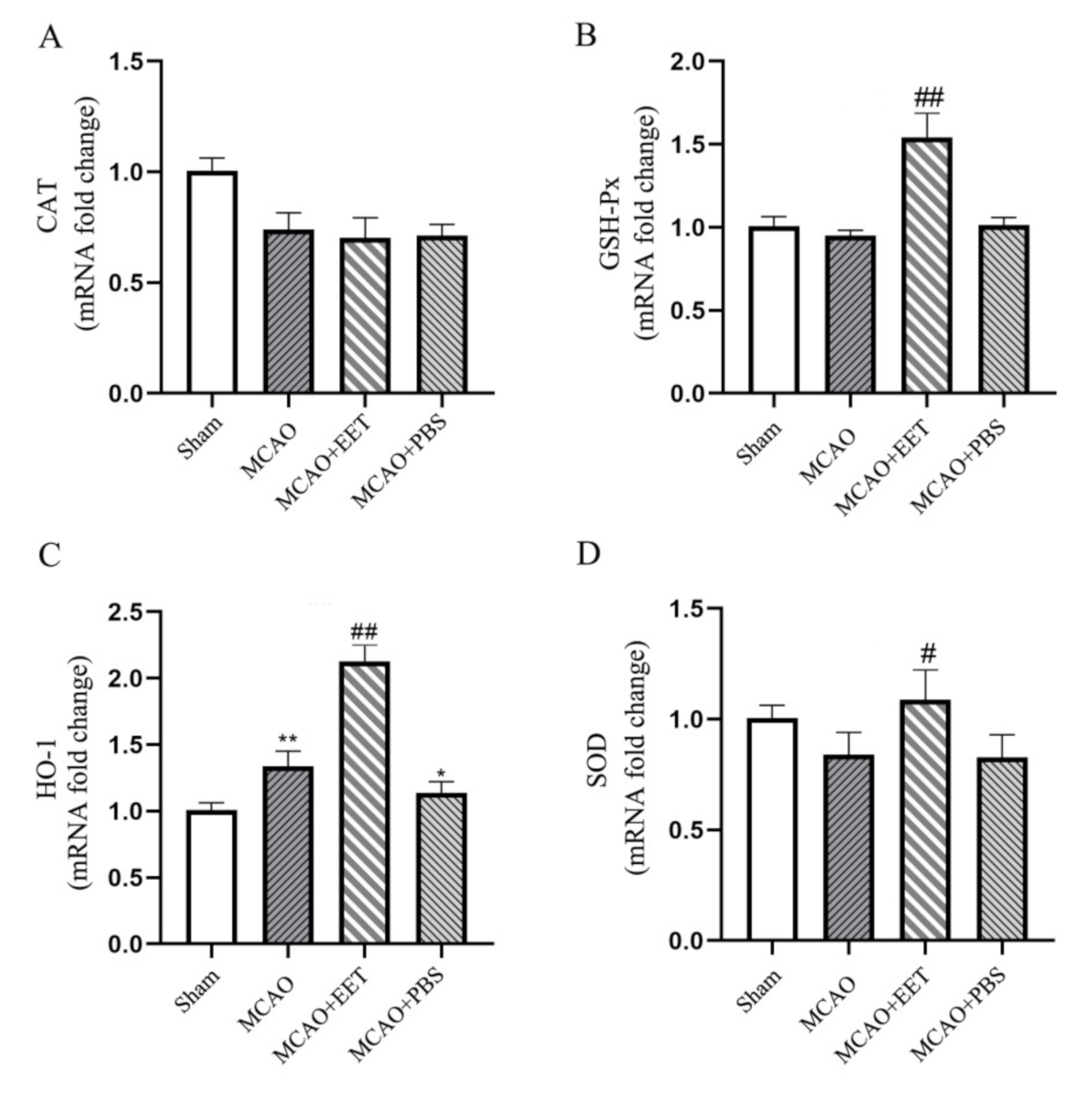
2.4. 14,15-EET UpRegulated the Transcription of Antioxidant Genes in Neurons after MCAO/R
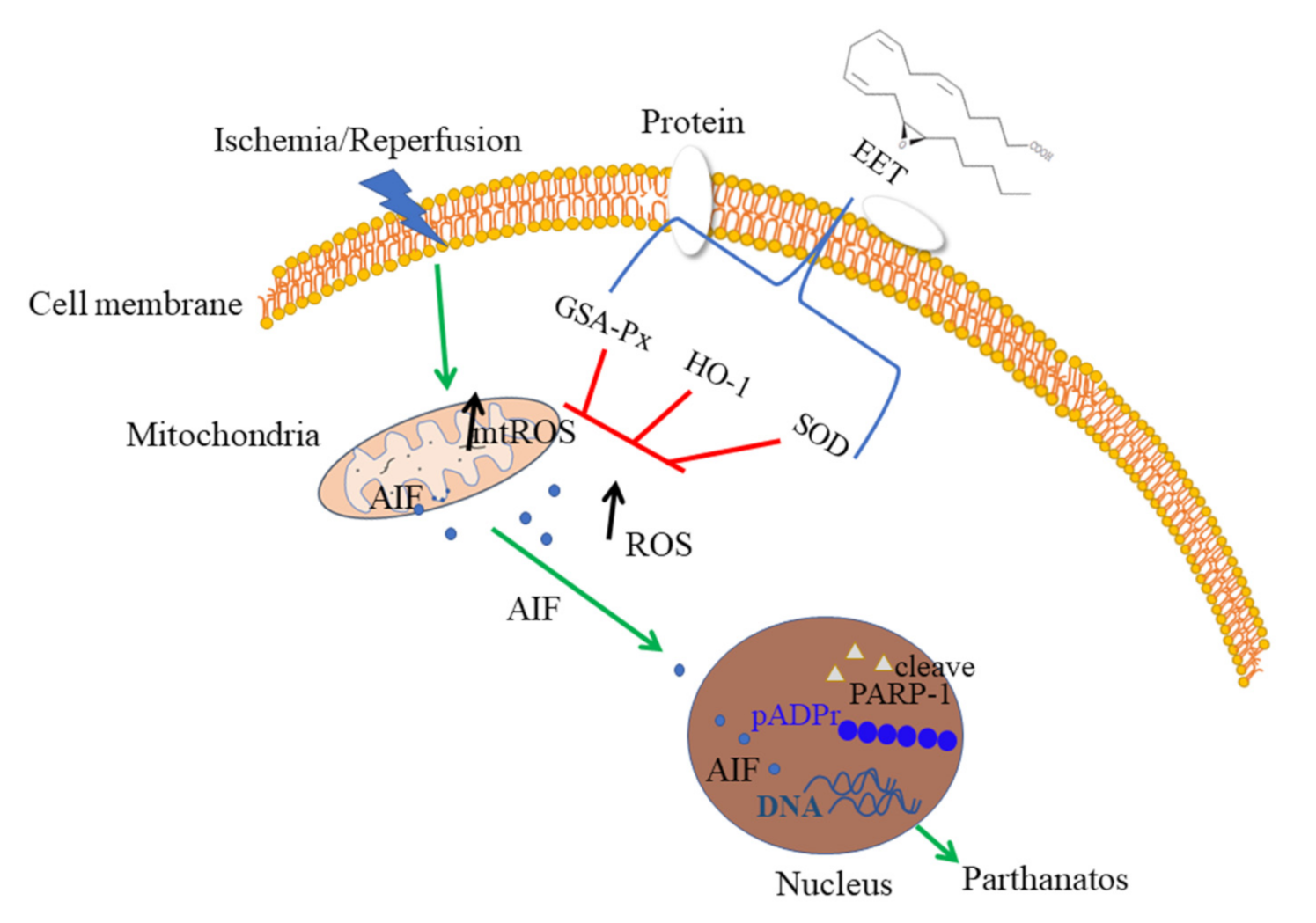
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Middle Cerebral Artery Occlusion and Reperfusion (MCAO/R)
4.3. Behavioral Assessment
4.4. Primary Cortical Neurons Culture
4.5. TTC Staining
4.6. Oxygen Glucose Deprivation/Reoxygenation (OGD/R)
4.7. Western Blot
4.8. Immunofluorescence
4.9. Measurement of ROS
4.10. Apoptosis Detection
4.11. Real-Time PCR (qPCR)
4.12. Statistics and Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Narne, P.; Pandey, V.; Simhadri, P.K.; Phanithi, P.B. Poly (ADP-ribose)polymerase-1 hyperactivation in neurodegenerative diseases: The death knell tolls for neurons. Semin. Cell Dev. Biol. 2017, 63, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Martire, S.; Mosca, L.; d′Erme, M. PARP-1 involvement in neurodegeneration: A focus on Alzheimer′s and Parkinson′s diseases. Mech. Ageing Dev. 2015, 146–148, 53–64. [Google Scholar] [CrossRef]
- Wang, X.; Ge, P. Parthanatos in the pathogenesis of nervous system diseases. Neuroscience 2020, 449, 241–250. [Google Scholar] [CrossRef]
- Koehler, R.C.; Dawson, V.L.; Dawson, T.M. Targeting Parthanatos in Ischemic Stroke. Front. Neurol. 2021, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Han, Y.; Guo, X.; Wen, J.; Wang, K.; Jiang, X.; Tian, X.; Ba, X.; Boldogh, I.; Zeng, X. PARP1 promotes gene expression at the post-transcriptiona level by modulating the RNA-binding protein HuR. Nat. Commun. 2017, 8, 14632. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Luo, G.; Zhang, L.F.; Geng, H.X. Neuroprotective effects of epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat. 2018, 138, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Otsuka, T.; Sugo, N.; Ardeshiri, A.; Alhadid, Y.K.; Iliff, J.J.; DeBarber, A.E.; Koop, D.R.; Alkayed, N.J. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke 2008, 39, 2073–2078. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Wang, M.; Zhu, M.; Xiong, W.; Qin, X.; Zhu, X. 14,15-Epoxyeicosatrienoic Acid Alleviates Pathology in a Mouse Model of Alzheimer′s Disease. J. Neurosci. 2020, 40, 8188–8203. [Google Scholar] [CrossRef]
- Geng, H.X.; Li, R.P.; Li, Y.G.; Wang, X.Q.; Zhang, L.; Deng, J.B.; Wang, L.; Deng, J.X. 14,15-EET Suppresses Neuronal Apoptosis in Ischemia-Reperfusion Through the Mitochondrial Pathway. Neurochem. Res. 2017, 42, 2841–2849. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Yuan, L.; Xiang, Y.; Zheng, R.; Zhu, S. 14,15-EET promotes mitochondrial biogenesis and protects cortical neurons against oxygen/glucose deprivation-induced apoptosis. Biochem. Biophys. Res. Commun. 2014, 450, 604–609. [Google Scholar] [CrossRef]
- Park, H.; Kam, T.I.; Dawson, T.M.; Dawson, V.L. Poly (ADP-ribose) (PAR)-dependent cell death in neurodegenerative diseases. Int. Rev. Cell Mol. Biol. 2020, 353, 1–29. [Google Scholar] [PubMed]
- Thapa, K.; Khan, H.; Sharma, U.; Grewal, A.K.; Singh, T.G. Poly (ADP-ribose) polymerase-1 as a promising drug target for neurodegenerative diseases. Life Sci. 2021, 267, 118975. [Google Scholar] [CrossRef]
- Salech, F.; Ponce, D.P.; Paula-Lima, A.C.; SanMartin, C.D.; Behrens, M.I. Nicotinamide, a Poly [ADP-Ribose] Polymerase 1 (PARP-1) Inhibitor, as an Adjunctive Therapy for the Treatment of Alzheimer′s Disease. Front Aging Neurosci. 2020, 12, 255. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Zhao, Y.; Zhang, J.; Liu, B.; Jiao, S.; Zhang, X. Astragaloside IV reduces neuronal apoptosis and parthanatos in ischemic injury by preserving mitochondrial hexokinase-II. Free Radic. Biol. Med. 2019, 131, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Yang, Y.L.; Cheng, X.; Liu, M.; Zhang, S.S.; Wang, Y.H.; Du, G.H. Baicalein attenuates caspase-independent cells death via inhibiting PARP-1 activation and AIF nuclear translocation in cerebral ischemia/reperfusion rats. Apoptosis 2020, 25, 354–369. [Google Scholar] [CrossRef]
- Spector, A.A.; Fang, X.; Snyder, G.D.; Weintraub, N.L. Epoxyeicosatrienoic acids (EETs): Metabolism and biochemical function. Prog. Lipid Res. 2004, 43, 55–90. [Google Scholar] [CrossRef]
- Dong, W.; Yang, B.; Wang, Y.; Yuan, J.; Fan, Y.; Song, E.; Song, Y. Polybrominated Diphenyl Ethers Quinone Induced Parthanatos-like Cell Death through a Reactive Oxygen Species-Associated Poly (ADP-ribose) Polymerase 1 Signaling. Chem. Res. Toxicol. 2018, 31, 1164–1171. [Google Scholar] [CrossRef]
- Turovskaya, M.V.; Gaidin, S.G.; Vedunova, M.V.; Babaev, A.A.; Turovsky, E.A. BDNF Overexpression Enhances the Preconditioning Effect of Brief Episodes of Hypoxia, Promoting Survival of GABAergic Neurons. Neurosci. Bull. 2020, 36, 733–760. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Lu, J.; Manaenko, A.; Tang, J.; Hu, Q. Mitochondria in Ischemic Stroke: New Insight and Implications. Aging Dis. 2018, 9, 924–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turovskaya, M.V.; Gaidin, S.G.; Mal′tseva, V.N.; Zinchenko, V.P.; Turovsky, E.A. Taxifolin protects neurons against ischemic injury in vitro via the activation of antioxidant systems and signal transduction pathways of GABAergic neurons. Mol. Cell Neurosci. 2019, 96, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Waldman, M.; Bellner, L.; Vanella, L.; Schragenheim, J.; Sodhi, K.; Singh, S.P.; Lin, D.; Lakhkar, A.; Li, J.; Hochhauser, E.; et al. Epoxyeicosatrienoic Acids Regulate Adipocyte Differentiation of Mouse 3T3 Cells, Via PGC-1α Activation, Which Is Required for HO-1 Expression and Increased Mitochondrial Function. Stem. Cells Dev. 2016, 25, 1084–1094. [Google Scholar] [CrossRef] [Green Version]
- Arad, M.; Waldman, M.; Abraham, N.G.; Hochhauser, E. Therapeutic approaches to diabetic cardiomyopathy: Targeting the antioxidant pathway. Prostaglandins Other Lipid Mediat. 2020, 150, 106454. [Google Scholar] [CrossRef]
- Zhang, C.H.; Zheng, L.; Gui, L.; Lin, J.-Y.; Zhu, Y.-M.; Deng, W.-S.; Luo, M. Soluble epoxide hydrolase inhibition with t-TUCB alleviates liver fibrosis and portal pressure in carbon tetrachloride-induced cirrhosis in rats. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, M.; Bellner, L.; Singh, S.P.; Favero, G.; Rezzani, R.; Rodella, L.F.; Falck, J.R.; Abraham, N.G.; Vanella, L. Epoxyeicosatrienoic intervention improves NAFLD in leptin receptor deficient mice by an increase in PGC1α-HO-1-PGC1α-mitochondrial signaling. Exp. Cell Res. 2019, 380, 180–187. [Google Scholar] [CrossRef]
- Singh, S.P.; Schragenheim, J.; Cao, J.; Falck, J.R.; Abraham, N.G.; Bellner, L. PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: Role of epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat. 2016, 125, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varennes, O.; Mentaverri, R.; Duflot, T.; Kauffenstein, G.; Objois, T.; Lenglet, G.; Avondo, C.; Morisseau, C.; Brazier, M.; Kamel, S.; et al. The Metabolism of Epoxyeicosatrienoic Acids by Soluble Epoxide Hydrolase Is Protective against the Development of Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 4313. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Li, Z.; Liu, T.; Chen, X.; Xu, Q.; Yao, W.; Zhang, C.; Zhang, Y. Epoxyeicosatrienoic acids: Emerging therapeutic agents for central post-stroke pain. Pharmacol. Res. 2020, 159, 104923. [Google Scholar] [CrossRef]
- Luther, J.M.; Brown, N.J. Epoxyeicosatrienoic acids and glucose homeostasis in mice and men. Prostaglandins Other Lipid Mediat. 2016, 125, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares-Rubio, H.F.; Espinosa-Aguirre, J.J. Role of epoxyeicosatrienoic acids in the lung. Prostaglandins Other Lipid Mediat. 2020, 149, 106451. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Fu, X.; Zhang, D.; Yu, L.; Li, N.; Gao, Y.; Liu, X.; Yin, C.; Ke, J.; et al. Alpha-7 Nicotinic Receptor Signaling Pathway Participates in the Neurogenesis Induced by ChAT-Positive Neurons in the Subventricular Zone. Transl. Stroke Res. 2017, 8, 484–493. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Tang, J.; Chen, H.; Gu, W.; Geng, H.; Wang, L.; Wang, Y. 14,15-EET Reduced Brain Injury from Cerebral Ischemia and Reperfusion via Suppressing Neuronal Parthanatos. Int. J. Mol. Sci. 2021, 22, 9660. https://doi.org/10.3390/ijms22189660
Zhao H, Tang J, Chen H, Gu W, Geng H, Wang L, Wang Y. 14,15-EET Reduced Brain Injury from Cerebral Ischemia and Reperfusion via Suppressing Neuronal Parthanatos. International Journal of Molecular Sciences. 2021; 22(18):9660. https://doi.org/10.3390/ijms22189660
Chicago/Turabian StyleZhao, Haipeng, Jing Tang, Hongyang Chen, Wei Gu, Huixia Geng, Lai Wang, and Yanming Wang. 2021. "14,15-EET Reduced Brain Injury from Cerebral Ischemia and Reperfusion via Suppressing Neuronal Parthanatos" International Journal of Molecular Sciences 22, no. 18: 9660. https://doi.org/10.3390/ijms22189660
APA StyleZhao, H., Tang, J., Chen, H., Gu, W., Geng, H., Wang, L., & Wang, Y. (2021). 14,15-EET Reduced Brain Injury from Cerebral Ischemia and Reperfusion via Suppressing Neuronal Parthanatos. International Journal of Molecular Sciences, 22(18), 9660. https://doi.org/10.3390/ijms22189660





