Anti-Inflammatory Effects of Metabolites from Antarctic Fungal Strain Pleosporales sp. SF-7343 in HaCaT Human Keratinocytes
Abstract
:1. Introduction
2. Results
2.1. Structural Determination of Isolated Metabolites
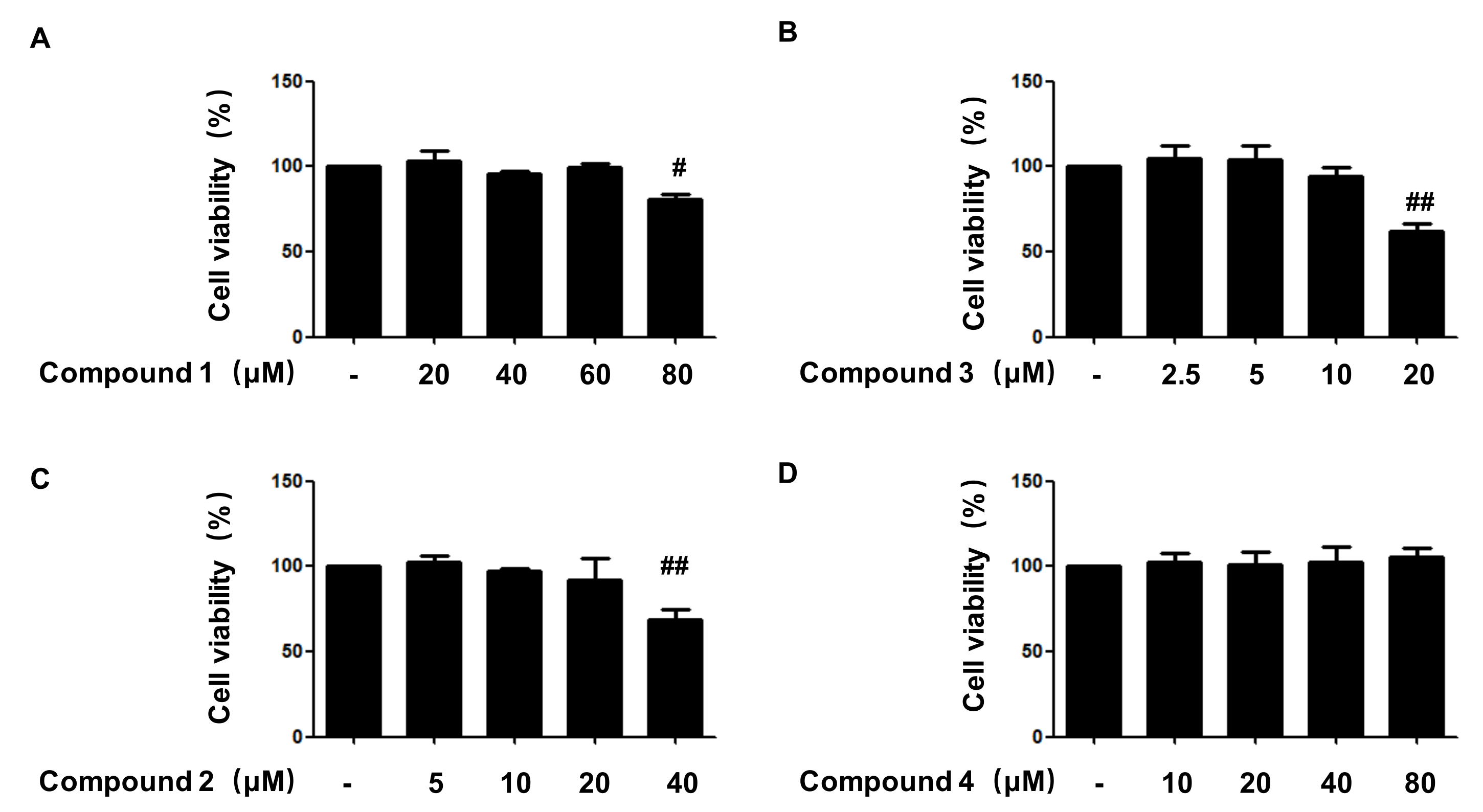
2.2. Effects of Compounds 1, 2, 3, and 4 on Cell Viability
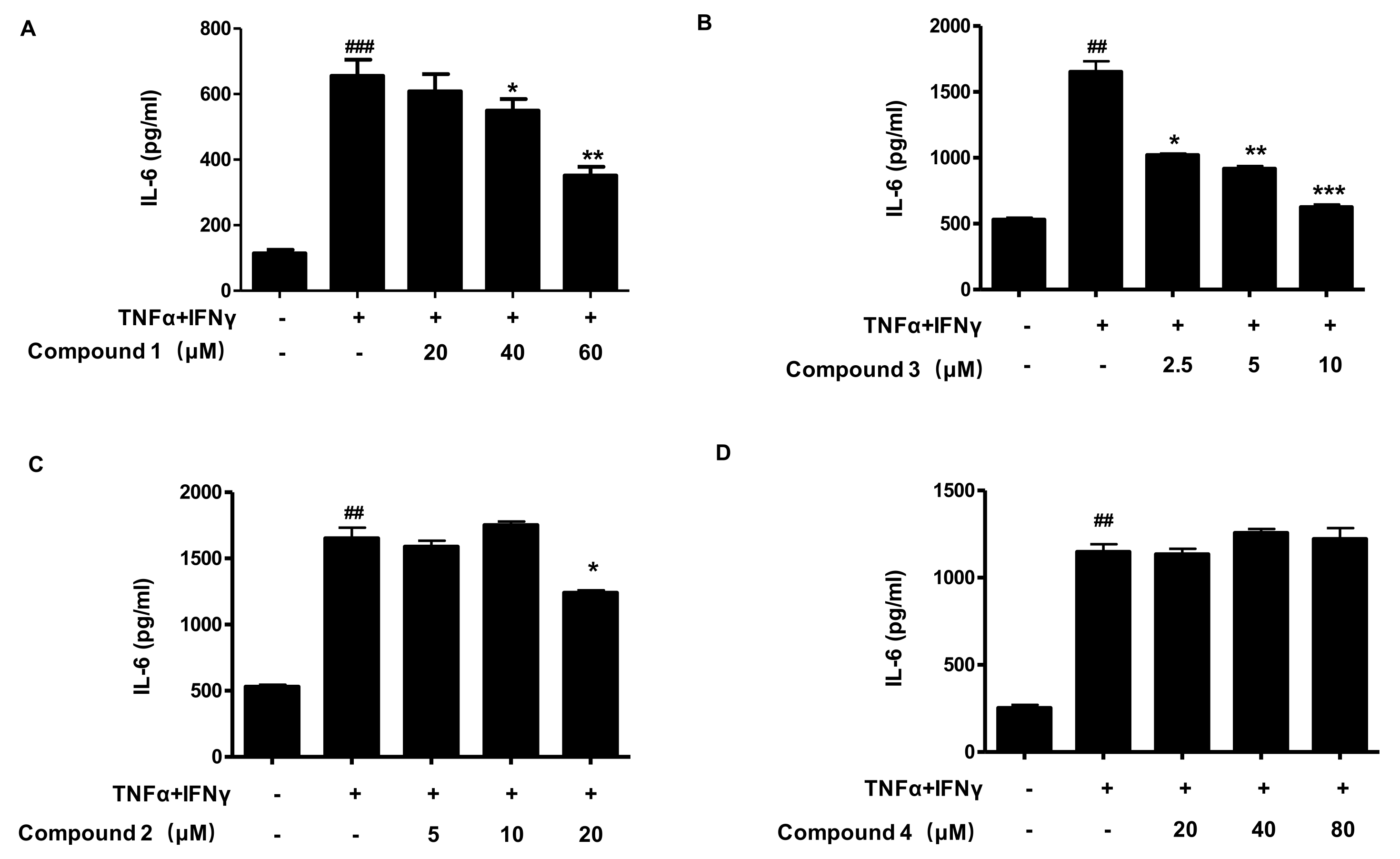
2.3. Effects of Compounds 1, 2, 3, and 4 on the Secretion of IL-6 in TNF-α/IFN-γ-Stimulated HaCaT Cells
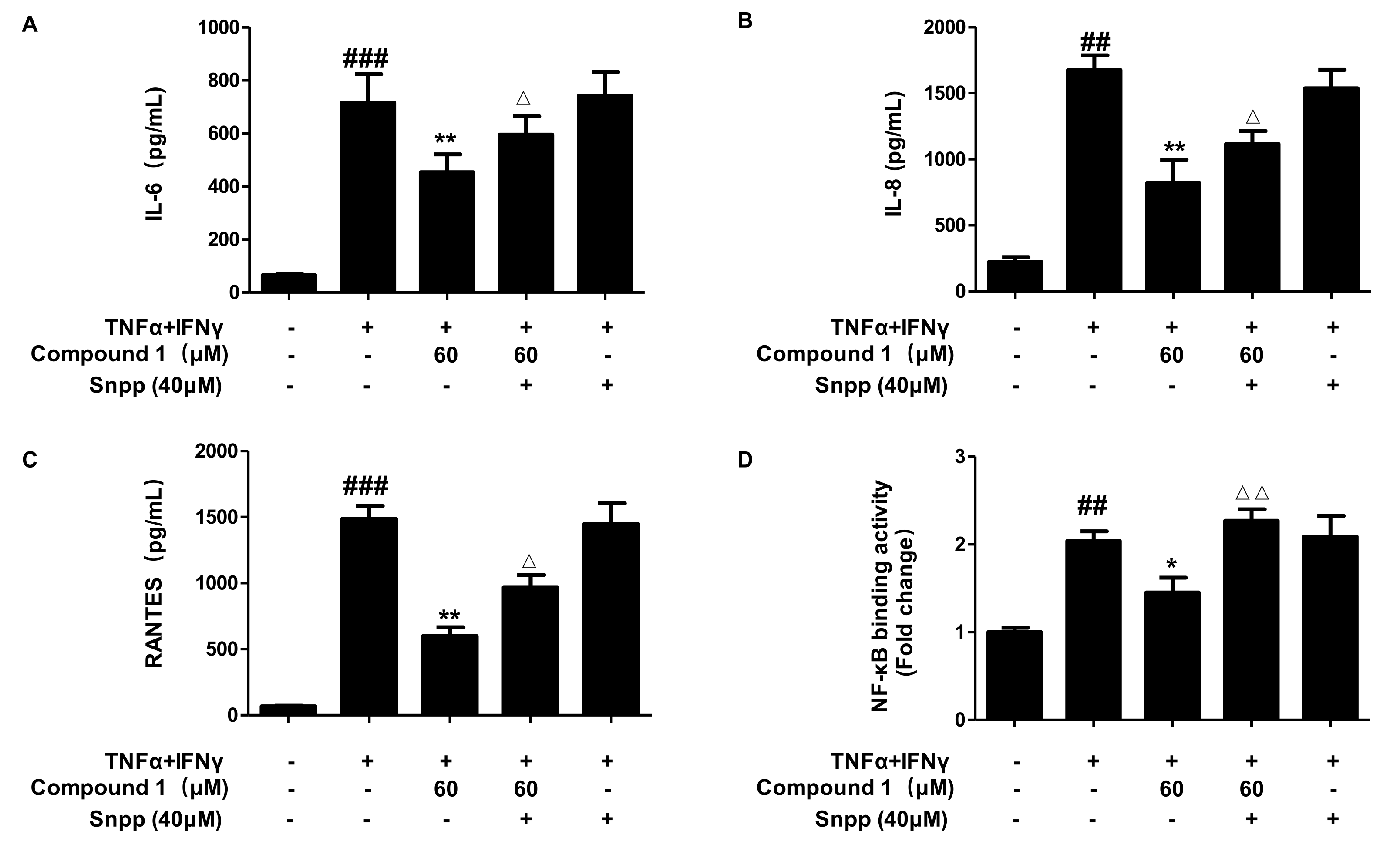
2.4. Effects of Compounds 1 and 3 on IL-8, MDC, and RANTES Secretion in TNF-α/IF-γ-Stimulated HaCaT Cells
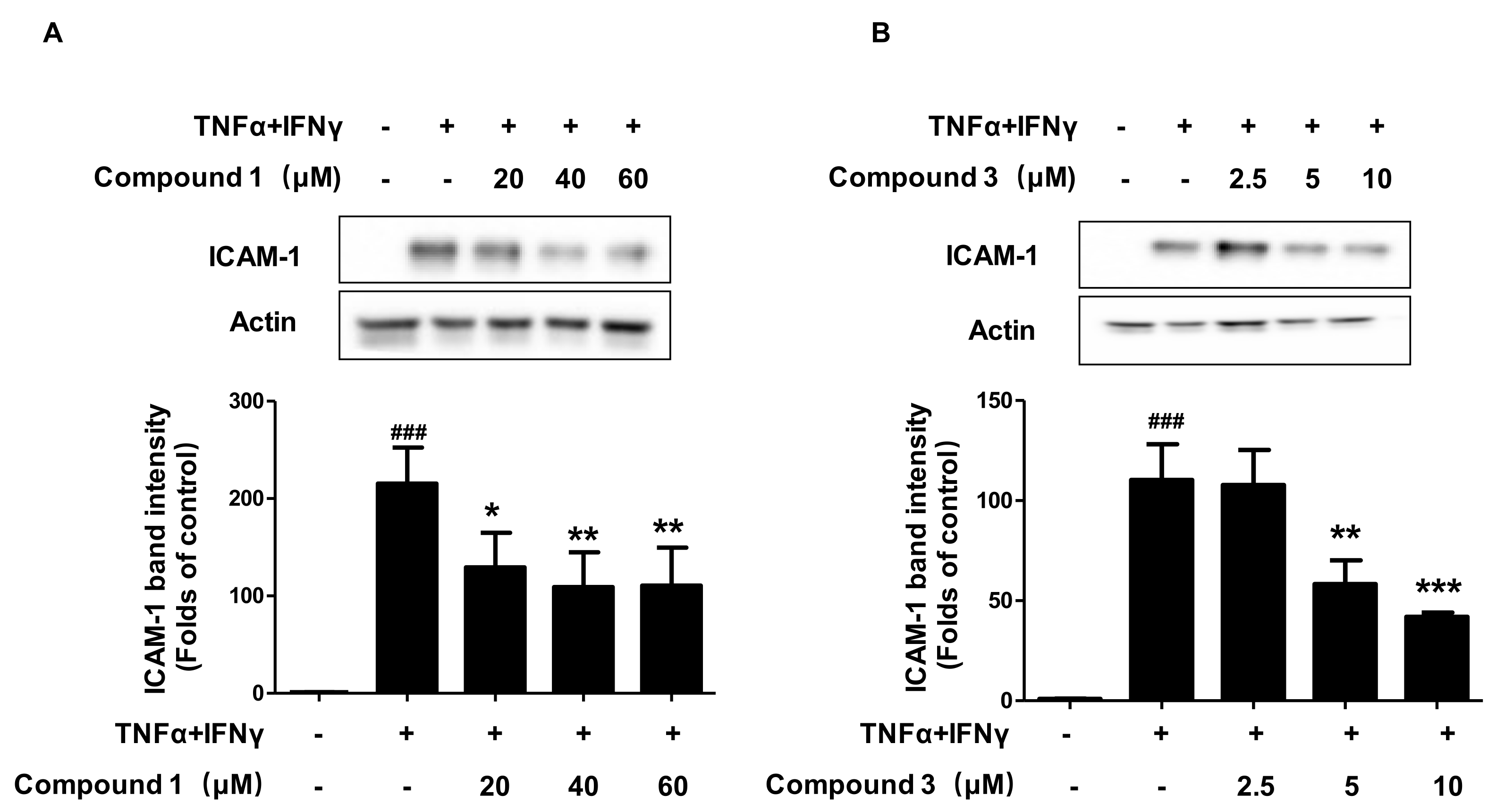
2.5. Effects of Compounds 1 and 3 on TNF-α/IFN-γ-Induced ICAM-1 Expression in HaCaT Cells
2.6. Effects of Compounds 1 and 3 on FLG and IVL Expression, in HaCaT Cells
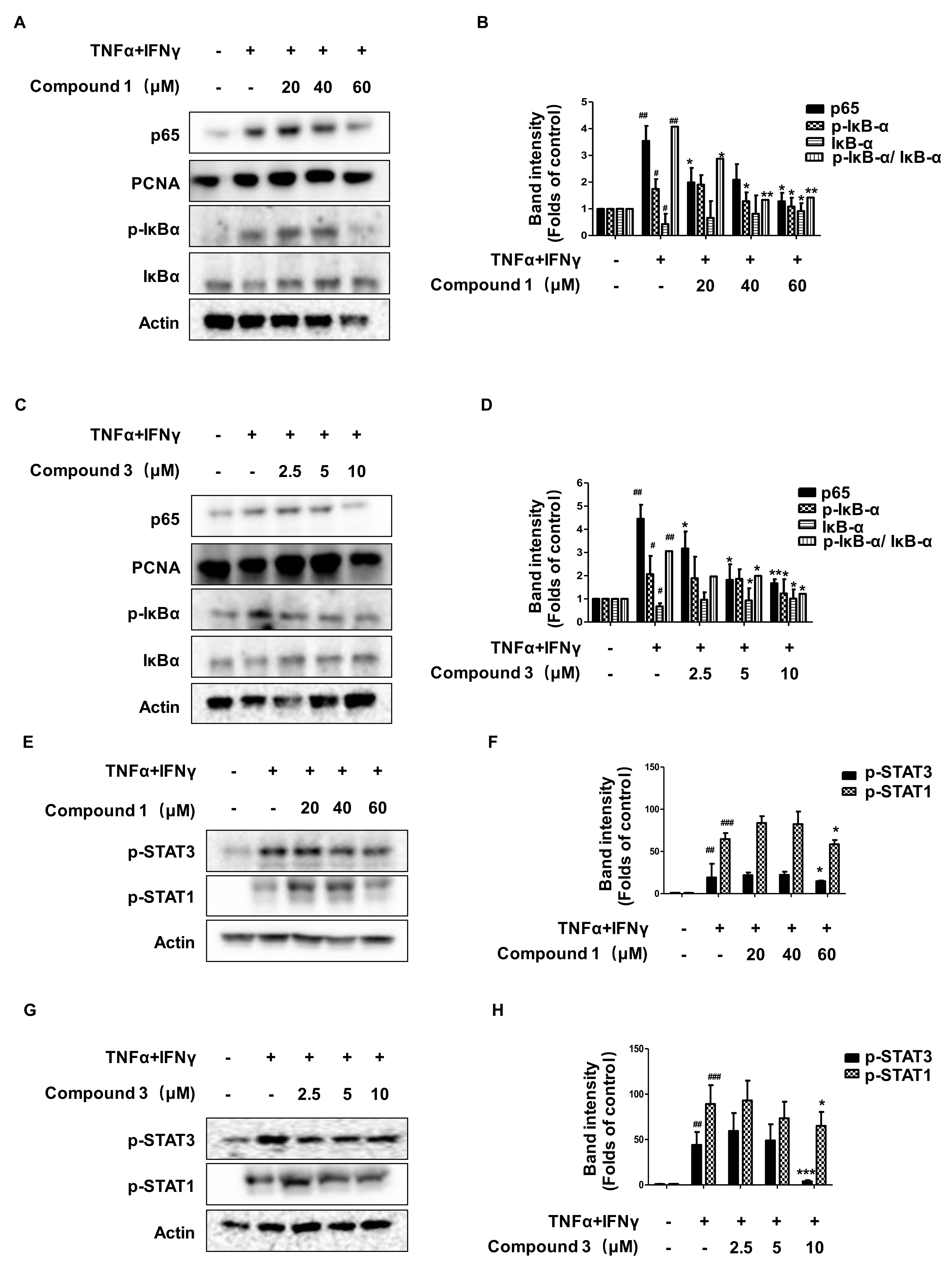
2.7. Effects of Compounds 1 and 3 on the NF-κB, p-STAT1, and p-STAT3 Signaling Pathways in HaCaT Cells
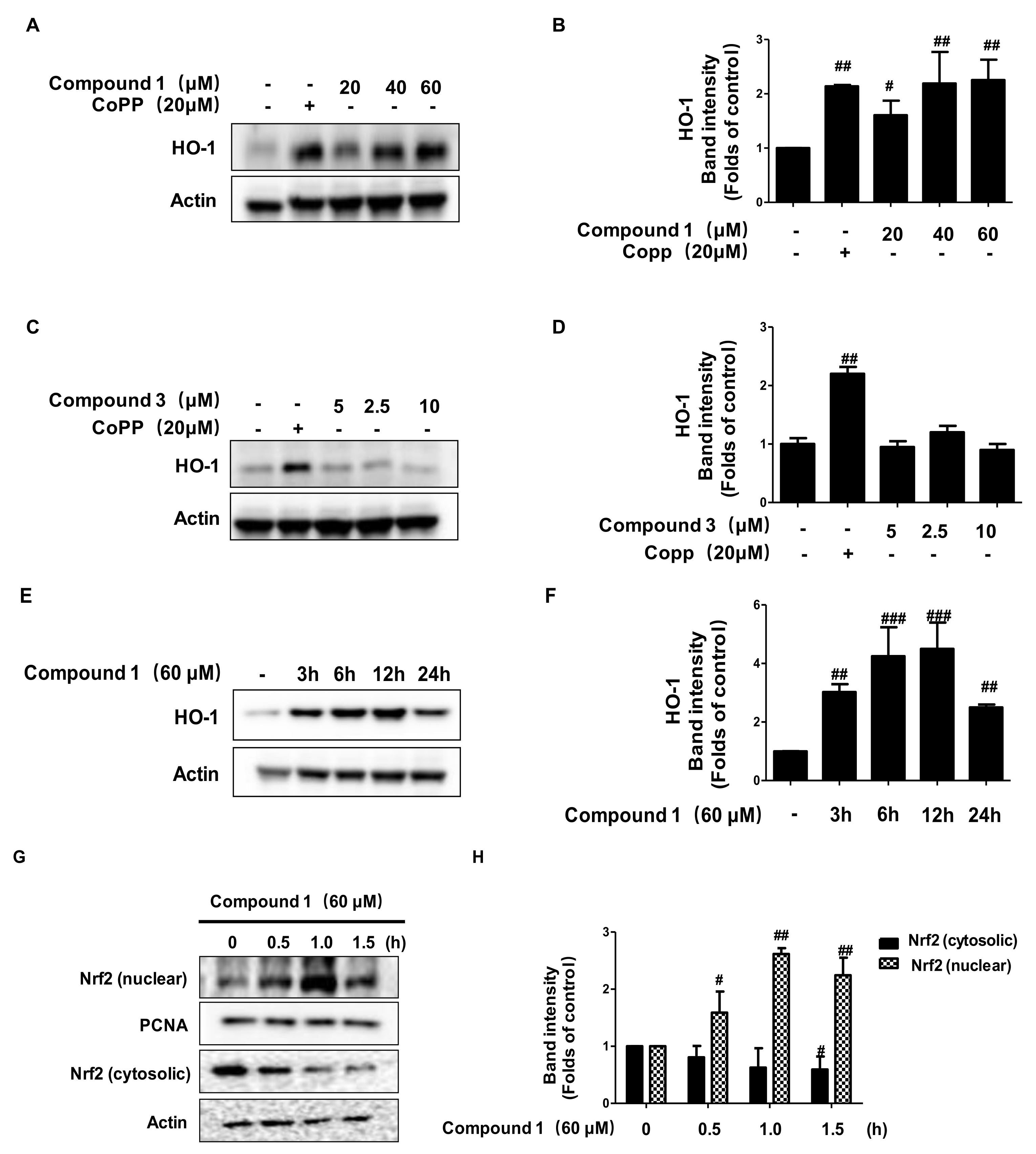
2.8. Effects of Compounds 1 and 3 on HO-1 Expression and Nrf2 Translocation
2.9. Anti-Inflammatory Effects of Compound 1 through HO-1 Regulation in TNF-α/IFN-γ-Stimulated HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Material and Fermentation
4.3. Extraction and Isolation of Metabolites
4.3.1. Compound 1
4.3.2. Compound 2
4.3.3. Compound 3
4.3.4. Compound 4
4.4. Cell Culture and Reagents
4.5. MTT Assay
4.6. Measurement of Cytokines and Chemokines
4.7. Extraction of Total, Nuclear, and Cytosolic Protein
4.8. Western Blot Analysis
4.9. NF-κB DNA Binding Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 10023, 1109–1122. [Google Scholar] [CrossRef]
- Lyons, J.J.; Milner, J.D.; Stone, K.D. Atopic dermatitis in children. Immunol. Allergy Clin. N. Am. 2016, 35, 293–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, C.A. Overview of atopic dermatitis. Am. J. Manag. Care 2017, 23, 115–123. [Google Scholar]
- Mounsey, S.; Agius, E. Atopic dermatitis. Br. J. Hosp. Med. 2017, 78, 183–187. [Google Scholar] [CrossRef]
- Furue, M.; Ulzii, D.; Vu, Y.H.; Tsuji, G.; Nakahara, T. Pathogenesis of Atopic Dermatitis: Current Paradigm. Iran. J. Immunol. 2019, 16, 97–107. [Google Scholar] [PubMed]
- Boguniewicz, M.; Leung, D.Y.M. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef]
- Wilson, V.G. Growth and differentiation of HaCaT keratinocytes. Methods Mol. Biol. 2014, 1195, 33–41. [Google Scholar] [PubMed]
- Ha, T.M.; Kim, D.C.; Sohn, J.H.; Yim, J.H.; Oh, Y.H. Anti-Inflammatory and Protein Tyrosine Phosphatase 1B Inhibitory Metabolites from the Antarctic Marine-Derived Fungal Strain Penicillium glabrum SF-7123. Mar. Drugs 2020, 18, 247. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Clarke, A. Antarctic marine biology. Curr. Biol. 2011, 21, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Chown, S.L. Antarctic marine biodiversity and deep-sea hydrothermal vents. PLoS Biol. 2012, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Zucconi, L.; Canini, F.; Temporiti, M.E.; Tosi, S. Extracellular Enzymes and Bioactive Compounds from Antarctic Terrestrial Fungi for Bioprospecting. Int. J. Environ. Res. Public Health 2020, 17, 6459. [Google Scholar] [CrossRef]
- Kollarova, J.; Cenk, E.; Schmutz, C.; Marko, D. The mycotoxin alternariol suppresses lipopolysaccharide-induced inflammation in THP-1 derived macrophages targeting the NF-κB signallingpathway. Arch. Toxicol. 2018, 92, 3347–3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.T.; Ma, Z.H.; Wang, G.K.; Xu, F.Q.; Yu, Y.; Wang, G.; Peng, D.Y.; Liu, J.S. Chemical constituents from plant endophytic fungus Alternaria alternata. Nat. Prod. Res. 2021, 35, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.; Toki, S.; Saitoh, Y.; Tsukuda, E.; Kawahara, K.; Ando, K.; Matsuda, Y. Isolation of myosin light chain kinase inhibitors from microorganisms: Dehydroaltenusin, altenusin, atrovenetinone, and cyclooctasulfur. Biosci. Biotech. Biochem. 1995, 7, 1333–1335. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.H.; Edrada-Ebel, R.; Indriani, I.D.; Wray, V.; Müller, W.E.G.; Totzke, F.; Zirrgiebel, U.; Schächtele, C.; Kubbutat, M.H.G.; Lin, W.H.; et al. Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J. Nat. Prod. 2008, 71, 972–980. [Google Scholar] [CrossRef]
- Bradburn, N.; Coker, R.D.; Blunden, G.; Turner, C.H.; Crabb, T.A. 5′-Epialtenuene and neoaltenuene, dibenzo-α-pyrones from Alternaria alternata cultured on rice. Phytochemistry 1994, 35, 665–669. [Google Scholar] [CrossRef]
- Kim, H.; Youn, G.S.; An, S.Y.; Kwon, H.Y.; Choi, S.Y.; Park, J. 2,3-Dimethoxy-2′-hydroxychalcone ameliorates TNF-α-induced ICAM-1 expression and subsequent monocyte adhesiveness via NF-kappaB inhibition and HO-1 induction in HaCaT cells. BMB Rep. 2016, 49, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Seong, G.S.; Choung, S.Y. Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function. Nutrients 2020, 12, 249. [Google Scholar] [CrossRef] [Green Version]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, M.; Sun, Y.; Xie, M.; Le, K.; Xu, M.; Huang, C. The potential of Diosgenin in treating psoriasis: Studies from HaCaT keratinocytes and imiquimod-induced murine model. Life Sci. 2020, 241, 117115. [Google Scholar] [CrossRef]
- Qin, X.; Chen, C.; Zhang, Y.; Zhang, L.; Mei, Y.; Long, X.; Tan, R.; Liang, W.; Sun, L. Acitretin modulates HaCaT cells proliferation through STAT1- and STAT3-dependent signaling. Saudi. Pharm. J. 2017, 25, 620–624. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.Y.; Jo, E.H.; Noh, H.M.; Park, S.; Park, M.C.; Kim, D.K. Chijabyukpi-Tang Inhibits Pro-Inflammatory Cytokines and Chemokines via the Nrf2/HO-1 Signaling Pathway in TNF-α/IFN-γ-Stimulated HaCaT Cells and Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice. Front. Pharmacol. 2020, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Peng, G.; Yang, F.; Zhang, Y.; Mu, Z.; Han, X. Sulforaphane has a therapeutic effect in an atopic dermatitis murine model and activates the Nrf2/HO-1 axis. Mol. Med. Rep. 2019, 20, 1761–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Cha, H.J.; Hong, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Kim, B.W.; Jeon, Y.J.; Choi, Y.H. Protective Effect of Phloroglucinol on Oxidative Stress-Induced DNA Damage and Apoptosis through Activation of the Nrf2/HO-1 Signaling Pathway in HaCaT Human Keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of coptisine against oxidative stress-induced DNA damage and apoptosis in HaCaT keratinocytes. Gen. Physiol. Biophys. 2019, 38, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, D.; Zhang, X.; Zhao, H. Protective Effect of Lemon Peel Polyphenols on Oxidative Stress-Induced Damage to Human Keratinocyte HaCaT Cells Through Activation of the Nrf2/HO-1 Signaling Pathway. Front. Nutr. 2021, 18, 606776. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Mascia, F.; Girolomoni, G. The contribution of keratinocytes to the pathogenesis of atopic dermatitis. Eur. J. Dermatol. 2006, 16, 125–131. [Google Scholar]
- Rahman, S.; Collins, M.; Williams, C.M.; Ma, H.L. The pathology and immunology of atopic dermatitis. Inflamm. Allergy Drug Targets 2011, 10, 486–496. [Google Scholar] [CrossRef]
- Talagas, M.; Lebonvallet, N.; Berthod, F.; Misery, L. Cutaneous nociception: Role of keratinocytes. Exp. Dermatol. 2019, 28, 1466–1469. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.G.; Luckett-Chastain, L.R.; Calhoun, K.N.; Frempah, B.; Bastian, A.; Gallucci, R.M. Interleukin 6 Function in the Skin and Isolated Keratinocytes Is Modulated by Hyperglycemia. J. Immunol. Res. 2019, 3, 5087847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, T.; Hanzawa, N.; Sugiyama, M. Effect of Lactobacillus strains on thymus and chemokine expression in keratinocytes and development of atopic dermatitis-like symptoms. Benef. Microbes 2018, 15, 643–652. [Google Scholar] [CrossRef]
- Tüzün, Y.; Antonov, M.; Dolar, N.; Wolf, R. Keratinocyte cytokine and chemokine receptors. Dermatol. Clin. 2007, 25, 467–476. [Google Scholar] [CrossRef]
- Nickoloff, B.J.; Xin, H.; Nestle, F.O.; Qin, J.Z. The cytokine and chemokine network in psoriasis. Clin. Dermatol. 2007, 25, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Deng, X.; Wang, J.; Li, Q. Baicalin Inhibits Cell Proliferation and Inflammatory Cytokines Induced by Tumor Necrosis Factor α (TNF-α) in Human Immortalized Keratinocytes (HaCaT) Human Keratinocytes by Inhibiting the STAT3/Nuclear Factor kappa B (NF-κB) Signaling Pathway. Med. Sci. Monit. 2020, 23, e1919392. [Google Scholar]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C. Keratinocytes in allergic skin diseases. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 452–456. [Google Scholar] [CrossRef]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [Green Version]
- Clausen, M.L.; Kezic, S.; Olesen, C.M.; Agner, T. Cytokine concentration across the stratum corneum in atopic dermatitis and healthy controls. Sci. Rep. 2020, 10, 21815. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Kim, H.J.; Cao, T.Q.; Liu, Z.; Lee, H.; Ko, W.; Kim, Y.-C.; Sohn, J.H.; Kim, T.K.; Yim, J.H.; et al. Anti-Inflammatory Effects of Metabolites from Antarctic Fungal Strain Pleosporales sp. SF-7343 in HaCaT Human Keratinocytes. Int. J. Mol. Sci. 2021, 22, 9674. https://doi.org/10.3390/ijms22189674
Dong L, Kim HJ, Cao TQ, Liu Z, Lee H, Ko W, Kim Y-C, Sohn JH, Kim TK, Yim JH, et al. Anti-Inflammatory Effects of Metabolites from Antarctic Fungal Strain Pleosporales sp. SF-7343 in HaCaT Human Keratinocytes. International Journal of Molecular Sciences. 2021; 22(18):9674. https://doi.org/10.3390/ijms22189674
Chicago/Turabian StyleDong, Linsha, Hye Jin Kim, Thao Quyen Cao, Zhiming Liu, Hwan Lee, Wonmin Ko, Youn-Chul Kim, Jae Hak Sohn, Tai Kyoung Kim, Joung Han Yim, and et al. 2021. "Anti-Inflammatory Effects of Metabolites from Antarctic Fungal Strain Pleosporales sp. SF-7343 in HaCaT Human Keratinocytes" International Journal of Molecular Sciences 22, no. 18: 9674. https://doi.org/10.3390/ijms22189674
APA StyleDong, L., Kim, H. J., Cao, T. Q., Liu, Z., Lee, H., Ko, W., Kim, Y.-C., Sohn, J. H., Kim, T. K., Yim, J. H., Lee, D.-S., & Oh, H. (2021). Anti-Inflammatory Effects of Metabolites from Antarctic Fungal Strain Pleosporales sp. SF-7343 in HaCaT Human Keratinocytes. International Journal of Molecular Sciences, 22(18), 9674. https://doi.org/10.3390/ijms22189674





