Unveiling the Metabolic Effects of Glycomacropeptide
Abstract
:1. Introduction
2. Results
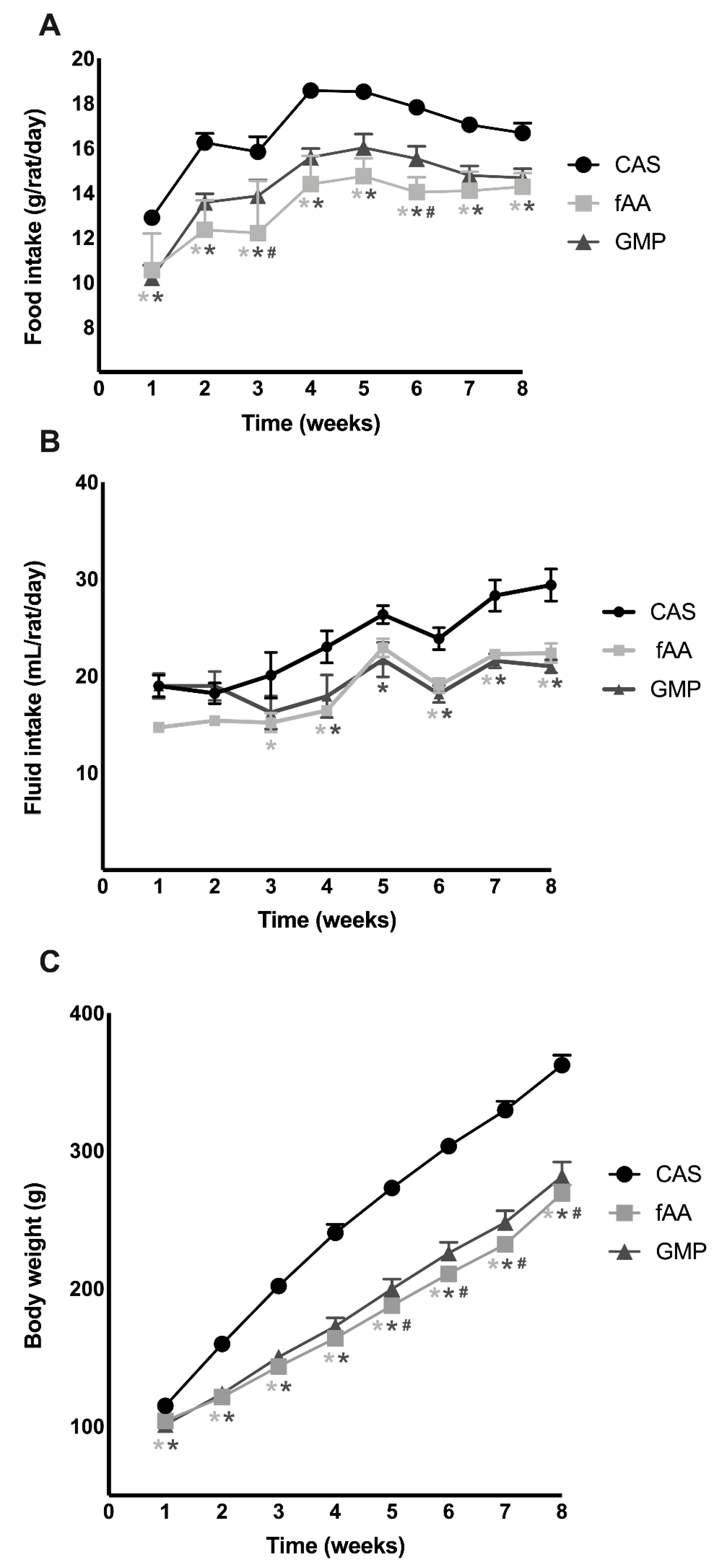
2.1. Food Intake, Fluid Intake and Body Weight
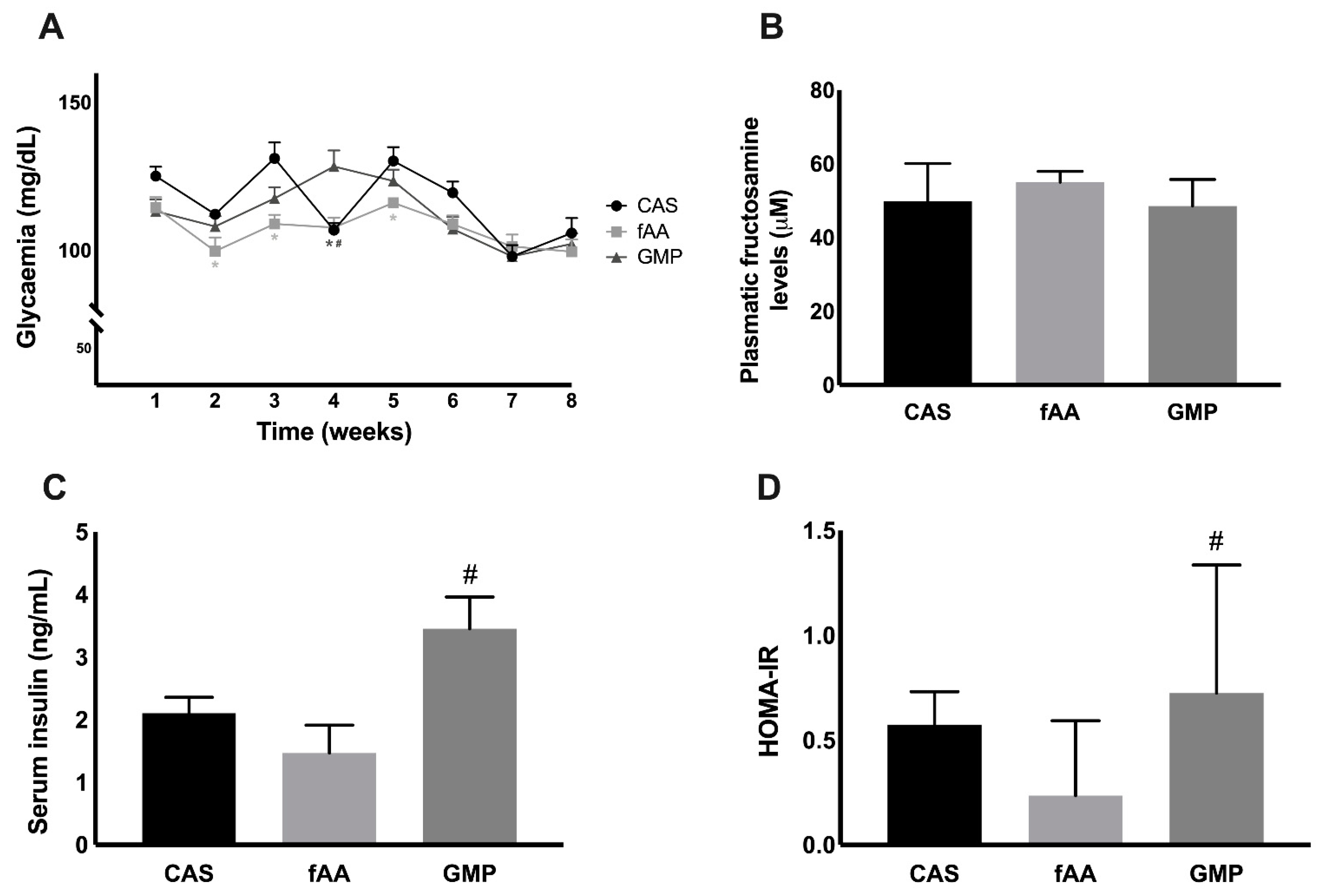
2.2. Markers of Glucose Metabolism
2.3. Local and Systemic GLP-1
2.4. Biochemical Markers
3. Discussion
4. Materials and Methods
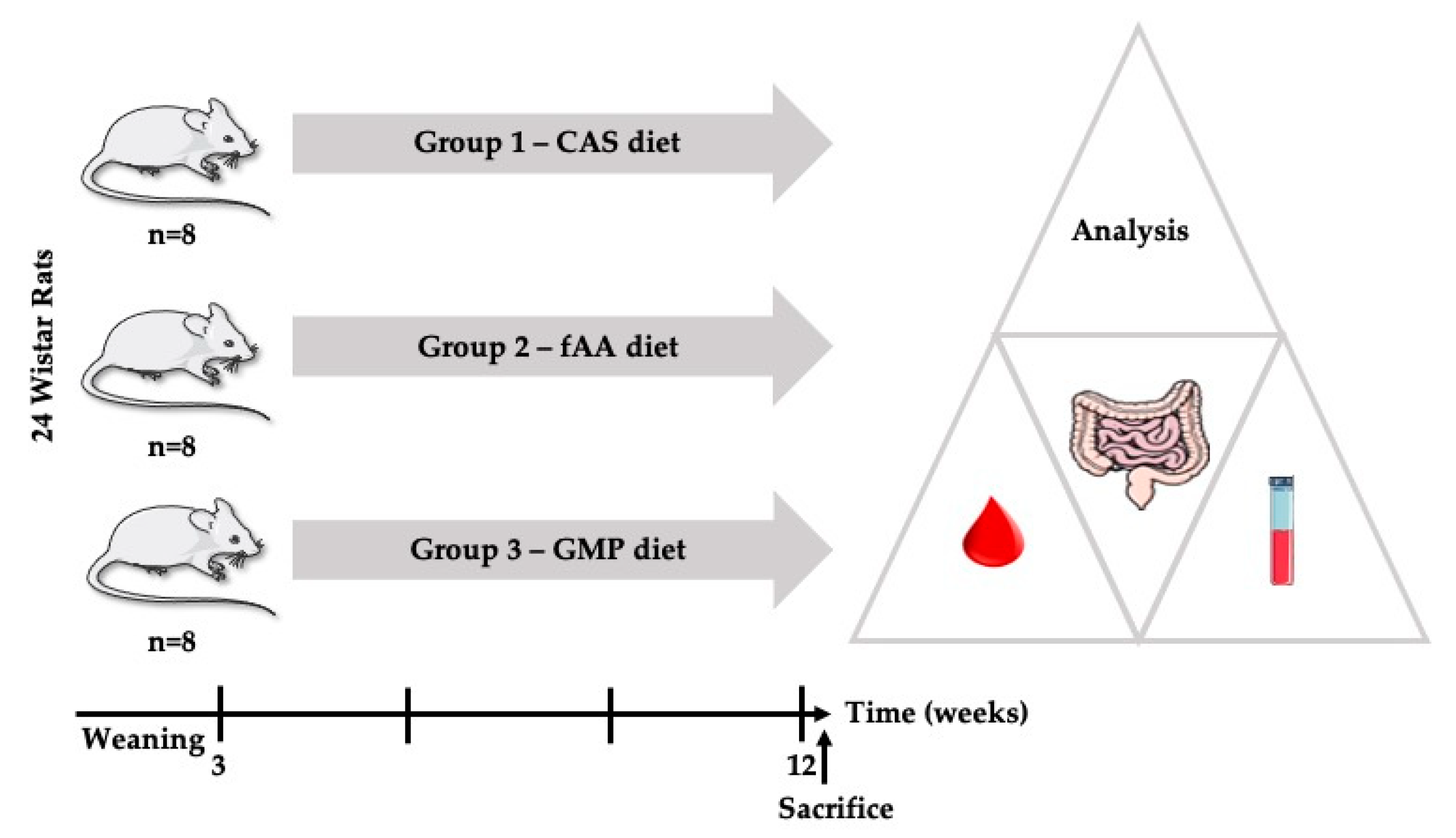
4.1. Animals and Housing
4.2. Diets
4.3. Experimental Design
4.4. Collection of Blood and Tissues
4.5. Glucose, Insulin and Total GLP-1
4.6. Biochemical Analyses
4.7. Immunochemistry for GLP-1
4.8. Computerised Image Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Belanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Gizewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.C.; MacDonald, A. Dietary intervention in the management of phenylketonuria: Current perspectives. Pediatric Health Med. Ther. 2016, 7, 155–163. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, A.; van Rijn, M.; Feillet, F.; Lund, A.M.; Bernstein, L.; Bosch, A.M.; Gizewska, M.; van Spronsen, F.J. Adherence issues in inherited metabolic disorders treated by low natural protein diets. Ann. Nutr. Metab. 2012, 61, 289–295. [Google Scholar] [CrossRef]
- Giarratana, N.; Gallina, G.; Panzeri, V.; Frangi, A.; Canobbio, A.; Reiner, G. A new Phe-free protein substitute engineered to allow a physiological absorption of free amino acids for phenylketonuria. J. Inborn Errors Metab. Screen. 2018, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, A.; Ashmore, C.; Daly, A.; Pinto, A.; Evans, S. An Observational Study Evaluating the Introduction of a Prolonged-Release Protein Substitute to the Dietary Management of Children with Phenylketonuria. Nutrients 2020, 12, 2686. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.C.; MacDonald, A. Treatment options and dietary supplements for patients with phenylketonuria. Expert Opin. Orphan Drugs 2018, 6, 667–681. [Google Scholar] [CrossRef]
- Daly, A.; Evans, S.; Chahal, S.; Santra, S.; Pinto, A.; Jackson, R.; Gingell, C.; Rocha, J.; Van Spronsen, F.J.; MacDonald, A. Glycomacropeptide: Long-term use and impact on blood phenylalanine, growth and nutritional status in children with PKU. Orphanet J. Rare Dis. 2019, 14, 44. [Google Scholar] [CrossRef]
- Pena, M.J.; Pinto, A.; Daly, A.; MacDonald, A.; Azevedo, L.; Rocha, J.C.; Borges, N. The Use of Glycomacropeptide in Patients with Phenylketonuria: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1794. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, A.; Singh, R.H.; Rocha, J.C.; van Spronsen, F.J. Optimising amino acid absorption: Essential to improve nitrogen balance and metabolic control in phenylketonuria. Nutr. Res. Rev. 2019, 32, 70–78. [Google Scholar] [CrossRef]
- Daly, A.; Evans, S.; Pinto, A.; Jackson, R.; Ashmore, C.; Rocha, J.C.; MacDonald, A. Preliminary Investigation to Review If a Glycomacropeptide Compared to L-Amino Acid Protein Substitute Alters the Pre- and Postprandial Amino Acid Profile in Children with Phenylketonuria. Nutrients 2020, 12, 2443. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; Rocha, J.C.; Borges, N. Amino acids, glucose metabolism and clinical relevance for phenylketonuria management. Ann. Nutr. Disord. Ther. 2015, 2, 1026. [Google Scholar]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Albrechtsen, N.W.; Windelov, J.A.; Svendsen, B.; Hartmann, B.; Holst, J.J. GLP-1 amidation efficiency along the length of the intestine in mice, rats and pigs and in GLP-1 secreting cell lines. Peptides 2014, 55, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Mönch, E.; Herrmann, M.E.; Brosicke, H.; Schoffer, A.; Keller, M. Utilisation of amino acid mixtures in adolescents with phenylketonuria. Eur. J. Pediatr. 1996, 155 (Suppl. S1), S115–S120. [Google Scholar] [CrossRef]
- Weigel, C.; Rauh, M.; Kiener, C.; Rascher, W.; Knerr, I. Effects of various dietary amino acid preparations for phenylketonuric patients on the metabolic profiles along with postprandial insulin and ghrelin responses. Ann. Nutr. Metab. 2007, 51, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Pena, M.J.; Guerreiro, S.G.; Rocha, J.C.; Morais, T.; Pereira, S.S.; Monteiro, M.P.; Borges, N. Acute effect of an amino acid mixture in the rat glycemic profile. J. Cell. Biochem. 2019, 120, 13056–13065. [Google Scholar] [CrossRef] [PubMed]
- Chungchunlam, S.M.; Henare, S.J.; Ganesh, S.; Moughan, P.J. Effect of whey protein and glycomacropeptide on measures of satiety in normal-weight adult women. Appetite 2014, 78, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Evans, S.; Pinto, A.; Jackson, R.; Ashmore, C.; Rocha, J.C.; MacDonald, A. The Impact of the Use of Glycomacropeptide on Satiety and Dietary Intake in Phenylketonuria. Nutrients 2020, 12, 2704. [Google Scholar] [CrossRef]
- Royle, P.J.; McIntosh, G.H.; Clifton, P.M. Whey protein isolate and glycomacropeptide decrease weight gain and alter body composition in male Wistar rats. Br. J. Nutr. 2008, 100, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Wernlund, P.G.; Hvas, C.L.; Dahlerup, J.F.; Bahl, M.I.; Licht, T.R.; Knudsen, K.E.B.; Agnholt, J.S. Casein glycomacropeptide is well tolerated in healthy adults and changes neither high-sensitive C-reactive protein, gut microbiota nor faecal butyrate: A restricted randomised trial. Br. J. Nutr. 2021, 125, 1374–1385. [Google Scholar] [CrossRef]
- Venos, E.; de Koning, L. Endocrine markers of diabetes and cardiovascular disease risk. In Endocrine Biomarkers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 251–299. [Google Scholar]
- Bernard, J.R.; Liao, Y.H.; Hara, D.; Ding, Z.; Chen, C.Y.; Nelson, J.L.; Ivy, J.L. An amino acid mixture improves glucose tolerance and insulin signaling in Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E752–E760. [Google Scholar] [CrossRef]
- Yang, J.; Dolinger, M.; Ritaccio, G.; Mazurkiewicz, J.; Conti, D.; Zhu, X.; Huang, Y. Leucine stimulates insulin secretion via down-regulation of surface expression of adrenergic alpha2A receptor through the mTOR (mammalian target of rapamycin) pathway: Implication in new-onset diabetes in renal transplantation. J. Biol. Chem. 2012, 287, 24795–24806. [Google Scholar] [CrossRef] [Green Version]
- Ahring, K.K.; Lund, A.M.; Jensen, E.; Jensen, T.G.; Brondum-Nielsen, K.; Pedersen, M.; Bardow, A.; Holst, J.J.; Rehfeld, J.F.; Moller, L.B. Comparison of Glycomacropeptide with Phenylalanine Free-Synthetic Amino Acids in Test Meals to PKU Patients: No Significant Differences in Biomarkers, Including Plasma Phe Levels. J. Nutr. Metab. 2018, 2018, 6352919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foisy Sauve, M.; Spahis, S.; Delvin, E.; Levy, E. Glycomacropeptide: A Bioactive Milk Derivative to Alleviate Metabolic Syndrome Outcomes. Antioxid. Redox Signal. 2021, 34, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Calkosinski, I.; Majda, J.; Terlecki, G.; Gostomska-Pampuch, K.; Malolepsza-Jarmolowska, K.; Sobolewska, S.; Calkosinska, A.; Kumala, A.; Gamian, A. Dynamic Analysis of Changes of Protein Levels and Selected Biochemical Indices in Rat Serum in the Course of Experimental Pleurisy. Inflammation 2016, 39, 1076–1089. [Google Scholar] [CrossRef] [Green Version]
- Solverson, P.; Murali, S.G.; Brinkman, A.S.; Nelson, D.W.; Clayton, M.K.; Yen, C.L.; Ney, D.M. Glycomacropeptide, a low-phenylalanine protein isolated from cheese whey, supports growth and attenuates metabolic stress in the murine model of phenylketonuria. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E885–E895. [Google Scholar] [CrossRef] [PubMed]
- Sawin, E.A.; De Wolfe, T.J.; Aktas, B.; Stroup, B.M.; Murali, S.G.; Steele, J.L.; Ney, D.M. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G590–G601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, A.L.; Fiorini Aguiar, S.L.; Goncalves Miranda, M.C.; Lemos, L.; Freitas Guimaraes, M.A.; Reis, D.S.; Vieira Barros, P.A.; Veloso, E.S.; Carvalho, T.G.; Ribeiro, F.M.; et al. Consumption of Diet Containing Free Amino Acids Exacerbates Colitis in Mice. Front. Immunol. 2017, 8, 1587. [Google Scholar] [CrossRef] [Green Version]
- Cavaillon, J.M. Pro- versus anti-inflammatory cytokines: Myth or reality. Cell. Mol. Biol. 2001, 47, 695–702. [Google Scholar]
- Lillefosse, H.H.; Tastesen, H.S.; Du, Z.-Y.; Ditlev, D.B.; Thorsen, F.A.; Madsen, L.; Kristiansen, K.; Liaset, B. Hydrolyzed casein reduces diet-induced obesity in male C57BL/6J mice. J. Nutr. 2013, 143, 1367–1375. [Google Scholar] [CrossRef] [Green Version]
- Ney, D.M.; Etzel, M.R. Designing medical foods for inherited metabolic disorders: Why intact protein is superior to amino acids. Curr. Opin. Biotechnol. 2017, 44, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Ney, D.M.; Murali, S.G.; Stroup, B.M.; Nair, N.; Sawin, E.A.; Rohr, F.; Levy, H.L. Metabolomic changes demonstrate reduced bioavailability of tyrosine and altered metabolism of tryptophan via the kynurenine pathway with ingestion of medical foods in phenylketonuria. Mol. Genet. Metab. 2017, 121, 96–103. [Google Scholar] [CrossRef]
- Stroup, B.M.; Nair, N.; Murali, S.G.; Broniowska, K.; Rohr, F.; Levy, H.L.; Ney, D.M. Metabolomic Markers of Essential Fatty Acids, Carnitine, and Cholesterol Metabolism in Adults and Adolescents with Phenylketonuria. J. Nutr. 2018, 148, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y.; et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front. Pharmacol. 2019, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Carbone, M.T.; Borghi, E.; Ottaviano, E.; Burlina, A.; Biasucci, G. Nutrition, Microbiota and Role of Gut-Brain Axis in Subjects with Phenylketonuria (PKU): A Review. Nutrients 2020, 12, 3319. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | CAS Diet | fAA Diet | GMP Diet |
|---|---|---|---|
| AST (U/L) | 89 ± 11 | 104 ± 16 | 68 ± 5 |
| ALT (U/L) | 17 (15–24) | 21 (17–22) | 17 (15–20) |
| ALP (U/L) | 146 ± 12 | 169 ± 11 | 119 ± 11 # |
| Creatinine (mg/dL) | 0.16 ± 0.01 | 0.18 ± 0.01 | 0.17 ± 0.01 |
| Urea (mg/dL) | 32 ± 2 | 24 ± 2 * | 24 ± 1 * |
| Uric acid (mg/dL) | <1.5 | <1.5 | <1.5 |
| Total cholesterol (mg/dL) | 74 ± 5 | 77 ± 4 | 61 ± 3 # |
| LDL-cholesterol (mg/dL) | 22 ± 3 | 24 ± 2 | 17 ± 2 |
| HDL-cholesterol (mg/dL) | 39 ± 4 | 42 ±2 | 36 ± 2 |
| Triglycerides (mg/dL) | 167 ± 35 | 55 ± 7 * | 68 ± 14 * |
| Total protein (g/L) | 56.5 ± 0.3 | 51.9 ± 0.3 * | 52.1 ± 0.9 * |
| Albumin (g/L) | 28.9 ± 0.5 | 26.8 ± 0.5 | 26.1 ± 0.9 * |
| Iron (µg/dL) | 187 (176–209) | 162 (151–174) * | 177 (161–189) |
| Transferrin (mg/dL) | 175 (167–177) | 158 (154–174) | 170 (166–175) |
| Transferrin saturation (%) | 78 (74–85) | 72 (68–77) | 73 (65–82) |
| Leptin (pg/mL) | 687 ± 143 | 692 ± 194 | 756 ± 136 |
| IL-1β (pg/mL) | 12.0 ± 2.4 | 5.9 ± 1.5 | 6.9 ± 1.6 |
| IL-6 (pg/mL) | 36 (17–47) | 71 (35–165) | 20 (10–74) |
| TNF-α (pg/mL) | 1.5 (0.9–2.2) | 2.2 (0.4–3.5) | 1.0 (0.4–1.3) |
| Ingredients (g/100 g) | CAS Diet | fAA Diet | GMP Diet |
|---|---|---|---|
| Casein | 20.0 | - | - |
| BiPRO® GMP | - | - | 20.3 |
| Additional L-AA | 3.5 | 20.9 | 3.2 |
| Protein | 20.9 | 20.9 | 20.9 |
| CHO | 61.9 | 61.9 | 61.9 |
| Fat | 7.0 | 7.0 | 7.0 |
| Fiber | 10.0 | 10.0 | 10.0 |
| Energy (kcal/g) | 107.2 | 104.6 | 107.3 |
| Amino Acid Profile (g/100 g) | CAS Diet | fAA Diet | GMP Diet * |
|---|---|---|---|
| L-cysteine | 0.42 | 0.02 | 0.02 (0.02 + 0.00) |
| L-isoleucine | 0.75 | 1.78 | 2.11 (2.11 + 0.00) |
| L-leucine | 2.09 | 1.07 | 0.94 (0.42 + 0.52) |
| L-lysine | 1.30 | 1.02 | 1.21 (1.21 + 0.00) |
| L-methionine | 1.18 | 0.98 | 1.04 (0.36 + 0.68) |
| L-phenylalanine | 1.81 | 1.02 | 1.02 (0.04 + 0.98) |
| L-threonine | 0.71 | 2.90 | 3.43 (3.43 + 0.00) |
| L-tryptophan | 0.39 | 0.20 | 0.20 (0.02 + 0.18) |
| L-valine | 0.92 | 1.57 | 1.86 (1.86 + 0.00) |
| L-histidine | 0.71 | 0.28 | 0.28 (0.02 + 0.26) |
| L-alanine | 0.50 | 1.04 | 1.23 (1.23 + 0.00) |
| L-arginine | 0.98 | 0.43 | 0.43 (0.04 + 0.39) |
| L-aspartic acid | 0.50 | 1.57 | 1.86 (1.86 + 0.00) |
| L-glutamic acid | 2.27 | 3.52 | 4.36 (4.16 + 0.20) |
| Glycine | 0.30 | 0.19 | 0.23 (0.23 + 0.00) |
| L-proline | 1.76 | 2.07 | 2.45 (2.45 + 0.00) |
| L-serine | 0.99 | 1.24 | 1.46 (1.46 + 0.00) |
| L-tyrosine | 0.90 | 0.01 | 0.01 (0.01 + 0.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pena, M.J.; Costa, R.; Rodrigues, I.; Martins, S.; Guimarães, J.T.; Faria, A.; Calhau, C.; Rocha, J.C.; Borges, N. Unveiling the Metabolic Effects of Glycomacropeptide. Int. J. Mol. Sci. 2021, 22, 9731. https://doi.org/10.3390/ijms22189731
Pena MJ, Costa R, Rodrigues I, Martins S, Guimarães JT, Faria A, Calhau C, Rocha JC, Borges N. Unveiling the Metabolic Effects of Glycomacropeptide. International Journal of Molecular Sciences. 2021; 22(18):9731. https://doi.org/10.3390/ijms22189731
Chicago/Turabian StylePena, Maria João, Raquel Costa, Ilda Rodrigues, Sandra Martins, João Tiago Guimarães, Ana Faria, Conceição Calhau, Júlio César Rocha, and Nuno Borges. 2021. "Unveiling the Metabolic Effects of Glycomacropeptide" International Journal of Molecular Sciences 22, no. 18: 9731. https://doi.org/10.3390/ijms22189731







