Water Dynamics in Whey-Protein-Based Composite Hydrogels by Means of NMR Relaxometry
Abstract
1. Introduction
2. Results
2.1. Theoretical Model of 1H Spin-Lattice Relaxation
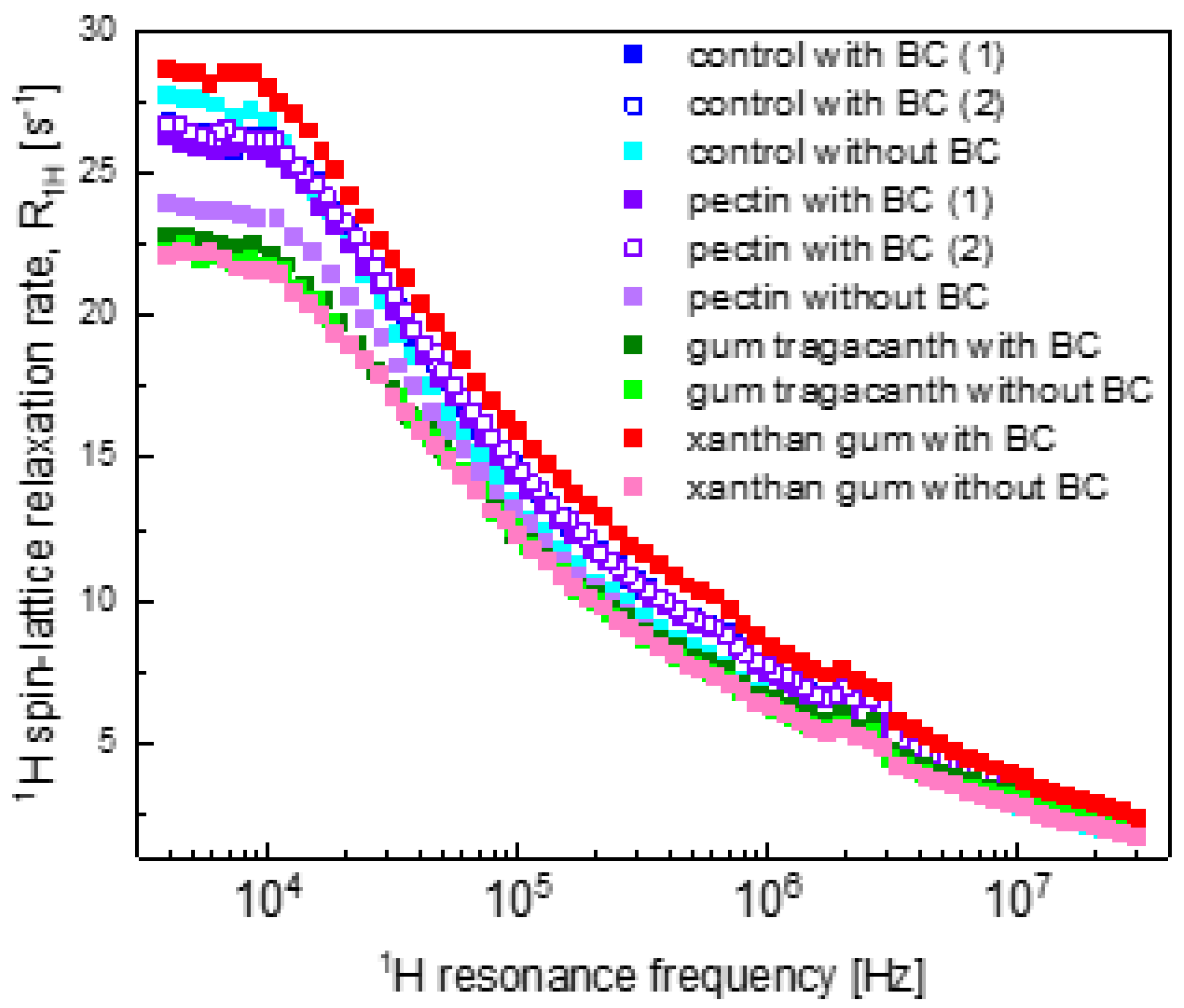
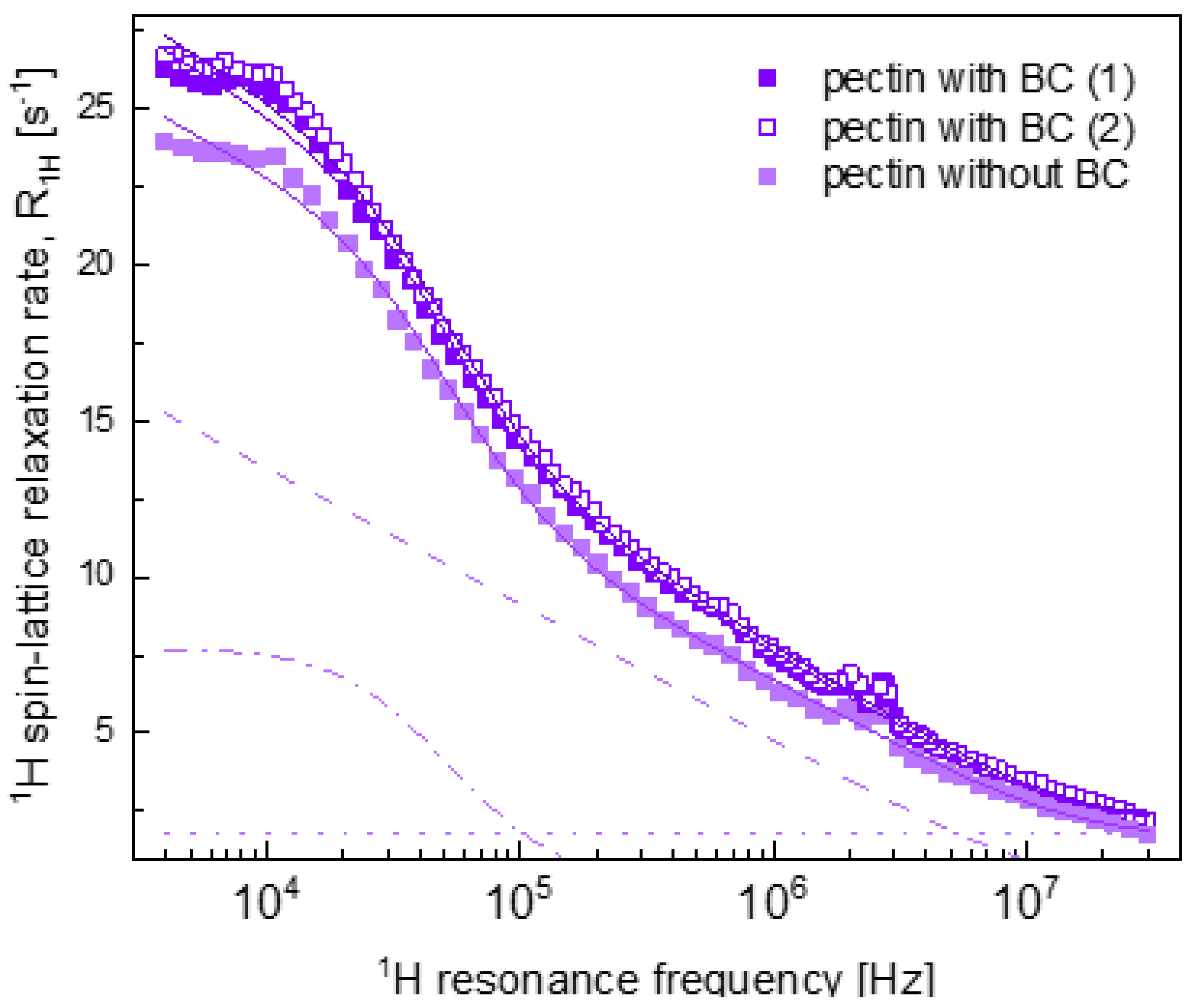
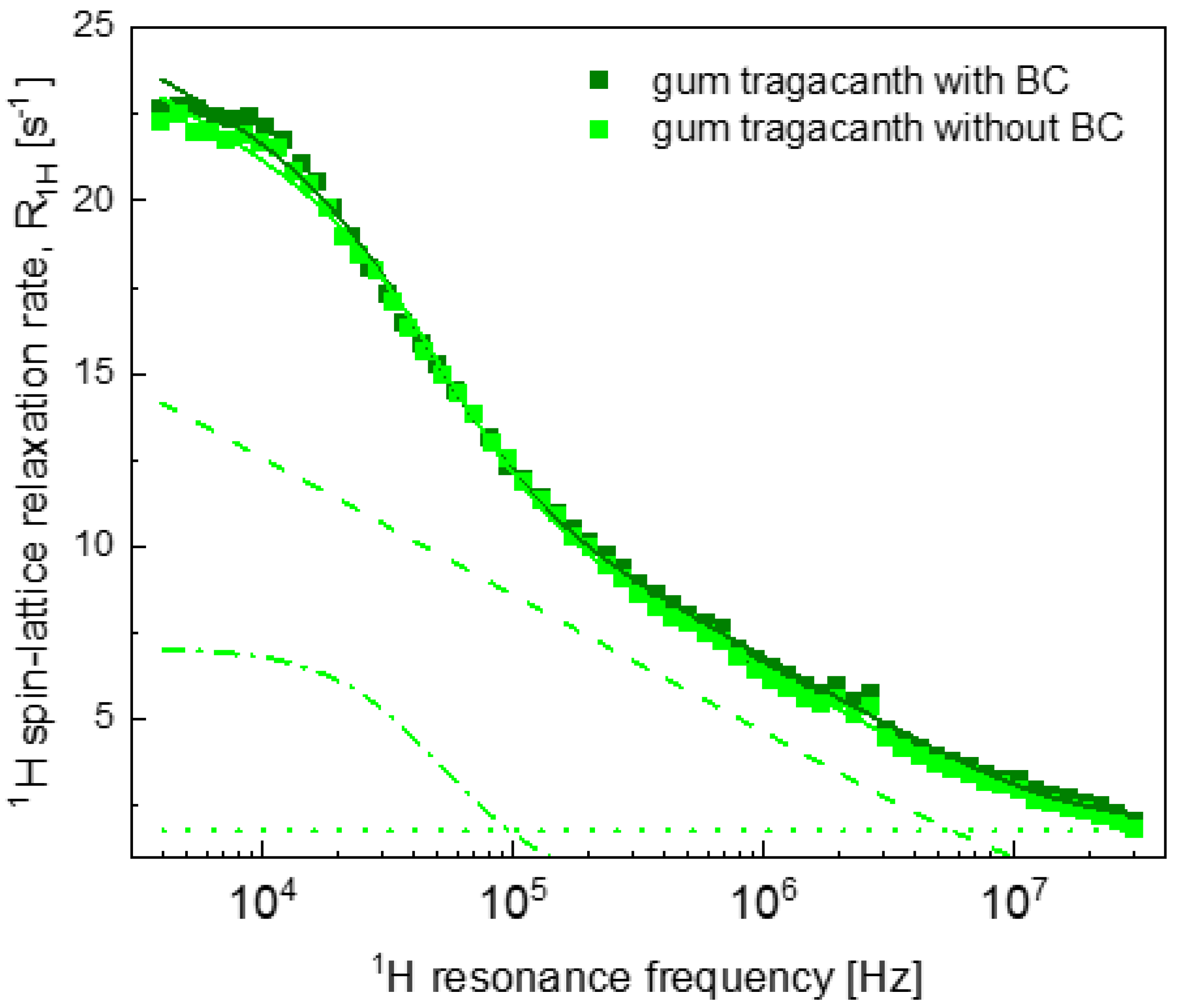
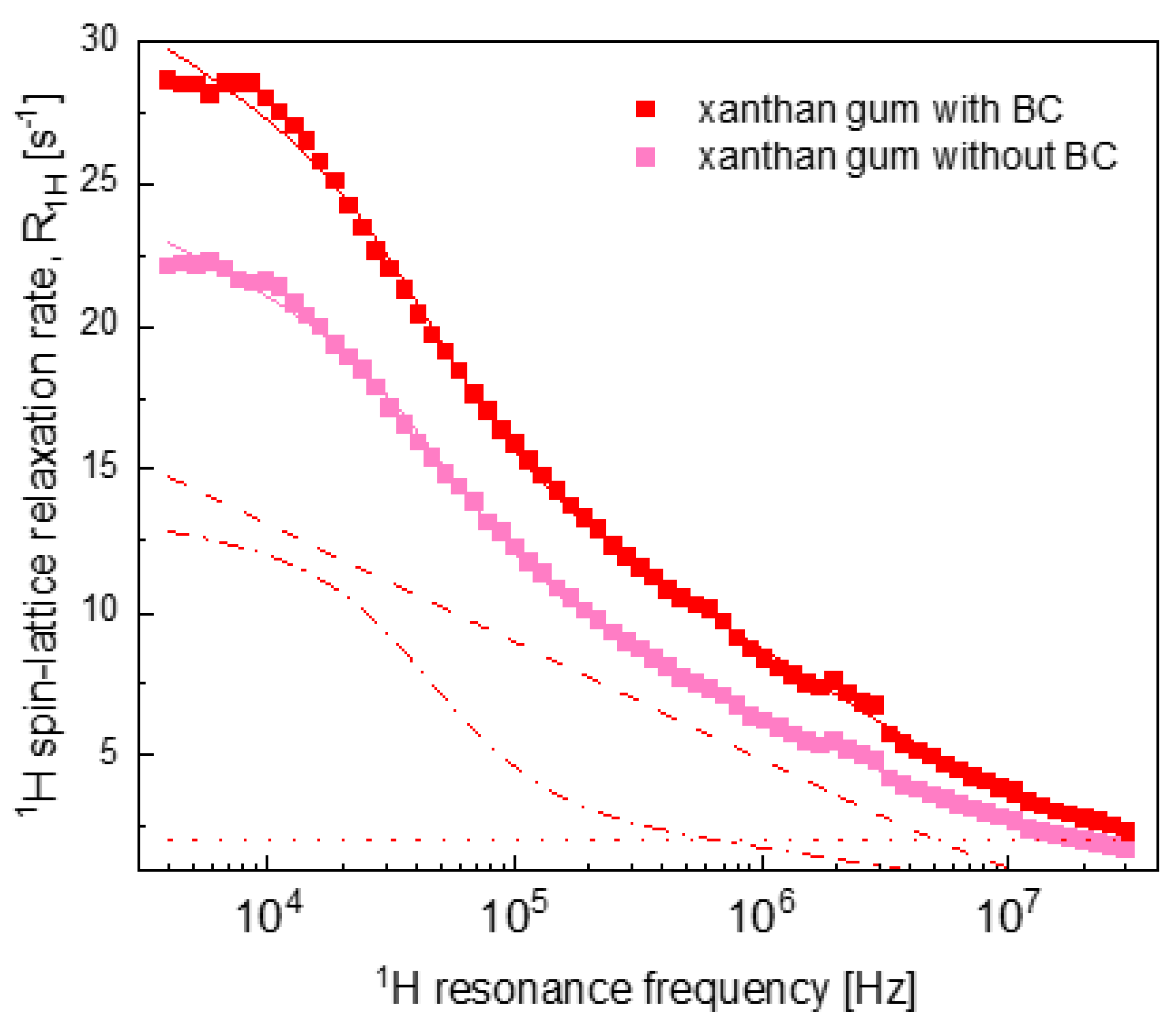
2.2. Relaxation Data
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Fast Field Cycling (FFC) NMR Relaxometry Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Argin, S.; Kofinas, P.; Lo, Y.M. The cell release kinetics and the swelling behavior of physically crosslinked xanthan-chitosan hydrogels in simulated gastrointestinal conditions. Food Hydrocoll. 2014, 40, 138–144. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Hydrogels: Characteristics and application as delivery systems of Phenolic and aroma compounds. Foods 2021, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Gulrez, K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterization and Applications. In Progress in Molecular and Environmental Bioengineering-From Analysis and Modeling to Technology Applications; Carpi, A., Ed.; IntechOpen Limited: London, UK, 2011; pp. 117–150. [Google Scholar]
- Garg, S.; Garg, A. Hydrogel: Classification, Properties, Preparation and Technical Features. Asian J. Biomater. Res. 2016, 2, 163–170. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Wichchukit, S.; Oztop, M.H.; McCarthy, M.J.; McCarthy, K.L. Whey protein/alginate beads as carriers of a bioactive component. Food Hydrocoll. 2013, 33, 66–73. [Google Scholar] [CrossRef]
- Si, L.; Zhao, Y.; Huang, J.; Li, S.; Zhai, X.; Li, G. Calcium pectinate gel bead intended for oral protein delivery: Preparation improvement and formulation development. Chem. Pharm. Bull. 2009, 57, 663–667. [Google Scholar] [CrossRef][Green Version]
- Lu, M.; Li, Z.; Liang, H.; Shi, M.; Zhao, L.; Li, W.; Chen, Y.; Wu, J.; Wang, S.; Chen, X.; et al. Controlled release of anthocyanins from oxidized konjac glucomannan microspheres stabilized by chitosan oligosaccharides. Food Hydrocoll. 2015, 51, 476–485. [Google Scholar] [CrossRef]
- Betz, M.; Kulozik, U. Whey protein gels for the entrapment of bioactive anthocyanins from bilberry extract. Int. Dairy J. 2011, 21, 703–710. [Google Scholar] [CrossRef]
- Maltais, A.; Remondetto, G.E.; Subirade, M. Soy protein cold-set hydrogels as controlled delivery devices for nutraceutical compounds. Food Hydrocoll. 2009, 23, 1647–1653. [Google Scholar] [CrossRef]
- Mession, J.L.; Blanchard, C.; Mint-Dah, F.V.; Lafarge, C.; Assifaoui, A.; Saurel, R. The effects of sodium alginate and calcium levels on pea proteins cold-set gelation. Food Hydrocoll. 2013, 31, 446–457. [Google Scholar] [CrossRef]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Betz, M.; Kulozik, U. Microencapsulation of bioactive bilberry anthocyanins by means of whey protein gels. Procedia Food Sci. 2011, 1, 2047–2056. [Google Scholar] [CrossRef]
- Akhtar, S.; Rauf, A.; Imran, M.; Qamar, M.; Riaz, M.; Mubarak, M.S. Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends Food Sci. Technol. 2017, 66, 36–47. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Kirca, A.; Cemeroǧlu, B. Degradation kinetics of anthocyanins in blood orange juice and concentrate. Food Chem. 2003, 81, 583–587. [Google Scholar] [CrossRef]
- Le, X.T.; Rioux, L.E.; Turgeon, S.L. Formation and functional properties of protein–polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef]
- Gupta, S.; D’Mello, R.; Chance, M.R. Structure and dynamics of protein waters revealed by radiolysis and mass spectrometry. Proc. Natl. Acad. Sci. USA 2012, 109, 14882–14887. [Google Scholar] [CrossRef] [PubMed]
- Eneh, C.I.; Bolen, M.J.; Suarez-Martinez, P.C.; Bachmann, A.L.; Zimudzi, T.J.; Hickner, M.A.; Batys, P.; Sammalkorpi, M.; Lutkenhaus, J.L. Fourier transform infrared spectroscopy investigation of water microenvironments in polyelectrolyte multilayers at varying temperatures. Soft Matter 2020, 16, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.D.; Oztop, M.H.; Mccarthy, M.J.; Mccarthy, K.L.; Lo, Y.M. Characterization of water distribution in xanthan-curdlan hydrogel complex using magnetic resonance imaging, nuclear magnetic resonance relaxometry, rheology, and scanning electron microscopy. J. Food Sci. 2011, 76, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, R.H.; Bradley, W.G.; Lisanti, C.J. MRI: The Basics, 3rd ed.; Lippincott Williams and Wilkins: Baltimore, MD, USA, 2010; ISBN 978-1-60831-115-6. [Google Scholar]
- Kirtil, E.; Oztop, M.H. 1H Nuclear Magnetic Resonance Relaxometry and Magnetic Resonance Imaging and Applications in Food Science and Processing. Food Eng. Rev. 2016, 8, 1–22. [Google Scholar] [CrossRef]
- Ozel, B.; Cikrikci, S.; Aydin, O.; Oztop, M.H. Polysaccharide blended whey protein isolate-(WPI) hydrogels: A physicochemical and controlled release study. Food Hydrocoll. 2017, 71, 35–46. [Google Scholar] [CrossRef]
- Mariette, F. Investigations of food colloids by NMR and MRI. Curr. Opin. Colloid Interface Sci. 2009, 14, 203–211. [Google Scholar] [CrossRef]
- Ozel, B.; Uguz, S.S.; Kilercioglu, M.; Grunin, L.; Oztop, M.H. Effect of different polysaccharides on swelling of composite whey protein hydrogels: A low field (LF) NMR relaxometry study. J. Food Process Eng. 2017, 40, 1–9. [Google Scholar] [CrossRef]
- Kimmich, R.; Anoardo, E. Field-cycling NMR relaxometry. Prog. Nucl. Magn. Reson. Spectrosc. 2004, 44, 257–320. [Google Scholar] [CrossRef]
- Kruk, D.; Meier, R.; Rachocki, A.; Korpała, A.; Singh, R.K.; Rössler, E.A. Determining diffusion coefficients of ionic liquids by means of field cycling nuclear magnetic resonance relaxometry. J. Chem. Phys. 2014, 140, 244509. [Google Scholar] [CrossRef] [PubMed]
- Rachocki, A.; Latanowicz, L.; Tritt-Goc, J. Dynamic processes and chemical composition of Lepidium sativum seeds determined by means of field-cycling NMR elaxometry and NMR spectroscopy. Anal. Bioanal. Chem. 2012, 404, 3155–3164. [Google Scholar] [CrossRef]
- Rachocki, A.; Tritt-Goc, J. Novel application of NMR relaxometry in studies of diffusion in virgin rape oil. Food Chem. 2014, 152, 94–99. [Google Scholar] [CrossRef]
- Steele, R.M.; Korb, J.P.; Ferrante, G.; Bubici, S. New applications and perspectives of fast field cycling NMR relaxometry. Magn. Reson. Chem. 2016, 54, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Capitani, D.; Sobolev, A.P.; Delfini, M.; Vista, S.; Antiochia, R.; Proietti, N.; Bubici, S.; Ferrante, G.; Carradori, S.; De Salvador, F.R.; et al. NMR methodologies in the analysis of blueberries. Electrophoresis 2014, 35, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Bajd, F.; Gradišek, A.; Apih, T.; Serša, I. Dry-cured ham tissue characterization by fast field cycling nmr relaxometry and quantitative magnetization transfer. Magn. Reson. Chem. 2016, 54, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Mineo, V.; Bubici, S.; De Pasquale, C.; Aboud, F.; MacCotta, A.; Planeta, D.; Alonzo, G. Dynamics of pistachio oils by proton nuclear magnetic resonance relaxation dispersion. Anal. Bioanal. Chem. 2011, 400, 1443–1450. [Google Scholar] [CrossRef]
- Kruk, D.; Florek-Wojciechowska, M.; Masiewicz, E.; Oztop, M.; Ploch-Jankowska, A.; Duda, P.; Wilczynski, S. Water mobility in cheese by means of Nuclear Magnetic Resonance relaxometry. J. Food Eng. 2021, 298, 110483. [Google Scholar] [CrossRef]
- Kruk, D.; Masiewicz, E.; Wojciechowski, M.; Florek-Wojciechowska, M.; Broche, L.M.; Lurie, D.J. Slow dynamics of solid proteins—Nuclear magnetic resonance relaxometry versus dielectric spectroscopy. J. Magn. Reson. 2020, 314, 106721. [Google Scholar] [CrossRef]
- Pocan, P.; Ilhan, E.; Florek-Wojciechowska, M.; Masiewicz, E.; Kruk, D.; Oztop, M.H. Exploring the water mobility in gelatin based soft candies by means of Fast Field Cycling (FFC) Nuclear Magnetic Resonance relaxometry. J. Food Eng. 2021, 294, 110422. [Google Scholar] [CrossRef]
- Hofmann, M.; Gainaru, C.; Cetinkaya, B.; Valiullin, R.; Fatkullin, N.; Rössler, E.A. Field-Cycling Relaxometry as a Molecular Rheology Technique: Common Analysis of NMR, Shear Modulus and Dielectric Loss Data of Polymers vs Dendrimers. Macromolecules 2015, 48, 7521–7534. [Google Scholar] [CrossRef]
- Meier, R.; Herrmann, A.; Hofmann, M.; Schmidtke, B.; Kresse, B.; Privalov, A.F.; Kruk, D.; Fujara, F.; Rössler, E.A. Iso-frictional mass dependence of diffusion of polymer melts revealed by 1H NMR relaxometry. Macromolecules 2013, 46, 5538–5548. [Google Scholar] [CrossRef]
- Meier, R.; Herrmann, A.; Kresse, B.; Privalov, A.F.; Kruk, D.; Fujara, F.; Rössler, E.A. Long-time diffusion in polymer melts revealed by 1H NMR relaxometry. ACS Macro Lett. 2013, 2, 96–99. [Google Scholar] [CrossRef]
- Kruk, D.; Rochowski, P.; Masiewicz, E.; Wilczynski, S.; Wojciechowski, M.; Broche, L.M.; Lurie, D.J. Mechanism of Water Dynamics in Hyaluronic Dermal Fillers Revealed by Nuclear Magnetic Resonance Relaxometry. Chem. Phys. Chem. 2019, 20, 2816–2822. [Google Scholar] [CrossRef]
- Alacik Develioglu, I.; Ozel, B.; Sahin, S.; Oztop, M.H. NMR Relaxometry and magnetic resonance imaging as tools to determine the emulsifying characteristics of quince seed powder in emulsions and hydrogels. Int. J. Biol. Macromol. 2020, 164, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Fries, P.H. Dipolar nuclear spin relaxation in liquids and plane fluids undergoing chemical reactions. Mol. Phys. 1983, 48, 503–526. [Google Scholar] [CrossRef]
- Kruk, D.; Meier, R.; Rössler, E.A. Nuclear magnetic resonance relaxometry as a method of measuring translational diffusion coefficients in liquids. Phys. Rev. E-Stat. Nonlinear Soft Matter Phys. 2012, 85, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kruk, D.; Herrmann, A.; Rössler, E.A. Field-cycling NMR relaxometry of viscous liquids and polymers. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 63, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, J.; Maler, L. Nuclear Spin Relaxation in Liquids: Theory, Experiments, and Applications, 1st ed.; Moore, J.H., Spencer, N.D., Eds.; Taylor & Francis: New York, NY, USA, 2006; ISBN 0750309644. [Google Scholar]
- Kowalewski, J.; Egorov, A.; Kruk, D.; Laaksonen, A.; Nikkhou Aski, S.; Parigi, G.; Westlund, P.O. Extensive NMRD studies of Ni(II) salt solutions in water and water-glycerol mixtures. J. Magn. Reson. 2008, 195, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kirca, A.; Ozkan, M.; Cemeroglu, B. Stability of black carrot anthocyanins in various fruit juices and nectars. Food Chem. 2006, 97, 598–605. [Google Scholar] [CrossRef]
- Ferreira, D.S.; Faria, A.F.; Grosso, C.R.F.; Mercadante, A.Z. Encapsulation of Blackberry Anthocyanins by Thermal Gelation of Curdlan. J. Braz. Chem. Soc. 2009, 20, 1908–1915. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.G.; Wu, N.F.; Fan, M.M.; Shen, X.L.; Chen, M.T.; Jiang, A.M.; Lai, L.S. Effect of hsian-tsao gum (HG) content upon rheological properties offilm-forming solutions (FFS) and physical properties of soy protein/hsian-tsao gum films. Food Hydrocoll. 2015, 50, 211–218. [Google Scholar] [CrossRef]
- Aspinall, G.O.; Baillie, J. Gum tragacanth. Part I. Fractionation of the gum and the structure of tragacanthic acid. J. Chem. Soc. 1963, 1702–1714. [Google Scholar] [CrossRef]
- Ozel, B.; Aydin, O.; Grunin, L.; Oztop, M.H. Physico-Chemical Changes of Composite Whey Protein Hydrogels in Simulated Gastric Fluid Conditions. J. Agric. Food Chem. 2018, 66, 9542–9555. [Google Scholar] [CrossRef] [PubMed]
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Mikac, U.; Sepe, A.; Kristl, J.; Baumgartner, S. A new approach combining different MRI methods to provide detailed view on swelling dynamics of xanthan tablets influencing drug release at different pH and ionic strength. J. Control. Release 2010, 145, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifar, M.A.; Musavi, S.M.; Kiumarsi, A.; Williams, P.A. Solution properties of targacanthin (water-soluble part of gum tragacanth exudate from Astragalus gossypinus). Int. J. Biol. Macromol. 2006, 38, 31–39. [Google Scholar] [CrossRef]
- Fabek, H.; Messerschmidt, S.; Brulport, V.; Goff, H.D. The effect of invitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocoll. 2014, 35, 718–726. [Google Scholar] [CrossRef]
- Masierak, W.; Florek-Wojciechowska, M.; Oglodek, I.; Jakubas, R.; Privalov, A.F.; Kresse, B.; Fujara, F.; Kruk, D. Dynamics of [C3H5N2]6[Bi4Br18] by means of 1H NMR relaxometry and quadrupole relaxation enhancement. J. Chem. Phys. 2015, 142, 204503. [Google Scholar] [CrossRef] [PubMed]
- Sunde, E.P.; Halle, B. Mechanism of 1H-14N cross-relaxation in immobilized proteins. J. Magn. Reson. 2010, 203, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Fries, P.H.; Belorizky, E. Simple expressions of the nuclear relaxation rate enhancement due to quadrupole nuclei in slowly tumbling molecules. J. Chem. Phys. 2015, 143, 044202. [Google Scholar] [CrossRef]

| Hydrogels | Water (%) * | BC (%) | WPI (%) | PC (%) | GT (%) | XG (%) |
|---|---|---|---|---|---|---|
| C with BC | 81 | 4 | 15 | - | - | - |
| PC with BC | 80.5 | 4 | 15 | 0.5 | - | - |
| GT with BC | 80.5 | 4 | 15 | - | 0.5 | - |
| XG with BC | 80.5 | 4 | 15 | - | - | 0.5 |
| C without BC | 85 | - | 15 | - | - | - |
| PC without BC | 84.5 | - | 15 | 0.5 | - | - |
| GT without BC | 84.5 | - | 15 | - | 0.5 | - |
| XG without BC | 84.5 | - | 15 | - | - | 0.5 |
| Sample | (107 Hz2) | (10−9 s) | (10−11 m2/s) * | (105 Hz2) | (10−6 s) | (s−1) | Relative Error (%) |
|---|---|---|---|---|---|---|---|
| C with BC | 2.87 | 7.24 | 1.08 | 8.57 | 2.39 | 1.6 | 3.1 |
| C without BC | 3.14 | 6.85 | 1.14 | 8.72 | 1.76 | 2.2 | 4.3 |
| PC with BC (1) | 3.12 | 6.65 | 1.18 | 9.14 | 1.72 | 2.1 | 3.7 |
| PC with BC (2) | 3.23 | 6.56 | 1.20 | 9.34 | 1.69 | 2.2 | 3.3 |
| PC without BC | 2.58 | 7.33 | 1.07 | 9.12 | 1.58 | 1.8 | 3.9 |
| GT with BC | 2.94 | 5.91 | 1.33 | 7.35 | 1.96 | 2.0 | 3.6 |
| GT without BC | 2.77 | 6.20 | 1.26 | 8.32 | 1.69 | 1.8 | 3.7 |
| XG with BC | 4.33 | 5.41 | 1.45 | 8.28 | 1.95 | 2.1 | 2.9 |
| XG without BC | 2.97 | 6.02 | 1.33 | 7.75 | 1.70 | 1.6 | 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozel, B.; Kruk, D.; Wojciechowski, M.; Osuch, M.; Oztop, M.H. Water Dynamics in Whey-Protein-Based Composite Hydrogels by Means of NMR Relaxometry. Int. J. Mol. Sci. 2021, 22, 9672. https://doi.org/10.3390/ijms22189672
Ozel B, Kruk D, Wojciechowski M, Osuch M, Oztop MH. Water Dynamics in Whey-Protein-Based Composite Hydrogels by Means of NMR Relaxometry. International Journal of Molecular Sciences. 2021; 22(18):9672. https://doi.org/10.3390/ijms22189672
Chicago/Turabian StyleOzel, Baris, Danuta Kruk, Milosz Wojciechowski, Maciej Osuch, and Mecit Halil Oztop. 2021. "Water Dynamics in Whey-Protein-Based Composite Hydrogels by Means of NMR Relaxometry" International Journal of Molecular Sciences 22, no. 18: 9672. https://doi.org/10.3390/ijms22189672
APA StyleOzel, B., Kruk, D., Wojciechowski, M., Osuch, M., & Oztop, M. H. (2021). Water Dynamics in Whey-Protein-Based Composite Hydrogels by Means of NMR Relaxometry. International Journal of Molecular Sciences, 22(18), 9672. https://doi.org/10.3390/ijms22189672






