Interactions between the Gut Microbiome, Lung Conditions, and Coronary Heart Disease and How Probiotics Affect These
Abstract
:1. Introduction
2. The Gut Microbiome and CVD
3. The Gut Microbiome and Lung Health
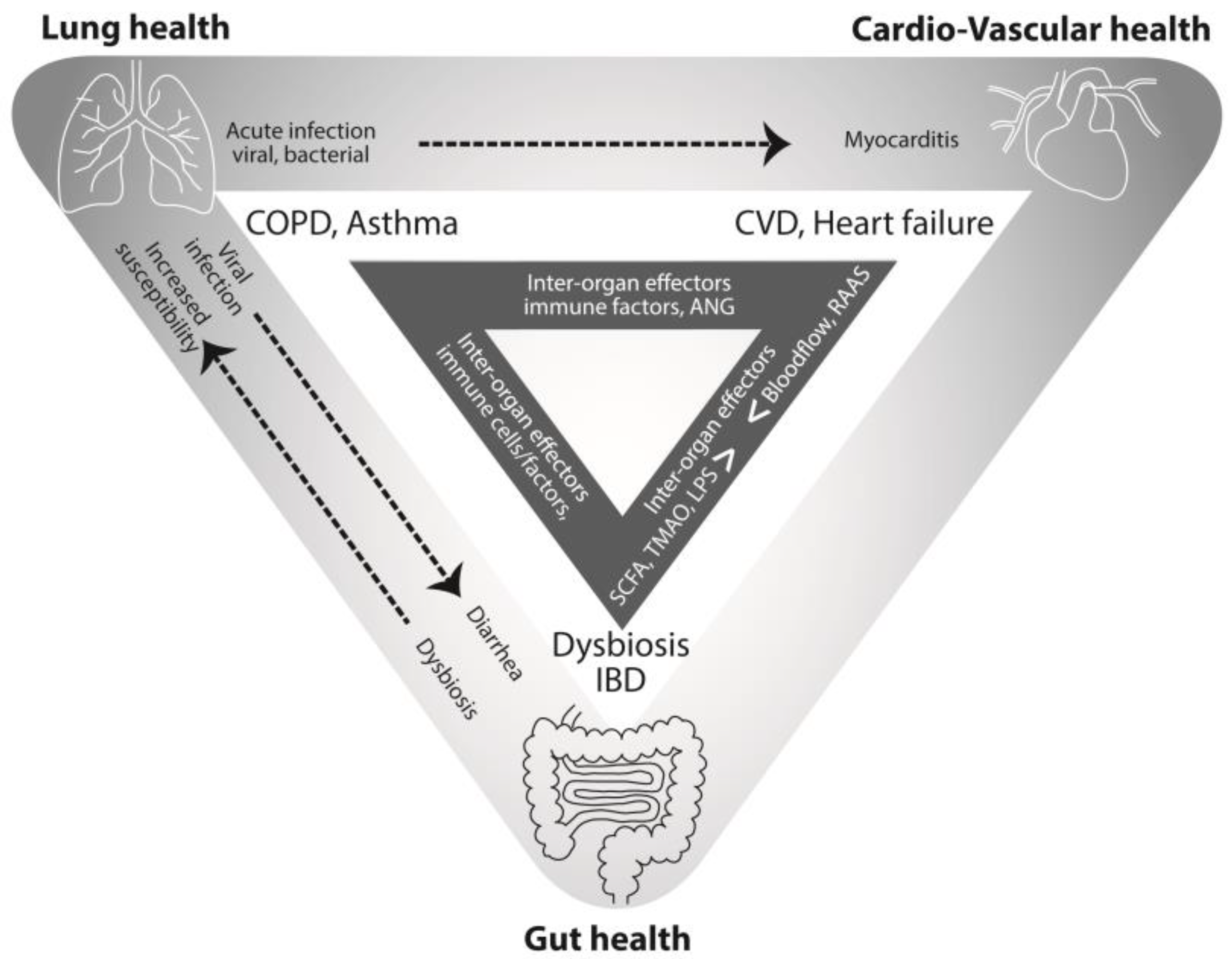
4. Connections between the Lung and the Heart
5. Learning from a Diseased State
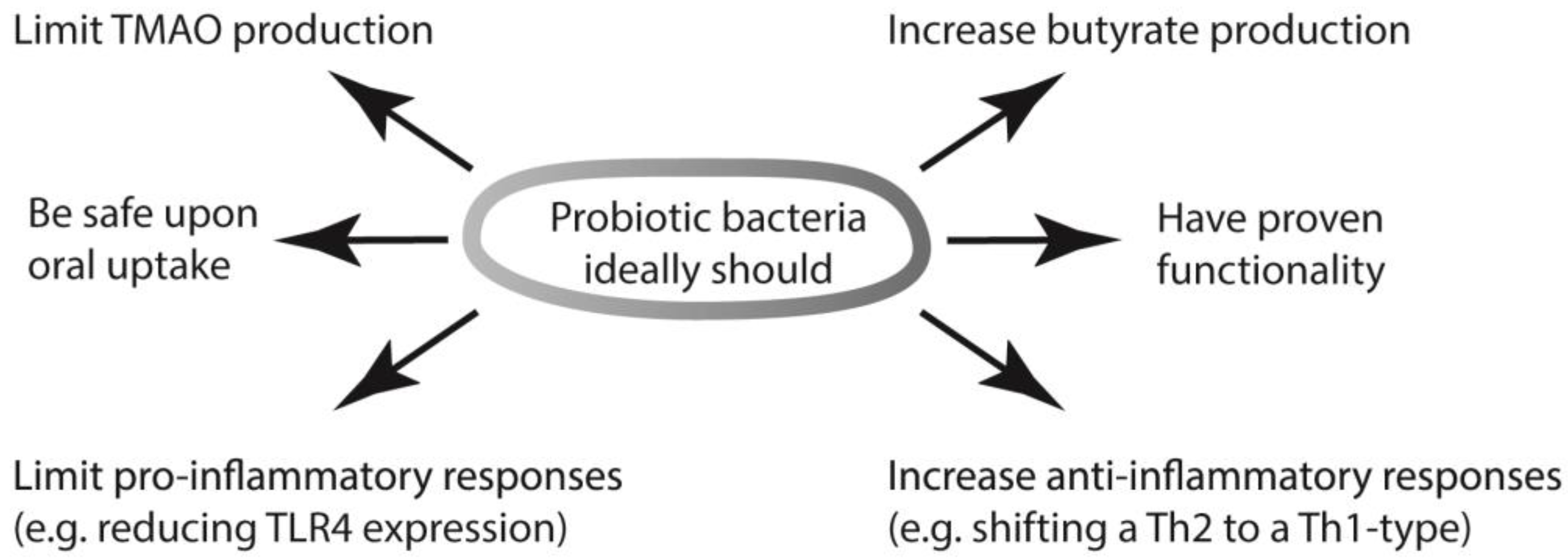
6. What Activities Would an Ideal Probiotic Need to Have?
7. Probiotics and COVID-19
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martínez, I.; Muller, C.E.; Walter, J. Long-Term Temporal Analysis of the Human Fecal Microbiota Revealed a Stable Core of Dominant Bacterial Species. PLoS ONE 2013, 8, e69621. [Google Scholar] [CrossRef]
- Pereira, F.; Berry, D. Microbial nutrient niches in the gut. Environ. Microbiol. 2017, 19, 1366–1378. [Google Scholar] [CrossRef] [Green Version]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.-A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015, 3, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, J.P.; Mak, J.W.Y.; Mullish, B.H.; Alexander, J.L.; Ng, S.C.; Marchesi, J.R. The gut microbiome: An under-recognised contributor to the COVID-19 pandemic? Ther. Adv. Gastroenterol. 2020, 13, 1756284820974914. [Google Scholar] [CrossRef] [PubMed]
- Bottari, B.; Castellone, V.; Neviani, E. Probiotics and COVID-19. Int. J. Food Sci. Nutr. 2021, 72, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Deidda, S.; Tora, L.; Firinu, D.; Del Giacco, S.; Campagna, M.; Meloni, F.; Orrù, G.; Chessa, L.; Carta, M.G.; Melis, A.; et al. Gastrointestinal coronavirus disease 2019: Epidemiology, clinical features, pathogenesis, prevention, and management. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L. SARS-CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, R.; Rani, A.; Metwally, A.; McGee, H.S.; Perkins, D.L. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 2016, 469, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Mitchell, S.; Smith, R. Dietary Precursors of Trimethylamine in Man: A Pilot Study. Food Chem. Toxicol. 1999, 37, 515–520. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisel, S.H.; Warrier, M. TrimethylamineN-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Fletcher, C. Trimethylamine N-oxide: Breathe new life. Br. J. Pharmacol. 2018, 175, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef]
- Trøseid, M. Gut microbiota and acute coronary syndromes: Ready for use in the emergency room? Eur. Heart J. 2017, 38, 825–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassenaar, T.M.; Zimmermann, K. Lipopolysaccharides in food, food supplements, and probiotics: Should we be worried? Eur. J. Microbiol. Immunol. 2018, 8, 63–69. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.; Ballantyne, C.M.; Couper, D.; Vigo, A.; Hoogeveen, R.; Folsom, A.R.; Heiss, G. Low-Grade Systemic Inflammation and the Development of Type 2 Diabetes: The Atherosclerosis Risk in Communities Study. Diabetes 2003, 52, 1799–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, J.M.G.; Costa, J.A.; Alfenas, R.C.G. Metabolic endotoxaemia and diabetes mellitus: A systematic review. Metabolism 2017, 68, 133–144. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes, Cardiovascular Disease, and Weight Gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, S.J.D.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawi, M.H.; Abdullah, A.; Ismail, A.; Sarbini, S.R. Manipulation of Gut Microbiota Using Acacia Gum Polysaccharide. ACS Omega 2021, 6, 17782–17797. [Google Scholar] [CrossRef]
- Moens, F.; Weckx, S.; De Vuyst, L. Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between bifidobacteria and Faecalibacterium prausnitzii. Int. J. Food Microbiol. 2016, 231, 76–85. [Google Scholar] [CrossRef]
- Mamic, P.; Chaikijurajai, T.; Tang, W.W. Gut microbiome—A potential mediator of pathogenesis in heart failure and its comorbidities: State-of-the-art review. J. Mol. Cell. Cardiol. 2021, 152, 105–117. [Google Scholar] [CrossRef]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Panigrahi, P. Is a fetus developing in a sterile environment? Lett. Appl. Microbiol. 2014, 59, 572–579. [Google Scholar] [CrossRef]
- Lal, C.V.; Travers, C.; Aghai, Z.H.; Eipers, P.; Jilling, T.; Halloran, B.; Carlo, W.A.; Keeley, J.; Rezonzew, G.; Kumar, R.; et al. The Airway Microbiome at Birth. Sci. Rep. 2016, 6, 31023. [Google Scholar] [CrossRef] [Green Version]
- Merk, V.M.; Phan, T.S.; Brunner, T. Regulation of Tissue Immune Responses by Local Glucocorticoids at Epithelial Barriers and Their Impact on Interorgan Crosstalk. Front. Immunol. 2021, 12, 672808. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Raftery, A.L.; Tsantikos, E.; Harris, N.L.; Hibbs, M.L. Links between Inflammatory Bowel Disease and Chronic Obstructive Pulmonary Disease. Front. Immunol. 2020, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- Chioma, O.S.; Hesse, L.E.; Chapman, A.; Drake, W.P. Role of the Microbiome in Interstitial Lung Diseases. Front. Med. 2021, 8, 595522. [Google Scholar] [CrossRef]
- Depner, M.; Taft, D.H.; Kirjavainen, P.V.; Kalanetra, K.M.; Karvonen, A.M.; Peschel, S.; Schmausser-Hechfellner, E.; Roduit, C.; Frei, R.; Lauener, R.; et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 2020, 26, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Saint-Criq, V.; Lugo-Villarino, G.; Thomas, M. Dysbiosis, malnutrition and enhanced gut–lung axis contribute to age-related respiratory diseases. Ageing Res. Rev. 2021, 66, 101235. [Google Scholar] [CrossRef] [PubMed]
- Favere, K.; Bosman, M.; Klingel, K.; Heymans, S.; Van Linthout, S.; Delputte, P.L.; De Sutter, J.; Heidbuchel, H.; Guns, P.J. Toll-Like Receptors: Are They Taking a Toll on the Heart in Viral Myocarditis? Viruses 2021, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Varagic, J.; Ahmad, S.; Nagata, S.; Ferrario, C.M. ACE2: Angiotensin II/Angiotensin-(1–7) Balance in Cardiac and Renal Injury. Curr. Hypertens. Rep. 2014, 16, 1–9. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, P.; Liang, T.; Chen, Y.; Liu, D.; Yu, H. Relationship between circulating levels of angiotensin-converting enzyme 2-angiotensin-(1–7)-MAS axis and coronary heart disease. Heart Vessel. 2020, 35, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prestes, T.R.R.; Rocha, N.P.; Miranda, A.S.; Teixeira, A.L.; Simoes-E-Silva, A.C. The Anti-Inflammatory Potential of ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Evidence from Basic and Clinical Research. Curr. Drug Targets 2017, 18, 1301–1313. [Google Scholar] [CrossRef]
- Ferreira-Duarte, M.; Estevinho, M.M.; Duarte-Araújo, M.; Magro, F.; Morato, M. Unraveling the Role of ACE2, the Binding Receptor for SARS-CoV-2, in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020, 26, 1787–1795. [Google Scholar] [CrossRef]
- Jia, H.P.; Look, D.C.; Shi, L.; Hickey, M.; Pewe, L.; Netland, J.; Farzan, M.; Wohlford-Lenane, C.; Perlman, S.; McCray, P.B. ACE2 Receptor Expression and Severe Acute Respiratory Syndrome Coronavirus Infection Depend on Differentiation of Human Airway Epithelia. J. Virol. 2005, 79, 14614–14621. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Glende, J.; Al-Falah, M.; De Vries, V.; Schwegmann-Wessels, C.; Qu, X.; Tan, L.; Tschernig, T.; Deng, H.; Naim, H.Y.; et al. Analysis of ACE2 in polarized epithelial cells: Surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J. Gen. Virol. 2006, 87, 1691–1695. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Shankar, E.M. SARS-CoV-2-Indigenous Microbiota Nexus: Does Gut Microbiota Contribute to Inflammation and Disease Severity in COVID-19? Front. Cell. Infect. Microbiol. 2021, 11, 590874. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Li, F.; Shi, Y. Main Clinical Features of COVID-19 and Potential Prognostic and Therapeutic Value of the Microbiota in SARS-CoV-2 Infections. Front. Microbiol. 2020, 11, 1302. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chen, Y.G.; Lin, C.L.; Huang, W.S.; Kao, C.H. Inflammatory Bowel Disease Increases the Risk of Peripheral Arterial Disease: A Nationwide Cohort Study. Medicine 2015, 94, e2381. [Google Scholar] [CrossRef]
- Frati, F.; Salvatori, C.; Incorvaia, C.; Bellucci, A.; Di Cara, G.; Marcucci, F.; Esposito, S. The Role of the Microbiome in Asthma: The gut–lung axis. Int. J. Mol. Sci. 2018, 20, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.; Shore, S.A. Obesity, Asthma, and the Microbiome. Physiology 2016, 31, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Womble, J.T.; Gunsch, C.K.; Ingram, J.L. The Gut/Lung Microbiome Axis in Obesity, Asthma, and Bariatric Surgery: A Literature Review. Obesity 2021, 29, 636–644. [Google Scholar] [CrossRef]
- Caselli, M.; Cassol, F.; Calò, G.; Holton, J.; Zuliani, G.; Gasbarrini, A. Actual concept of “probiotics”: Is it more functional to science or business? World J. Gastroenterol. 2013, 19, 1527–1540. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Gueimonde, M.; Duncan, S.H.; Flint, H.J.; de los Reyes-Gavilan, C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015, 362, fnv176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Henson, M.A. Metabolic modeling of bacterial co-culture systems predicts enhanced carbon monoxide-to-butyrate conversion compared to monoculture systems. Biochem. Eng. J. 2019, 151, 107338. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M. Insights from 100 years of research with probiotic E. coli. Eur. J. Microbiol. Immunol. 2016, 6, 147–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.; Xie, M. A review of a potential and promising probiotic candidate—Akkermansia muciniphila. J. Appl. Microbiol. 2021, 130, 1813–1822. [Google Scholar] [CrossRef]
- Martens, K.; Pugin, B.; De Boeck, I.; Spacova, I.; Steelant, B.; Seys, S.F.; Lebeer, S.; Hellings, P.W. Probiotics for the airways: Potential to improve epithelial and immune homeostasis. Allergy 2018, 73, 1954–1963. [Google Scholar] [CrossRef] [Green Version]
- Badi, S.A.; Tarashi, S.; Fateh, A.; Rohani, P.; Masotti, A.; Siadat, S.D. From the Role of Microbiota in gut–lung axis to SARS-CoV-2 Pathogenesis. Mediat. Inflamm. 2021, 2021, 6611222. [Google Scholar]
- Heyman, M. Effect of Lactic Acid Bacteria on Diarrheal Diseases. J. Am. Coll. Nutr. 2000, 19, 137S–146S. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Seksik, P.; Lepage, P.; Doré, J. Cellular and physiological effects of probiotics and prebiotics. Mini-Rev. Med. Chem. 2004, 4, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.Y.; Tao, J.; Chan, O.S.; Li, H.-B.; Pang, H. Preventing Respiratory Tract Infections by Synbiotic Interventions: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 979–988. [Google Scholar] [CrossRef]
- Wang, F.; Pan, B.; Xu, S.; Xu, Z.; Zhang, T.; Zhang, Q.; Bao, Y.; Wang, Y.; Zhang, J.; Xu, C.; et al. A meta-analysis reveals the effectiveness of probiotics and prebiotics against respiratory viral infection. Biosci. Rep. 2021, 41, 20203638. [Google Scholar] [CrossRef]
- Tanaka, M.; Itoh, H. Hypertension as a Metabolic Disorder and the Novel Role of the Gut. Curr. Hypertens. Rep. 2019, 21, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gui, Q.; Wang, A.; Zhao, X.; Huang, S.; Tan, Z.; Xiao, C.; Yang, Y. Effects of probiotic supplementation on natural killer cell function in healthy elderly individuals: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2020, 74, 1630–1637. [Google Scholar] [CrossRef]
- Roshan, H.; Ghaedi, E.; Rahmani, J.; Barati, M.; Najafi, M.; Karimzedeh, M.; Nikpayam, O. Effects of probiotics and synbiotic supplementation on antioxidant status: A meta-analysis of randomized clinical trials. Clin. Nutr. ESPEN 2019, 30, 81–88. [Google Scholar] [CrossRef]
- Milajerdi, A.; Mousavi, S.M.; Sadeghi, A.; Moghaddam, A.S.; Parohan, M.; Larijani, B.; Esmaillzadeh, A. The effect of probiotics on inflammatory biomarkers: A meta-analysis of randomized clinical trials. Eur. J. Nutr. 2020, 59, 633–649. [Google Scholar] [CrossRef]
- Qu, H.; Zhang, Y.; Chai, H.; Gao, Z.-Y.; Shi, D.-Z. Effects of microbiota-driven therapy on inflammatory responses in elderly individuals: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0211233. [Google Scholar] [CrossRef] [Green Version]
- Jafarabadi, M.A.; Dehghani, A.; Khalili, L.; Barzegar, A.; Mesrizad, M.; Hassanalilou, T. A Meta-analysis of Randomized Controlled Trials of the Effect of Probiotic Food or Supplement on Glycemic Response and Body Mass Index in Patients with Type 2 Diabetes, Updating the Evidence. Curr. Diabetes Rev. 2021, 17, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, T.; Molnár, B.; Németh, D.; Hegyi, P.; Szakács, Z.; Bálint, A.; Garami, A.; Soós, A.; Márta, K.; Solymár, M. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: A meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Tao, Y.W.; Gu, Y.L.; Mao, X.Q.; Zhang, L.; Pei, Y.F. Effects of probiotics on type II diabetes mellitus: A meta-analysis. J. Transl. Med. 2020, 18, 30. [Google Scholar] [CrossRef] [Green Version]
- Ardeshirlarijani, E.; Tabatabaei-Malazy, O.; Mohseni, S.; Qorbani, M.; Larijani, B.; Jalili, R.B. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: A meta-analysis of randomized trials. DARU J. Pharm. Sci. 2019, 27, 827–837. [Google Scholar] [CrossRef]
- Tabrizi, R.; Ostadmohammadi, V.; Lankarani, K.B.; Akbari, M.; Akbari, H.; Vakili, S.; Shokrpour, M.; Kolahdooz, F.; Rouhi, V.; Asemi, Z. The effects of probiotic and synbiotic supplementation on inflammatory markers among patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2019, 852, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Guo, J.; Jia, Q.; Huang, Y.S.; Huang, W.-J.; Zhang, W.; Zhang, F.; Liu, W.J.; Wang, Y. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 142, 303–313. [Google Scholar] [CrossRef]
- He, J.; Zhang, F.; Han, Y. Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes: A meta-analysis of RCTs. Medicine 2017, 96, e9166. [Google Scholar] [CrossRef]
- Yao, K.; Zeng, L.; He, Q.; Wang, W.; Lei, J.; Zou, X. Effect of Probiotics on Glucose and Lipid Metabolism in Type 2 Diabetes Mellitus: A Meta-Analysis of 12 Randomized Controlled Trials. Med. Sci. Monit. 2017, 23, 3044–3053. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-M.; Zhou, F.; Yuan, Y.; Xu, Y.-C. Effects of probiotics supplement in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. Med. Clin. (Engl. Ed.) 2017, 148, 362–370. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Han, H.; Cui, H.; Peng, M.; Wang, G.; Wang, Z. Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: A meta-analysis of randomized, controlled trials. Medicine 2016, 95, e4088. [Google Scholar] [CrossRef]
- Nikbakht, E.; Khalesi, S.; Singh, I.; Williams, L.T.; West, N.P.; Colson, N. Effect of probiotics and synbiotics on blood glucose: A systematic review and meta-analysis of controlled trials. Eur. J. Nutr. 2018, 57, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, E.A.; Bersch-Ferreira, Â.C.; Torreglosa, C.R.; Da Silva, J.T.; Coqueiro, A.Y.; Kuntz, M.G.F.; Chrispim, P.P.; Weber, B.; Cavalcanti, A.B. Effects of oral supplementation with probiotics or synbiotics in overweight and obese adults: A systematic review and meta-analyses of randomized trials. Nutr. Rev. 2019, 77, 430–450. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Ghavami, A.; Hadi, A.; Askari, G.; Symonds, M.; Miraghajani, M. Effects of pro-/synbiotic supplementation on anthropometric and metabolic indices in overweight or obese children and adolescents: A systematic review and meta-analysis. Complement. Ther. Med. 2019, 44, 269–276. [Google Scholar] [CrossRef]
- Yan, S.; Tian, Z.; Li, M.; Li, B.; Cui, W. Effects of probiotic supplementation on the regulation of blood lipid levels in overweight or obese subjects: A meta-analysis. Food Funct. 2019, 10, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesaeth, J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018, 19, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Hadi, A.; Alizadeh, K.; Hajianfar, H.; Mohammadi, H.; Miraghajani, M. Efficacy of synbiotic supplementation in obesity treatment: A systematic review and meta-analysis of clinical trials. Crit. Rev. Food Sci. Nutr. 2018, 60, 584–596. [Google Scholar] [CrossRef]
- Koutnikova, H.; Genser, B.; Monteiro-Sepulveda, M.; Faurie, J.-M.; Rizkalla, S.; Schrezenmeir, J.; Clément, K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2019, 9, e017995. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, S.R.; Maraj, B.; Harding-Theobald, E.; Vittinghoff, E.; Terrault, N.A. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Am. J. Clin. Nutr. 2019, 110, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, R.; Ghavami, A.; Khalesi, S.; Ghiasvand, R.; Mokari_Yamchi, A. The effect of probiotic fermented milk products on blood lipid concentrations: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 997–1015. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, X.; Yang, Y. Effect of probiotics on lipid profiles in hypercholesterolaemic adults: A meta-analysis of randomized controlled trials. Med. Clin. 2019, 152, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Ma, J.; Song, M.; Jin, Y.; Ji, C.; Ge, W.; Guo, C. Effects of products designed to modulate the gut microbiota on hyperlipidaemia. Eur. J. Nutr. 2019, 58, 2713–2729. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Q.; Ren, Y.; Ruan, Z. Effect of probiotic Lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PLoS ONE 2017, 12, e0178868. [Google Scholar] [CrossRef]
- Sharma, S.; Kurpad, A.V.; Puri, S. Potential of probiotics in hypercholesterolemia: A meta-analysis. Indian J. Public Health 2016, 60, 280–286. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, M.; Chen, L.; Bhochhibhoya, A. Probiotic Foods and Supplements Interventions for Metabolic Syndromes: A Systematic Review and Meta-Analysis of Recent Clinical Trials. Ann. Nutr. Metab. 2019, 74, 224–241. [Google Scholar] [CrossRef]
- Iheozor-Ejiofor, Z.; Kaur, L.; Gordon, M.; Baines, P.A.; Sinopoulou, V.; Akobeng, A.K. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020, 2020, CD007443. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Xu, M.; Liu, D.; Zhou, D.; Yang, W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0228846. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; Macdonald, J.K.; Gordon, M.; Mullin, G.E. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 2, CD012839. [Google Scholar] [CrossRef]
- Asha, M.Z.; Khalil, S.F.H. Efficacy and Safety of Probiotics, Prebiotics and Synbiotics in the Treatment of Irritable Bowel Syndrome: A systematic review and meta-analysis. Sultan Qaboos Univ. Med. J. 2020, 20, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, H.-L.; Xiao, J.-Y. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int. J. Surg. 2020, 75, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Tong, X.; Wang, R.; Song, X. The clinical effects of probiotics for inflammatory bowel disease: A meta-analysis. Medicine 2018, 97, e13792. [Google Scholar] [CrossRef]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef]
- Wen, Y.; Li, J.; Long, Q.; Yue, C.-C.; He, B.; Tang, X.-G. The efficacy and safety of probiotics for patients with constipation-predominant irritable bowel syndrome: A systematic review and meta-analysis based on seventeen randomized controlled trials. Int. J. Surg. 2020, 79, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.P.; Bibbò, S.; Fresi, G.; Bassotti, G.; Pes, G.M. Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 2913. [Google Scholar] [CrossRef] [Green Version]
- Limketkai, B.N.; Akobeng, A.K.; Gordon, M.; Adepoju, A.A. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2020, 7, CD006634. [Google Scholar] [CrossRef]
- Liang, D.; Longgui, N.; Guoqiang, X. Efficacy of different probiotic protocols in irritable bowel syndrome: A network meta-analysis. Medicine 2019, 98, e16068. [Google Scholar] [CrossRef]
- Yuan, F.; Ni, H.; Asche, C.V.; Kim, M.; Walayat, S.; Ren, J. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: A meta-analysis. Curr. Med. Res. Opin. 2017, 33, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Guo, C.; Mu, D.; Feng, B.; Zuo, X.; Li, Y. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: A meta-analysis. BMC Gastroenterol. 2016, 16, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, X.; Ge, T.; Xiao, Y.; Liao, Y.; Cui, Y.; Zhang, Y.; Ho, W.; Yu, G.; Zhang, T. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4509. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, L.; Wu, S.; Yuan, L.; Tang, S.; Xiang, Y.; Qu, X.; Liu, H.; Qin, X.; Liu, C. Efficacy of probiotic supplementary therapy for asthma, allergic rhinitis, and wheeze: A meta-analysis of randomized controlled trials. Allergy Asthma Proc. 2019, 40, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Jiang, P.; Liu, J.; Sun, R.; Zhu, L. Association between probiotic supplementation and asthma incidence in infants: A meta-analysis of randomized controlled trials. J. Asthma 2020, 57, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Güvenç, I.A.; Muluk, N.B.; Mutlu, F.Ş.; Eşki, E.; Altıntoprak, N.; Oktemer, T.; Cingi, C. Do probiotics have a role in the treatment of allergic rhinitis? A comprehensive systematic review and meta-analysis. Am. J. Rhinol. Allergy. 2016, 30, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Mikhael, A.-M.; Davoodvandi, A.; Jafarnejad, S. Effect of Lactobacillusplantarum containing probiotics on blood pressure: A systematic review and meta-analysis. Pharmacol. Res. 2020, 153, 104663. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, M.J.; Gao, Q.; Yang, J.F.; Yang, L.; Pang, X.L.; Jiang, X.J. The effects of probiotics on total cholesterol: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e9679. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Boeing, H.; Stelmach-Mardas, M.; Gottschald, M.; Dietrich, S.; Hoffmann, G.; Chaimani, A. Dietary Supplements and Risk of Cause-Specific Death, Cardiovascular Disease, and Cancer: A Systematic Review and Meta-Analysis of Primary Prevention Trials. Adv. Nutr. 2017, 8, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-L.; Shih, P.-C.; Liu, S.-J.; Lin, C.-H.; Liu, J.-M.; Lei, W.-T.; Lin, C.-Y. The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: A systematic review and meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2018, 12, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Baud, D.; Dimopoulou Agri, V.; Gibson, G.R.; Reid, G.; Giannoni, E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health 2020, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Din, A.U.; Mazhar, M.; Waseem, M.; Ahmad, W.; Bibi, A.; Hassan, A.; Ali, N.; Gang, W.; Qian, G.; Ullah, R.; et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed. Pharmacother. 2021, 133, 110947. [Google Scholar] [CrossRef]
- Mirzaei, R.; Attar, A.; Papizadeh, S.; Jeda, A.S.; Hosseini-Fard, S.R.; Jamasbi, E.; Kazemi, S.; Amerkani, S.; Talei, G.R.; Moradi, P.; et al. The emerging role of probiotics as a mitigation strategy against coronavirus disease 2019 (COVID-19). Arch. Virol. 2021, 166, 1819–1840. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, D.; Robba, C.; Fedele, A.; Trancǎ, S.; Sukkar, S.G.; Di Pilato, V.; Bassetti, M.; Giacobbe, D.R.; Vena, A.; Patroniti, N.; et al. The Role of Dysbiosis in Critically Ill Patients with COVID-19 and Acute Respiratory Distress Syndrome. Front. Med. 2021, 8, 671714. [Google Scholar] [CrossRef]
- Allali, I.; Bakri, Y.; Amzazi, S.; Ghazal, H. Gut–lung axis in COVID-19. Interdiscip. Perspect. Infect. Dis. 2021, 2021, 6655380. [Google Scholar] [CrossRef] [PubMed]
- Mahooti, M.; Miri, S.M.; Abdolalipour, E.; Ghaemi, A. The immunomodulatory effects of probiotics on respiratory viral infections: A hint for COVID-19 treatment? Microb. Pathog. 2020, 148, 104452. [Google Scholar] [CrossRef]
- Ettinger, G.; Macdonald, K.; Reid, G.; Burton, J. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes 2014, 5, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A. The impact of probiotics on interactions within the microbiota-gut-lung triad in COVID-19. Int. J. Food Sci. Nutr. 2021, 72, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-K.; Paik, H.-D. Prophylactic effects of probiotics on respiratory viruses including COVID-19: A review. Food Sci. Biotechnol. 2021, 4, 1–9. [Google Scholar] [CrossRef]
- Cabrera Martimbianco, A.L.; Pacheco, R.L.; Bagattini, Â.M.; Riera, R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Int. J. Clin. Pract. 2021, e14357. [Google Scholar] [CrossRef]

| Target Condition | Reported Effects (1) | Nr of Trials Included [Reference] |
|---|---|---|
| Improving natural killer cell function | No significant effect | 6 [65] |
| Oxidative stress | Improved antioxidant markers | 16 [66] |
| Inflammatory markers | Reduced pro-inflammatory markers | 42 [67] |
| Inflammation in elderly | No significant effect | 10 [68] |
| Glycemic response, BMI, T2D | Improved glucose metabolism, reduced BMI | 14 [69] |
| T2D | Reduced dyslipemia, improve metabolic control | 32 [70] |
| T2D | Reduced insulin resistance | 15 [71] |
| T2D | Improved oxidative stress biomarkers | 13 [72] |
| T2D | Improved inflammatory markers | 18 [73] |
| T2D | Improved inflammatory and oxidative stress markers | 16 [74] |
| T2D | Improved blood lipid profile, reduced BP | 10 [75] |
| T2D | Reduced insulin resistance | 12 [76] |
| T2D | Reduced insulin resistance and blood lipid profile | 12 [77] |
| T2D | Lowered fasting glucose levels | 12 [78], 14 [79] |
| High BMI | Reduced waist circumference, no effect on BMI | 19 [80] |
| High BMI | No significant effect | 9 [81] |
| High BMI | Reduced total cholesterol, LDL | 12 [82] |
| High BMI | Reduced body weight | 15 [83] |
| High BMI (synbiotic treatment) | Reduced waist circumference and body weight | 23 [84] |
| Obesity, T2D, NAFLD | Minor improvements of metabolic risk factors | 15 [85] |
| NAFLD | Improved liver-specific markers | 21 [86] |
| Not specified | Reduced LDL, total cholesterol | 39 [87] |
| Lipidaemia | Reduced LDL, total cholesterol | 19 [88], 21 [89], 15 [90], 12 [91] |
| Metabolic syndrome | No significant effect for most biomarkers, but reduced LDL | 18 [92] |
| UC | Induced remission | 14 [93], 3 [94] |
| Crohn’s Disease and UC | No significant effect | 18 [95] |
| IBD | Improved symptoms | 33 [96], 35 [97] |
| IBD | No significant effect | 10 [98] |
| IBD | Induced remission | 22 [99] |
| IBD (Constipation-dominant) | Increased stool frequency | 17 [100] |
| IBD, side effect assessment | Increased abdominal pain | 9 [101] |
| Crohn’s disease | No clear effect | 2 [102] |
| IBS | Improved symptoms | 14 [103], 5 [104], 22 [105] |
| Prevention of RTI | Reduced incidence of RTI | 16 [62] |
| Pediatric RTIs | Reduced risk of RTI | 23 [106] |
| Pediatric asthma, allergic rhinitis | No significant reduction | 17 [107] |
| Pediatric asthma | No significant reduction | 19 [108] |
| Allergic rhinitis | Reduced nasal and ocular symptom scores | 5 [109] |
| Hypertension | Lowered systolic BP | 7 [110] |
| CVD, cholestrol | Reduced total cholesterol | 32 [111] |
| CVD | No significant effect | unclear [112] |
| Vaccination efficiency (influenzavirus) | Enhanced antibody titers | 12 [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wassenaar, T.M.; Juncos, V.A.; Zimmermann, K. Interactions between the Gut Microbiome, Lung Conditions, and Coronary Heart Disease and How Probiotics Affect These. Int. J. Mol. Sci. 2021, 22, 9700. https://doi.org/10.3390/ijms22189700
Wassenaar TM, Juncos VA, Zimmermann K. Interactions between the Gut Microbiome, Lung Conditions, and Coronary Heart Disease and How Probiotics Affect These. International Journal of Molecular Sciences. 2021; 22(18):9700. https://doi.org/10.3390/ijms22189700
Chicago/Turabian StyleWassenaar, Trudy M., Valentina A. Juncos, and Kurt Zimmermann. 2021. "Interactions between the Gut Microbiome, Lung Conditions, and Coronary Heart Disease and How Probiotics Affect These" International Journal of Molecular Sciences 22, no. 18: 9700. https://doi.org/10.3390/ijms22189700
APA StyleWassenaar, T. M., Juncos, V. A., & Zimmermann, K. (2021). Interactions between the Gut Microbiome, Lung Conditions, and Coronary Heart Disease and How Probiotics Affect These. International Journal of Molecular Sciences, 22(18), 9700. https://doi.org/10.3390/ijms22189700





