A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults
Abstract
1. Introduction
2. Vitamin B12 and Anemia
3. Vitamin B12 and Neurological Disorders
4. Vitamin B12 Deficiency and Hyperhomocysteinemia
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADMA | 8-isoprostaglandin F2α dimethy larginine |
| CT | computed tomography |
| DRS-R-98 | Delirium Rating Scale—Revised-98 |
| Hb | hemoglobin |
| Hcy | homocysteine |
| HHcy | hyperhomocysteinemia |
| MICD | metformin induced cobalamin deficiency |
| MADRS | Montgomery Asberg Depression rating scale |
| MCV | mean corpuscular volume |
| MMA | methylmalonic acid |
| MR | magnetic resonance |
| MMSE | Mini-Mental State Examination |
| MTHFR | 5,10-methylenetetrahydrofolate reductase gene |
| PPI | proton-pump inhibitor |
| RDAs | recommended dietary allowances |
| RBCs | red blood cells |
| SCD | sub-acute combined degeneration |
| SDMA | arginine and symmetric dimethyl arginine |
| TPHA | Treponema pallidum hematoglutinin assay |
References
- Lanska, D.J. Chapter 30 Historical aspects of the major neurological vitamin deficiency disorders: The water-soluble B vitamins. Handb. Clin. Neurol. 2009, 95, 445–476. [Google Scholar] [CrossRef]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef]
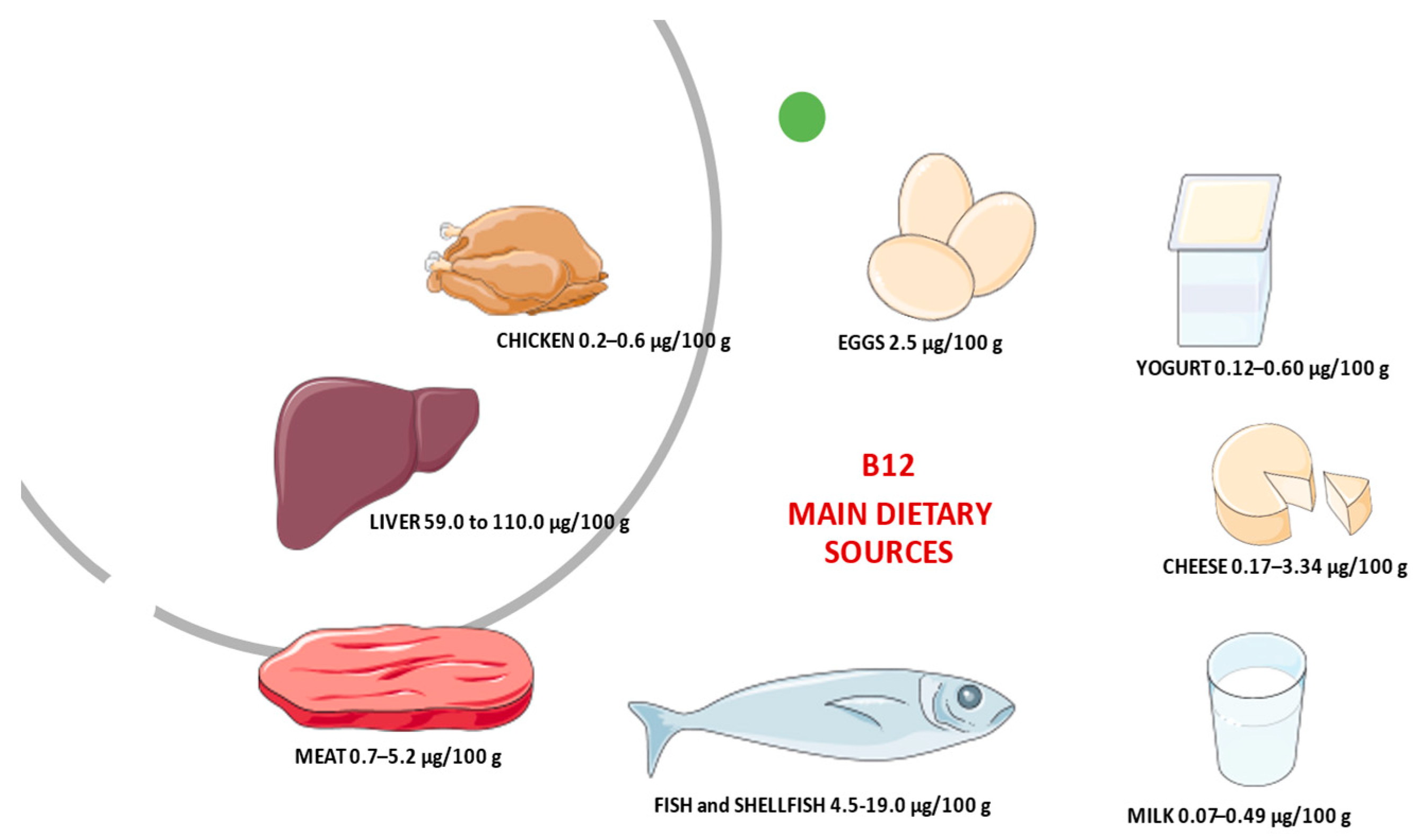
- Watanabe, F.; Bito, T. Vitamin B12 sources and microbial interaction. Exp. Biol. Med. 2018, 243, 148–158. [Google Scholar] [CrossRef]
- Doets, E.L.; Veld, P.H.I.’T.; Szczecińska, A.; Dhonukshe-Rutten, R.A.; Cavelaars, A.E.; Veer, P.V.’T.; Brzozowska, A.; de Groot, L. Systematic Review on Daily Vitamin B12 Losses and Bioavailability for Deriving Recommendations on Vitamin B12 Intake with the Factorial Approach. Ann. Nutr. Metab. 2013, 62, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. Bioavailability of Vitamin B12. Int. J. Vitam. Nutr. Res. 2010, 80, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Smith, D.; Nurk, E.; Berstad, P.; A Drevon, C.; Ueland, P.M.; E Vollset, S.; Tell, G.S.; Refsum, H. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture, Agricultural Research Service. What We Eat in America, 2017–2018. External Link Disclaimer; U.S. Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2020.
- Carmel, R. How I treat cobalamin (vitamin B12) deficiency. Blood 2008, 112, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef]
- Stabler, S.P. Vitamin B12Deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef]
- Green, R.; Mitra, A.D. Megaloblastic Anemias. Med. Clin. N. Am. 2017, 101, 297–317. [Google Scholar] [CrossRef]
- Sakyi, S.A.; Laing, E.F.; Mantey, R.; Kwarteng, A.; Owiredu, E.-W.; Dadzie, R.E.; Amoani, B.; Opoku, S.; Afranie, B.O.; Boakye, D. Profiling immuno-metabolic mediators of vitamin B12 deficiency among metformin-treated type 2 diabetic patients in Ghana. PLoS ONE 2021, 16, e0249325. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Britto, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; E Lester, S.; Babatunde, T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: A review of literature. Eur. J. Clin. Nutr. 2014, 68, 541–548. [Google Scholar] [CrossRef]
- Berkins, S.; Schiöth, H.B.; Rukh, G. Depression and Vegetarians: Association between Dietary Vitamin B6, B12 and Folate In-take and Global and Subcortical Brain Volumes. Nutrients 2021, 13, 1790. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R. Vitamin B12 status is a risk factor for bone fractures among vegans. Med. Hypotheses 2021, 153, 110625. [Google Scholar] [CrossRef]
- Desmond, M.A.; Sobiecki, J.G.; Jaworski, M.; Płudowski, P.; Antoniewicz, J.; Shirley, M.K.; Eaton, S.; Książyk, J.; Cortina-Borja, M.; De Stavola, B.; et al. Growth, body composition, and cardiovascular and nutritional risk of 5- to 10-y-old children consuming vegetarian, vegan, or omnivore diets. Am. J. Clin. Nutr. 2021, 113, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Sukumar, N.; Adaikalakoteswari, A.; Goljan, I.; Venkataraman, H.; Gopinath, A.; Bagias, C.; Yajnik, C.S.; Stallard, N.; Ghebremichael-Weldeselassie, Y.; et al. Association of maternal vitamin B12 and folate levels in early pregnancy with gestational diabetes: A prospective UK cohort study (PRiDE study). Diabetologia 2021, 64, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- Behere, R.V.; Deshmukh, A.S.; Otiv, S.; Gupte, M.D.; Yajnik, C.S. Maternal Vitamin B12 Status During Pregnancy and Its Association With Outcomes of Pregnancy and Health of the Offspring: A Systematic Review and Implications for Policy in India. Front. Endocrinol. 2021, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Nalder, L.; Zheng, B.; Chiandet, G.; Middleton, L.T.; de Jager, C.A. Vitamin B12 and Folate Status in Cognitively Healthy Older Adults and Associations with Cognitive Performance. J. Nutr. Heal. Aging 2021, 25, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Aslinia, F.; Mazza, J.J.; Yale, S.H. Megaloblastic anemia and other causes of macrocytosis. Clin. Med. Res. 2006, 4, 236–241. [Google Scholar] [CrossRef] [PubMed]
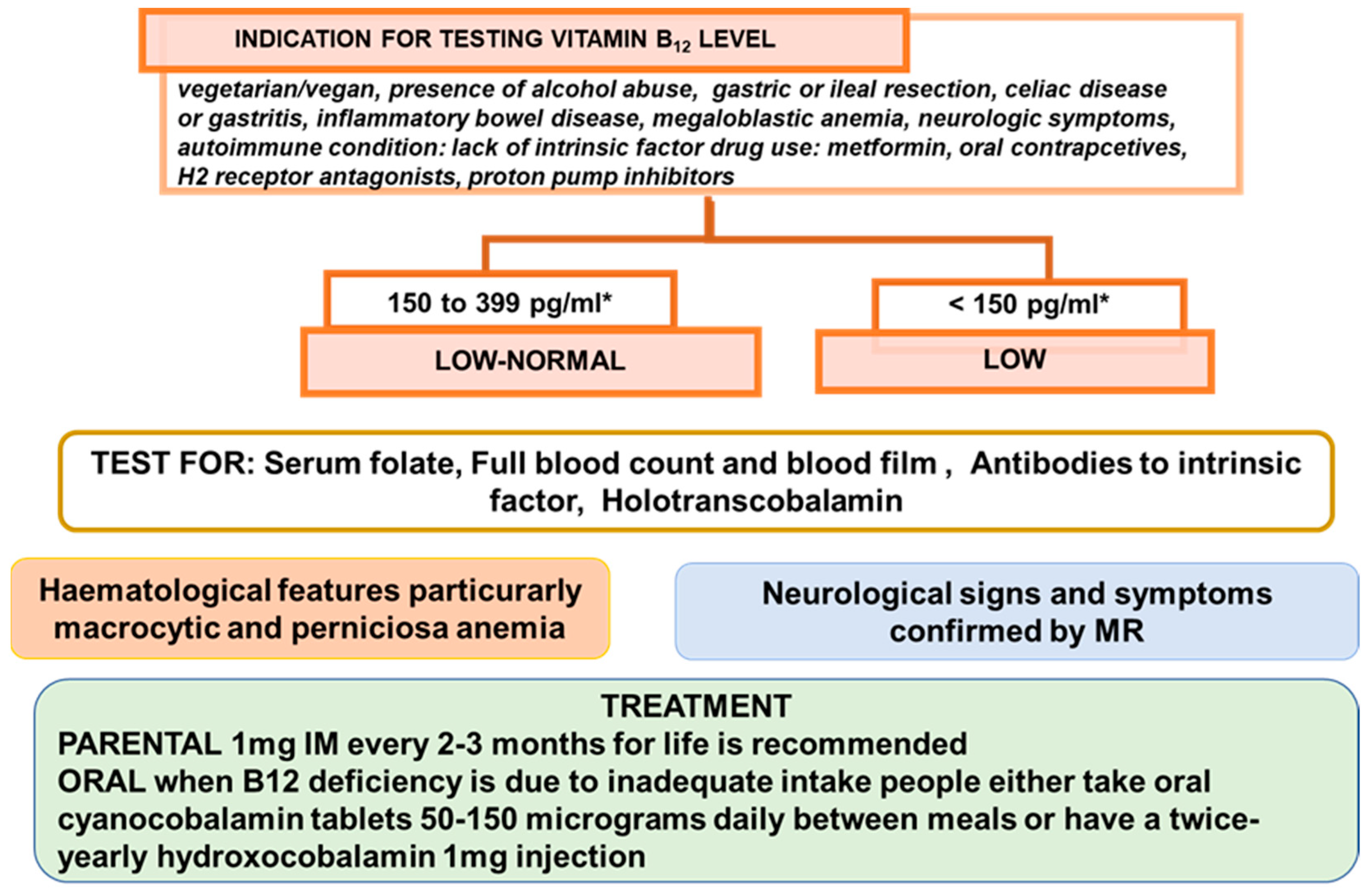
- Sukumar, N.; Saravanan, P. Investigating vitamin B12 deficiency. BMJ 2019, 365, l1865. [Google Scholar] [CrossRef] [PubMed]
- Socha, D.S.; DeSouza, S.I.; Flagg, A.; Sekeres, M.; Rogers, H.J. Severe megaloblastic anemia: Vitamin deficiency and other causes. Clevel. Clin. J. Med. 2020, 87, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.G.; Cardoso, M.F.P.; Faria, O.; Gomez, R.S.; Mesquita, R.A. A Case Report of Pernicious Anemia and Recurrent Aph-thous Stomatitis. J. Contemp. Dent. Pract. 2009, 10, 83–89. [Google Scholar]
- Pontes, H.A.; Neto, N.C.; Ferreira, K.B.; Fonseca, F.P.; Vallinoto, G.M.; Pontes, F.S.; Pinto, D.S. Oral manifestations of vitamin B12 deficiency: A case report. J. Can. Dent. Assoc. 2009, 75, 533–537. [Google Scholar]
- Pahadiya, H.R.; Lakhotia, M.; Choudhary, S.; Prajapati, G.R.; Pradhan, S. Reversible ecchymosis and hyperpigmented. lesions: A rare presentation of dietary Vitamin B12 deficiency. J. Fam. Med. Prim. Care 2016, 5, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Surani, S.R.; Sharma, M. A Case of Pancytopenia Caused by an Unusual Habit of Eating Pasta. Cureus 2019, 11, e6028. [Google Scholar] [CrossRef]
- Hussain, S.A.; Din, M.A.U.; Boppana, L.K.T.; Kapoor, A.; Jamshed, S. Suspected Metformin-induced Cobalamin Deficiency Mimicking Thrombotic Thrombocytopenic Purpura. Cureus 2020, 12, e6921. [Google Scholar] [CrossRef]
- Sasi, S.; Yassin, M.A. A Rare Case of Acquired Hemolytic Anemia and Pancytopenia Secondary to Pernicious Anemia. Case Rep. Oncol. 2020, 13, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, B.; Glocker, F.X.; Schumacher, M.; Deuschl, G.; Lücking, C.H. Subacute combined degeneration: Clinical, electro-physiological, and magnetic resonance imaging finding. J. Neurol. Neurosurg. Psychiatry. 1998, 65, 822–827. [Google Scholar] [CrossRef]
- Maamar, M.; Tazi-Mezalek, Z.; Harmouche, H.; Ammouri, W.; Zahlane, M.; Adnaoui, M.; Aouni, M.; Mohattane, A.; Maaouni, A. Neurological manifestations of vitamin B12 deficiency: A retrospective study of 26 cases. Rev. Med. Interne 2006, 27, 442–447. [Google Scholar] [CrossRef]
- Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Lindenbaum, J.; Healton, E.B.; Savage, D.G.; Brust, J.C.; Garrett, T.J.; Podell, E.R.; Marcello, P.D.; Stabile, S.; Allen, R.H. Neuro-psychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N. Engl. J. Med. 1988, 318, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Ralapanawa, D.M.P.U.K.; Jayawickreme, K.P.; Ekanayake, E.M.M.; Jayalath, W.A.T.A. B12 deficiency with neurological manifestations in the absence of anaemia. BMC Res. Notes 2015, 8, 458. [Google Scholar] [CrossRef]
- Ekabe, C.J.; Kehbila, J.; Abanda, M.H.; Kadia, B.M.; Sama, C.-B.; Monekosso, G.L. Vitamin B12 deficiency neuropathy; a rare diagnosis in young adults: A case report. BMC Res. Notes 2017, 10, 72. [Google Scholar] [CrossRef]
- Maamar, M.; Mezalek, Z.T.; Harmouche, H.; Adnaoui, M.; Aouni, M.; Maaouni, A. Contribution of spinal MRI for unsuspected cobalamin deficiency in isolated sub-acute combined degeneration. Eur. J. Intern. Med. 2008, 19, 143–145. [Google Scholar] [CrossRef]
- Senol, M.G.; Sonmez, G.; Ozdag, F.; Saracoglu, M. Reversible myelopathy with vitamin B12 deficiency. Singap. Med. J. 2008, 49, e330–e332. [Google Scholar]
- Srikanth, S.G.; Jayakumar, P.N.; Vasudev, M.K.; Taly, A.B.; Chandrashekar, H.S. MRI in subacute combined degeneration of spinal cord: A case report and review of literature. Neurol. India 2002, 50, 310. [Google Scholar]
- Kumar, S. Recurrent seizures: An unusual manifestation of vitamin B12 deficiency. Neurol. India 2004, 52, 122–123. [Google Scholar] [PubMed]
- Mavrommati, K.; Sentissi, O. Delirium as a result of vitamin B12 deficiency in a vegetarian female patient. Eur. J. Clin. Nutr. 2013, 67, 996–997. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Remacha, A.F.; Souto, J.C.; Piñana, J.L.; Sardà, M.P.; Queraltó, J.M.; Martí-Fàbregas, J.; Garcia-Moll, X.; Fernández, C.; Rodríguez, Á.; Cuesta, J. Vitamin B12 deficiency, hyperhomocysteinemia and thrombosis: A case and control study. Int. J. Hematol. 2011, 93, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Romero, M.; Cannegieter, S.C.; Heijer, M.D.; Doggen, C.J.M.; Rosendaal, F.R.; Lijfering, W.M. Hyperhomocysteinemia and Risk of First Venous Thrombosis: The Influence of (Unmeasured) Confounding Factors. Am. J. Epidemiol. 2018, 187, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Taniguchi, T.; Saito, N.; Kimura, T. Inferior vena cava thrombus due to hyperhomocysteinemia. J. Cardiol. Cases 2018, 18, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.; D’Cruz, S.; Kaur, R. An uncommon presentation of hyperhomocysteinemia and vitamin B12 deficiency: A case report. J. Med. Case Rep. 2019, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Ammouri, W.; Tazi, Z.M.; Harmouche, H.; Maamar, M.; Adnaoui, M. Venous thromboembolism and hyperhomocysteinemia as first manifestation of pernicious anemia: A case series. J. Med. Case Rep. 2017, 11, 250. [Google Scholar] [CrossRef]
- Ulrich, A.; Muller, D.; Linnebank, M.; Tarnutzer, A.A. Pitfalls in the diagnostic evaluation of subacute combined degeneration. BMJ Case Rep. 2015, 2015, bcr2014208622. [Google Scholar] [CrossRef]
- Kovalenko, O.; Kassem, A.N.; Jenkins, M. Hyperhomocysteinemia and Pulmonary Embolism in a Young Male. Cureus 2020, 12, e7818. [Google Scholar] [CrossRef]
- Goette, A.; Hammwöhner, M.; Dierkes, J.; Lachmuth, J.; Frölich, J.C.; Klein, H.; Bode-Böger, S.M. Aortic thrombus and pulmonary embolism in a patient with hyperhomocysteinemia. Nat. Clin. Pr. Neurol. 2006, 3, 396–399. [Google Scholar] [CrossRef]
- Ruscin, J.M.; Page, R.L.; Valuck, R.J. Vitamin B12 Deficiency Associated with Histamine2-Receptor Antagonists and a Proton-Pump Inhibitor. Ann. Pharmacother. 2002, 36, 812–816. [Google Scholar] [CrossRef] [PubMed]
- McCaddon, A. Homocysteine and cognitive impairment; a case series in a General Practice setting. Nutr. J. 2006, 5, 6. [Google Scholar] [CrossRef]
- BSH Guidance on B12 Replacement during the COVID-19 Pandemic, British Society for Haematology. Available online: https://b-s-h.org.uk/media/18275/bsh-guidance-b12-replacement-covid-1901052020finalv.pdf (accessed on 1 May 2021).
| References | Laboratory and Clinical Investigation | Main Changes after Treatment |
|---|---|---|
| Socha et al. [23] | Full blood profile, Folate levels, Vitamin B12 levels Parietal cell antibody, Conventional cytogenetics, Hematologic neoplasm next-generation-sequencing panel (62 genes) for disease-associated mutations. | Case 1: Abnormal complete blood cell count findings improved, as did neurologic symptoms. Case 2: rapid improvement of hematologic symptoms and slower but complete resolution of neurologic symptoms. |
| Garcia et al. [24] | Hb, MCV, Folate levels, iron, ferritin, vitamins B2, B6, and B12 levels, gastroduodenoscopy and gastric biopsy, Antibodies against intrinsic factor and Helicobacter pylori detection | At 12 months the patient was free of the Recurrent aphthous stomatitis with normal levels of hemoglobin, MCV, and vitamin B12. |
| Pontes et al. [25] | Full blood count, Folate levels and vitamin B12 levels | After 14 days of treatment complete remission of all symptoms. |
| Pahadiya et al. [26] | Full blood profile, vitamin B12 levels, LDH, bilirubin Bone marrow aspiration, antinuclear antibody and Coomb’s test, Coagulation profile, iron profile Renal function tests, urinalysis and electrolytes Gastroscopy, electrocardiograph, ultrasonography of abdomen, and chest X-ray | At the follow-up of 1 month, hematological parameters were within normal limits and ecchymosis disappeared. |
| Surani and Sharma [27] | Full blood profile, Folate levels, vitamin B12 levels | Hemoglobin improved to 10.3 gm/dL after four days. Complete blood count showed a complete resolution of pancytopenia at two months follow up. Vitamin B12 and folate level normalized. |
| Hussain et al. [28]. | Full blood profile, Vitamin B12, Folate, Haptoglobin, MMA, Intrinsic factor antibody | At six-month follow-up clinical and laboratory analysis improvement (e.g., hemoglobin improved to 11.9 gm/d). |
| Sasi and Yassin [29]. | Full blood profile, B12 level, Bilirubin, LDH Haptoglobin, direct antiglobulin (DAT) Serum iron, thyroid functions | Blood cell counts started showing an upward trend on day 4 after starting the treatment. On discharge, after 10 days of hospital stay, improvement of blood profile and vitamin B12 (from values <37 pmol/L to 369 pmol/L), remission of all symptoms. |
| References | Laboratory and Clinical Investigations | Main Changes after Treatment |
|---|---|---|
| Ralapanawa et al. [34] | Full blood profile, serum creatinine, plasma glucose, thyroid stimulating hormone levels, vitamin B12 levels, nerve conduction studies | After 3 months, clinical improvement, with repeated B12 levels being elevated up to 308.6 pg/mL. Follow up at 1 and 3 years showed improvement of nerve conduction. |
| Ekabe et al. [35] | Full blood profile, HIV test, Treponema pallidum hematoglutinin assay (TPHA), erythrocyte sedimentation rate and peripheral blood smear analysis, stool exam and urinalysis | At 1 months follow up good clinical recovery, improvement in neurological symptoms and a follow up MCV of 97 fl, red blood cell count of 4.1 million/µL, and reticulocyte count of 0.95%. |
| Maamar et al. [36] | Full blood profile, Somatosensorial evoked potential (SEP), MRI, vitamin B12 levels, Folate levels, bone marrow biopsy | Correction of the neurological signs (paresthesis and sphincter disorders). |
| Senol et al. [37] | Blood glucose, AST, ALT, blood urea nitrogen, creatinine, Hb, MCV, white blood cell count, sedimentation rate, Vitamin B12 levels, HbA1C level, Somatosensorial evoked potential (SEP), Electromyography, Gastric endoscopy and biopsy, Brain MR, Cervical spine MR imaging | At two months follow up complete resolution of symptoms, MR imaging abnormalities significantly improved; impairment of the Somatosensorial evoked potential continued. |
| Srikanth et al. [38] | Full blood profile, bone marrow biopsy, Visual evoked potential and brain stem evoked potential studies, Gastric endoscopy and biopsy, workups for infections, para infectious myelitis, multiple sclerosis and connective tissue disorders, Folate levels, vitamin B12 levels, cervical MR examination | At 10 months follow-up, MRI revealed total resolution of cord abnormality. |
| Kumar [39] | Full blood profile, bone marrow biopsy, vitamin B12 levels, Folate levels, Anti-intrinsic factor antibody Gastric endoscopy and biopsy, Brain CT scan, EEG | At 24 months follow-up resolution of seizure and functional independence. |
| Mavromati & Sentissi [40] | Full blood profile, Electrolytes, vitamin B12 levels, Folate levels, Lyme’s test brain MRI, Neuropsychiatric tests | At 1 week normalization of vitamin B12 level (330 pmol/L); at 2 weeks important diminution of the cognitive deficiency and a partial remission of the depressive symptoms (MADRS score 22, MMSE 28/30 and DRS-R-98 4; the clock test was normalised). Four weeks after the episode total remission of the depressive symptoms (MADRS score: 4) and stable mental status. |
| References | Laboratory and Clinical Investigation | Main Changes after Treatment |
|---|---|---|
| Tanaka et al. [43] | Full blood profile, prothrombin time, protein C, protein S levels, total homocysteine, folic acid, vitamin B12 (Antinuclear antibody (fluorescent antibody technique), immunoglobulin G anticardiolipin antibodies (IgG ACA), phospholipid (GPL), Lupus anticoagulant (diluted Russell’s viper venom time rate). Tumor marker, carcinoembryonic antigen (CEA carbohydrate antigen 19-9, and a-fetoprotein (AFP), CT | Serum homocysteine level decreased (total homocysteine: 12.4 mmol/L), and swelling of his leg improved with significant resolution of thrombus by CT. |
| Kapur [44] | Full blood profile, Peripheral blood film, serum cobalamin levels, prothrombin time, protein S, antithrombin III, fibrinogen levels, factor V Leiden assay and prothrombin gene mutation, fasting total serum homocysteine levels, neurological examination, Cerebrospinal fluid examination, CT, MRI | Significant improvement of neurological symptoms. At 6 months normal serum cobalamin 364 pg/mL (200–600) and fasting total homocysteine levels 8.4 μmol/L (5.0–13.9). The rest of the thrombophilia profile was within normal limits. |
| Ammouri [45] | Full blood profile, prothrombin time, partial thromboplastin time, fibrinogen level, protein C, protein S levels, antithrombin III function, genetic testing for factor V Leiden and factor II mutation, plasma homocysteine level, cobalamin plasma level, folate plasma, antibodies to intrinsic factor, bone marrow biopsy, chest radiographs, ECG, TC, Ultrasonography | Case 1: After a 1-year follow up total remission of psychiatric disorders and thrombotic events. Hemoglobin and homocysteine plasma levels were within normal range. Case 2: At 6-month follow-up period, hemoglobin and homocysteine plasma levels were within normal range. No thrombotic events for 3 years after the follow-up. Case 3: At 6-month follow-up period, hemoglobin and homocysteine plasma levels were within normal range. No thrombotic events during 4 years of follow-up. Case 4: At 3-year follow-up no psychiatric disorders and thrombotic events. Homocysteine plasma level was within normal range. |
| Ulrich [46]. | Full blood profile, holotranscobalamin plasma levels, total homocysteine, MMA, Folate, zinc and copper, Electroneurography, CT, MRI. | Cyanocobalamin, MMA and homocysteine levels continuously decreased, and were normal again after 1 month; improvement of sensory disturbances and gait ataxia; At 2 months follow-up MRI showed significant regression of the dorsal column hyperintensities. |
| Kovalenko et al. [47] | Full blood profile, troponin, blood urea nitrogen, creatinine, serum electrolytes, B-type natriuretic peptide level, Factor V Leiden, prothrombin mutation, cardiolipin antibody, lupus anticoagulant, anti-B2 glycoprotein, protein C, protein S levels, Homocysteine level, vitamin B12, folate levels, chest radiographs, ECG, echocardiogram, Pulmonary angiography | Serum Hcy levels did not decrease to normal values. |
| Goette et al. [48]. | Full blood profile, lipid profile, Liver function tests (γ-glutamyl transpeptidase, Alanine transaminase and aspartate aminotransferase, bilirubin), activated partial thromboplastin time, international normalized ratio, thrombin time, activated recalcification, fibrinogen, clotting factors II, XII and VIII levels, protein C, protein S, anti-phospholipid antibodies, vitamin B12, folate, Hcy, analyses of cofactors and enzymes involved in homocysteine metabolism, serum levels of 8-isoprostaglandin F2α dimethy larginine (ADMA), Plasma concentrations of arginine and symmetric dimethyl arginine (SDMA), serum level of creatinine, urine analysis 5,10-methylenetetrahydrofolate reductase (MTHFR) gene, TC, computed tomography angiography, ultrasound, echocardiogram | At 2 weeks follow-up level of homocysteine had decreased to 57.6 μmol/L. Three weeks later homocysteine level was 18.1 μmol/L, and after 3 months it was 5.5 μmol/L. After completing his the following metabolites had decreased: ADMA, to 0.363 μmol/L; SDMA, to 0.32 μmol/L; arginine, to 62.8 μmol/L; light reflex rheography and oscillography shown normal perfusion; improvement of pain, paraesthesia in right leg and increasing of pain-free walking distance. |
| Ruscin et al. [49] | Full blood profile, vitamin B12, methylmalonic acid (MMA), total serum homocysteine, serum folate, serum creatinine, renal function test. | At first follow-up vitamin B12 has increased, MMA and HCYS was reduced at 351 nmol/L and 23.7 µmol/L respectively. At second follow-up vitamin B12 was normal; MMA and HCYS were further reduced but remain slight elevated. |
| McCaddon [50] | Full blood profile, vitamin B12, serum and red cell folate, plasma folate, parietal cell antibodies, total serum homocysteine, cognitive tests. | Case 1: improvement in memory and cognitive tests. Case 2: Within one month tHcy fell to 7.5 μmol/L; no significant cognitive deficits. Case 3: No improvement; the patient died from a bronchopneumonia several weeks later. Case 4: At six-months follow up tHcy fell to 6.6 μmol/L; marked improvement in general behaviour observed also three years later. Case 5: improvement in cognitive tests. Case 6: tHcy fell to 9.6 μmol/L; improvement in cognitive tests. Case 7: At one month follow-up tHcy fell to 8.3 μmol/L; improvement in cognitive tests. At one year follow up MRI scan showed no significant progression in the extent or size of the focal areas of abnormality in the deep white matter, and no change in ventricular configuration. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzini, E.; Raguzzini, A.; Polito, A. A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults. Int. J. Mol. Sci. 2021, 22, 9694. https://doi.org/10.3390/ijms22189694
Azzini E, Raguzzini A, Polito A. A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults. International Journal of Molecular Sciences. 2021; 22(18):9694. https://doi.org/10.3390/ijms22189694
Chicago/Turabian StyleAzzini, Elena, Anna Raguzzini, and Angela Polito. 2021. "A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults" International Journal of Molecular Sciences 22, no. 18: 9694. https://doi.org/10.3390/ijms22189694
APA StyleAzzini, E., Raguzzini, A., & Polito, A. (2021). A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults. International Journal of Molecular Sciences, 22(18), 9694. https://doi.org/10.3390/ijms22189694








