Open MHC Class I Conformers: A Look through the Looking Glass
Abstract
:1. Introduction
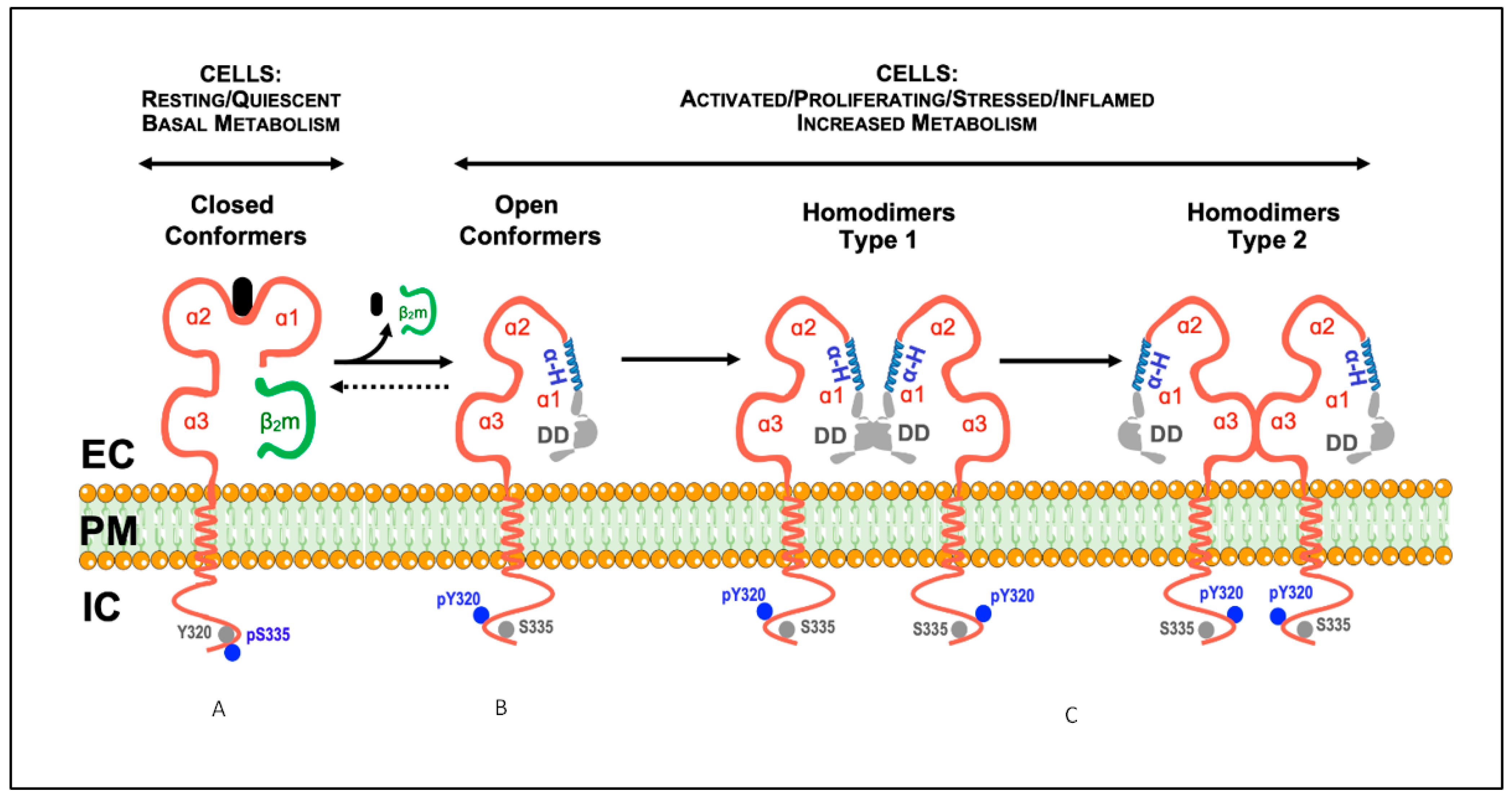
2. Open MHC-I Conformers: Structure, Origin and the Search for a Physiological Function
2.1. Current Knowledge
2.2. Forward-Looking Perspective
3. Homo-Associations of Open MHC-I Conformers: Modulation of (Auto)Immune Responses
3.1. Current Knowledge
3.2. Forward-Looking Perspective
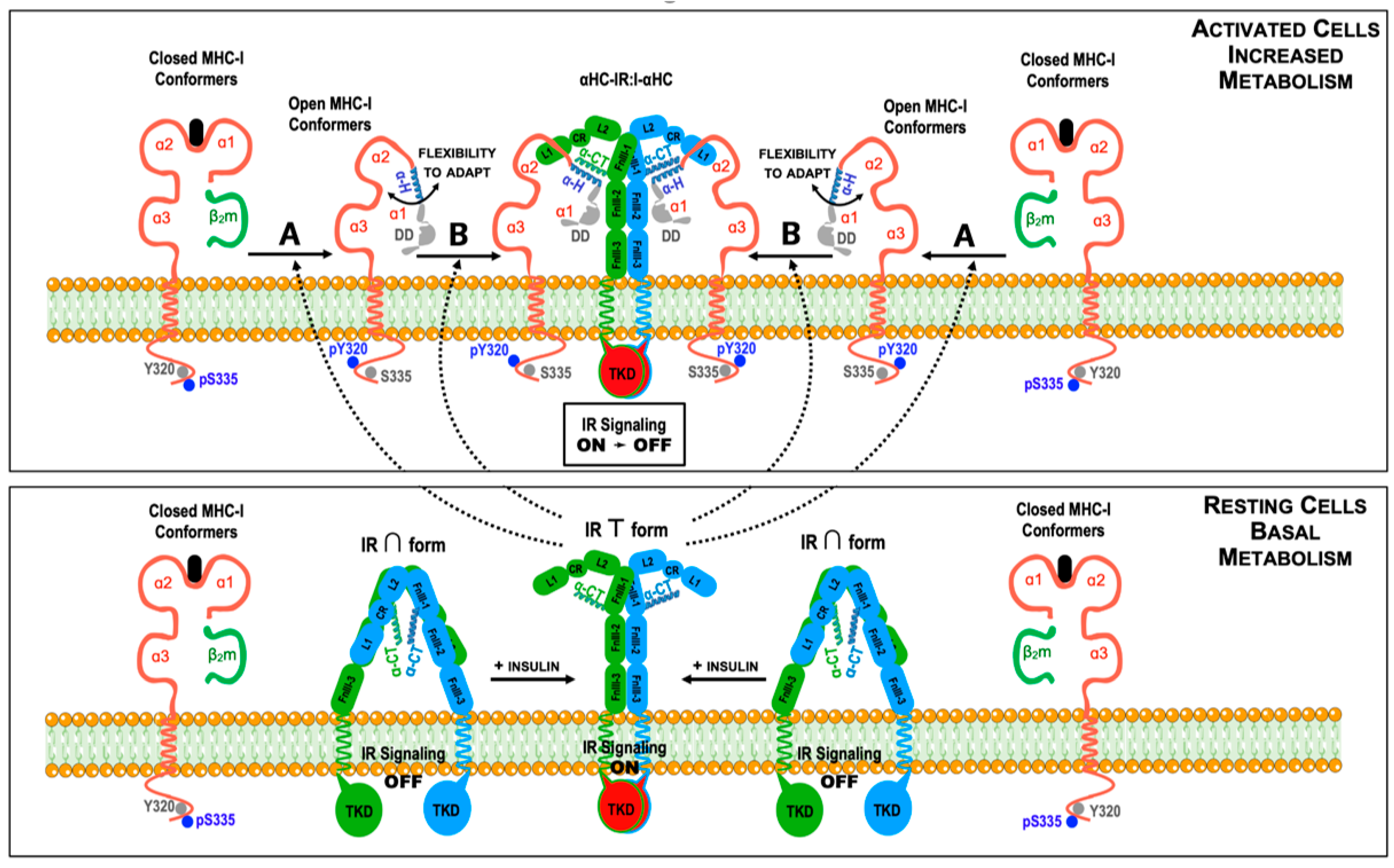
4. Hetero-Associations of Open MHC-I Conformers: Modulation of Cell Signaling and (Tumor) Cell Growth
4.1. Current Knowledge
4.2. Forward-Looking Perspective
- The cells express baseline levels of MHC-I molecules and the open MHC-I conformers will homeostatically regulate IR-mediated signaling and glucose uptake as needed.
- The cells show an increased expression of MHC-I molecules at the plasma membrane (above baseline levels, for example, caused by inflammatory cytokines). In this setting, many αHC-IR:I-αHC complexes will be formed at the cell surface with a subsequent increase in IR-mediated signaling. As a result, extracellular glucose levels may temporarily decrease, leading to hypoglycemia.
- The cells show a diminished expression of MHC-I molecules at the plasma membrane (below baseline levels, for example, caused by genetic or microenvironmental factors). In this setting, very few αHC-IR:I-αHC complexes will form and the number of IR present at the plasma membrane will increase due to a reduced endocytosis. As a result, IR-mediated signaling will be constitutively turned on. Although, initially, extracellular glucose levels may decrease, the cells will become refractory to insulin, which may ultimately lead to hyperglycemia and type II diabetes.
- Whether uptake of glucose by the cells is dependent or independent of insulin signaling;
- Whether the cells are part of a tissue/organ or are circulating lymphomyeloid cells;
- Whether they are normal or malignant cells.
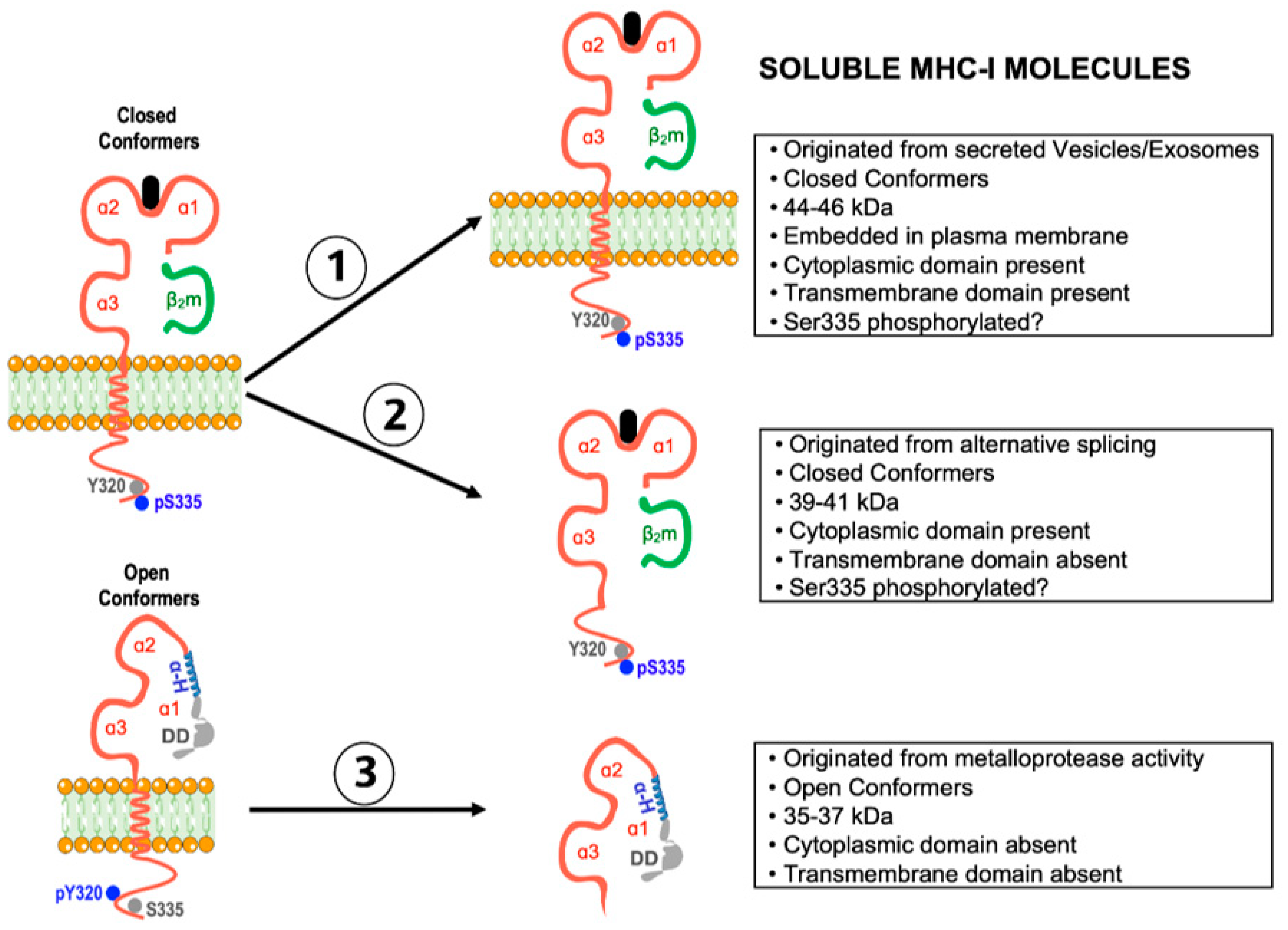
5. Shedding of Open MHC-I Conformers: Modulation of Tumor Cell Growth and Allograft Rejection
5.1. Current Knowledge
5.2. Forward-Looking Perspective
6. Open MHC-I Conformers: Unforeseen Modulators of Neuronal Development and Synaptic Plasticity?
6.1. Current Knowledge
6.2. Forward-Looking Perspective
7. Concluding Remarks and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, J. The Natural History of the Major Histocompatibility Complex; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Bjorkman, P.J.; Parham, P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu. Rev. Biochem. 1990, 59, 253–288. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.G.; Williams, D.B. Intracellular Assembly and Trafficking of MHC Class I Molecules. Traffic 2009, 10, 1745–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, T.H.; Lybarger, L.; Yu, L.; Mitaksov, V.; Fremont, D.H. Recognition of open conformers of classical MHC by chaper-ones and monoclonal antibodies. Immunol. Rev. 2005, 207, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Arosa, F.A.; Santos, S.; Powis, S. Open conformers: The hidden face of MHC-I molecules. Trends Immunol. 2007, 28, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Reits, E.; Neefjes, J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef] [Green Version]
- Saunders, P.M.; Vivian, J.; O’Connor, G.; Sullivan, L.; Pymm, P.G.; Rossjohn, J.; Brooks, A.G. A bird’s eye view of NK cell receptor interactions with their MHC class I ligands. Immunol. Rev. 2015, 267, 148–166. [Google Scholar] [CrossRef] [Green Version]
- Guild, B.C.; Strominger, J.L. Human and murine class I MHC antigens share conserved serine 335, the site of HLA phos-phorylation in vivo. J. Biol. Chem. 1984, 259, 9235–9240. [Google Scholar] [CrossRef]
- Peyron, J.F.; Fehlmann, M. Phosphorylation of class I histocompatibility antigens in human B lymphocytes. Regulation by phorbol esters and insulin. Biochem. J. 1988, 256, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.H.; Barber, B.H. The conformational flexibility of class I H-2 molecules as revealed by anti-peptide antibodies specific for intracytoplasmic determinants: Differential reactivity of beta2-microglobulin “bound” and “free” H-2Kb heavy chains. Mol. Immunol. 1990, 27, 169–180. [Google Scholar] [CrossRef]
- Capps, G.G.; Zúñiga, M.C. The cytoplasmic domain of the H-2Ld class I major histocompatibility complex molecule is differentially accessible to immunological and biochemical probes during transport to the cell surface. J. Biol. Chem. 1993, 268, 21263–21270. [Google Scholar] [CrossRef]
- Thor, G.; Sepulveda, H.; Chada, S.; Dutton, R.W. Monoclonal antibody that distinguishes between a phosphorylated, beta 2-microglobulin-associated, and a free, nonphosphorylated, chain of MHC class I. J. Immunol. 1993, 151, 211–224. [Google Scholar] [PubMed]
- Little, A.M.; Nössner, E.; Parham, P. Dissociation of beta 2-microglobulin from HLA class I heavy chains correlates with acquisition of epitopes in the cytoplasmic tail. J. Immunol. 1995, 154, 5205–5215. [Google Scholar] [PubMed]
- Santos, S.; Powis, S.; Arosa, F.A. Misfolding of Major Histocompatibility Complex Class I Molecules in Activated T Cells Allows cis-Interactions with Receptors and Signaling Molecules and Is Associated with Tyrosine Phosphorylation. J. Biol. Chem. 2004, 279, 53062–53070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, S.G.; Antoniou, A.N.; Sampaio, P.; Powis, S.J.; Arosa, F.A. Lack of Tyrosine 320 Impairs Spontaneous Endocytosis and Enhances Release of HLA-B27 Molecules. J. Immunol. 2006, 176, 2942–2949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svejgaard, A.; Ryder, L. Interaction of HLA molecules with non-immunological ligands as an explanation of HLA and disease associations. Lancet 1976, 308, 547–549. [Google Scholar] [CrossRef]
- Ohno, S. The Original Function of MHC Antigens as the General Plasma Membrane Anchorage Site of Organogenesis-Directing Proteins. Immunol. Rev. 1977, 33, 59–69. [Google Scholar] [CrossRef]
- Simonsen, M.; Olsson, L. Compound receptors in the cell membrane: Ruminations from the borderland of immunology and physiology. Prog. Allergy 1985, 36, 151–176. [Google Scholar]
- Edidin, M. Major histocompatibility complex haplotypes and the cell physiology of peptide hormones. Hum. Immunol. 1986, 15, 357–365. [Google Scholar] [CrossRef]
- Tsai, W.C.; Chen, C.J.; Yen, J.H.; Ou, T.T.; Tsai, J.J.; Liu, C.S.; Liu, H.W. Free HLA class I heavy chain-carrying monocytes--a po-tential role in the pathogenesis of spondyloarthropathies. J. Rheumatol. 2002, 29, 966–972. [Google Scholar]
- Cardoso, E.M.; Esgalhado, A.; Patrao, L.; Santos, M.; Neves, V.P.; Martinez, J.; Patto, M.A.V.; Silva, H.; Arosa, F.A. Distinctive CD8+ T cell and MHC class I signatures in polycythemia vera patients. Ann. Hematol. 2018, 97, 1563–1575. [Google Scholar] [CrossRef]
- Bix, M.; Raulet, D. Functionally conformed free class I heavy chains exist on the surface of beta 2 microglobulin negative cells. J. Exp. Med. 1992, 176, 829–834. [Google Scholar] [CrossRef] [Green Version]
- Martayan, A.; Fiscella, M.; Setini, A.; Ciccarelli, G.; Gambari, R.; Feriotto, G.; Beretta, A.; Siccardi, A.G.; Appella, E.; Giacomini, P. Conformation and surface expression of free HLA-CW1 heavy chains in the absence of beta2m. Hum. Immunol. 1997, 53, 23–33. [Google Scholar] [CrossRef]
- Hochman, J.H.; Jiang, H.; Matyus, L.; Edidin, M.; Pernis, B. Endocytosis and dissociation of class I MHC molecules labeled with fluorescent beta-2 microglobulin. J. Immunol. 1991, 146, 1862–1867. [Google Scholar]
- Pickl, W.F.; Holter, W.; Stöckl, J.; Majdic, O.; Knapp, W. Expression of beta 2-microglobulin-free HLA class I alpha-chains on activated T cells requires internalization of HLA class I heterodimers. Immunology 1996, 88, 104–109. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, S.; Schwab, R.; Bushkin, Y. The origin and fate of beta 2m-free MHC class I molecules induced on activated T cells. Cell Immunol. 1992, 142, 103–113. [Google Scholar] [CrossRef]
- Carreno, B.M.; Hansen, T.H. Exogenous peptide ligand influences the expression and half-life of free HLA class I heavy chains ubiquitously detected at the cell surface. Eur. J. Immunol. 1994, 24, 1285–1292. [Google Scholar] [CrossRef]
- Mahmutefendic, H.; Blagojević, G.; Tomas, M.I.; Kučić, N.; Lucin, P. Segregation of open Major Histocompatibility Class I conformers at the plasma membrane and during endosomal trafficking reveals conformation-based sorting in the endosomal system. Int. J. Biochem. Cell Biol. 2011, 43, 504–515. [Google Scholar] [CrossRef]
- Schnabl, E.; Stockinger, H.; Majdic, O.; Gaugitsch, H.; Lindley, I.J.; Maurer, D.; Hajek-Rosenmayr, A.; Knapp, W. Activated human T lymphocytes express MHC class I heavy chains not associated with beta 2-microglobulin. J. Exp. Med. 1990, 171, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Arosa, F.A.; de Jesus, O.; Porto, G.; Carmo, A.M.; de Sousa, M. Calreticulin is expressed on the cell surface of activated hu-man peripheral blood T lymphocytes in association with major histocompatibility complex class I molecules. J. Biol. Chem. 1999, 274, 16917–16922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monos, D.S.; Cooper, H.L. Rapid turnover of HLA proteins in quiescent lymphocytes: Proposed connection with immu-nologic surveillance. J. Immunol. 1983, 131, 341–346. [Google Scholar]
- Pernis, B. Internalization of lymphocyte membrane components. Immunol. Today 1985, 6, 45–49. [Google Scholar] [CrossRef]
- Machy, P.; Truneh, A.; Gennaro, D.; Hoffstein, S. Major histocompatibility complex class I molecules internalized via coat-ed pits in T lymphocytes. Nature 1987, 328, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Parham, P.; Adams, E.J.; Arnett, K. The Origins of HLA-A,B,C Polymorphism. Immunol. Rev. 1995, 143, 141–180. [Google Scholar] [CrossRef]
- Lizée, G.; Basha, G.; Tiong, J.; Julien, J.-P.; Tian, M.; Biron, K.E.; Jefferies, W.A. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat. Immunol. 2003, 4, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, M.; Wiley, N.C. Structural characterization of a soluble and partially folded class I major histocompatibility heavy chain/beta2m heterodimer. Nat. Struct Biol. 1998, 5, 377–384. [Google Scholar] [CrossRef]
- Saini, S.K.; Abualrous, E.; Tigan, A.-S.; Covella, K.; Wellbrock, U.; Springer, S. Not all empty MHC class I molecules are molten globules: Tryptophan fluorescence reveals a two-step mechanism of thermal denaturation. Mol. Immunol. 2013, 54, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Edidin, M.; Achilles, S.; Zeff, R.; Wei, T. Probing the stability of class I major histocompatibility complex (MHC) molecules on the surface of human cells. Immunogenetics 1997, 46, 41–45. [Google Scholar] [CrossRef]
- Zacharias, M.; Springer, S. Conformational Flexibility of the MHC Class I alpha1-alpha2 Domain in Peptide Bound and Free States: A Molecular Dynamics Simulation Study. Biophys. J. 2004, 87, 2203–2214. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.D.; Lie, W.-R.; Gorka, J.; Kindle, C.S.; Myers, N.B.; Hansen, T.H. Disparate interaction of peptide ligand with nascent versus mature class I major histocompatibility complex molecules: Comparisons of peptide binding to alternative forms of Ld in cell lysates and the cell surface. J. Exp. Med. 1992, 175, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Catipovi, B.; Talluri, G.; Oh, J.; Wei, T.; Su, X.-M.; Johansen, T.E.; Edidin, M.; Schneck, J.P. Analysis of the structure of empty and peptide-loaded major histocompatibility complex molecules at the cell surface. J. Exp. Med. 1994, 180, 1753–1761. [Google Scholar] [CrossRef] [Green Version]
- Dirscherl, C.; Hein, Z.; Ramnarayan, V.R.; Jacob-Dolan, C.; Springer, S. A two-hybrid antibody micropattern assay reveals specific in cis interactions of MHC I heavy chains at the cell surface. eLife 2018, 7, 34150. [Google Scholar] [CrossRef]
- Bychkova, V.E.; Semisotnov, G.; Balobanov, V.A.; Finkelstein, A.V. The Molten Globule Concept: 45 Years Later. Biochemistry 2018, 83, S33–S47. [Google Scholar] [CrossRef]
- Kitazawa, S.; Kameda, T.; Yagi-Utsumi, M.; Sugase, K.; Baxter, N.J.; Kato, K.; Williamson, M.P.; Kitahara, R. Solution Structure of the Q41N Variant of Ubiquitin as a Model for the Alternatively Folded N2 State of Ubiquitin. Biochemistry 2013, 52, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.R.; Park, C. Ligand binding to a high-energy partially unfolded protein. Protein Sci. 2015, 24, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mage, M.G.; Dolan, M.A.; Wang, R.; Boyd, L.F.; Revilleza, M.J.; Robinson, H.; Natarajan, K.; Myers, N.B.; Hansen, T.H.; Margulies, D.H. The Peptide-Receptive Transition State of MHC Class I Molecules: Insight from Structure and Molecular Dynamics. J. Immunol. 2012, 189, 1391–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjanappa, R.; Garcia-Alai, M.; Kopicki, J.-D.; Lockhauserbäumer, J.; Aboelmagd, M.; Hinrichs, J.; Nemtanu, I.M.; Uetrecht, C.; Zacharias, M.; Springer, S.; et al. Structures of peptide-free and partially loaded MHC class I molecules reveal mechanisms of peptide selection. Nat. Commun. 2020, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, M.T.; Classon, B.J.; Hudson, P.J.; Margulies, D.H. Direct binding of the MHC class I molecule H-2Ld to CD8: Interac-tion with the amino terminus of a mature cell surface protein. J. Immunol. 1988, 160, 2809–2814. [Google Scholar]
- Correia, M.P.; Cardoso, E.M.; Pereira, C.-F.; Neves, R.; Uhrberg, M.; Arosa, F.A. Hepatocytes and IL-15: A Favorable Microenvironment for T Cell Survival and CD8+T Cell Differentiation. J. Immunol. 2009, 182, 6149–6159. [Google Scholar] [CrossRef] [Green Version]
- Antunes, R.F.; Brandão, C.; Maia, M.; Arosa, F.A. Red blood cells release factors with growth and survival bioactivities for normal and leukemic T cells. Immunol. Cell Biol. 2010, 89, 111–121. [Google Scholar] [CrossRef]
- Ramalingam, T.S.; Chakrabarti, A.; Edidin, M. Interaction of Class I Human Leukocyte Antigen (HLA-I) Molecules with Insulin Receptors and Its Effect on the Insulin-Signaling Cascade. Mol. Biol. Cell 1997, 8, 2463–2474. [Google Scholar] [CrossRef] [Green Version]
- Assa-Kunik, E.; Fishman, D.; Kellman-Pressman, S.; Tsory, S.; Elhyany, S.; Baharir, O.; Segal, S. Alterations in the Expression of MHC Class I Glycoproteins by B16BL6 Melanoma Cells Modulate Insulin Receptor-Regulated Signal Transduction and Augments Resistance to Apoptosis. J. Immunol. 2003, 171, 2945–2952. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.; Santi, M.; Rajan, B.; Rushing, E.J.; Choi, M.R.; Rood, B.R.; Cornelison, R.; MacDonald, T.J.; Vukmanovic, S. A novel role of HLA class I in the pathology of medulloblastoma. J. Transl. Med. 2009, 7, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.; Santi, M.; Rushing, E.J.; Cornelison, R.; Macdonald, T.J.; Vukmanovic, S. Characterization of signaling function and expression of HLA class I molecules in medulloblastoma. J. Neuro-Oncol. 2011, 103, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmutefendic, H.; Zagorac, G.B.; Tomas, M.I.; Groettrup, M.; Momburg, F.; Lucin, P. Endosomal trafficking of open Major Histocompatibility Class I conformers—Implications for presentation of endocytosed antigens. Mol. Immunol. 2013, 55, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodridge, J.P.; Lee, N.; Burian, A.; Pyo, C.-W.; Tykodi, S.S.; Warren, E.H.; Yee, C.; Riddell, S.R.; Geraghty, D.E. HLA-F and MHC-I Open Conformers Cooperate in a MHC-I Antigen Cross-Presentation Pathway. J. Immunol. 2013, 191, 1567–1577. [Google Scholar] [CrossRef] [Green Version]
- Adiko, A.C.; Ebabdor, J.; Martínez, E.E.; Guermonprez, P.; Esaveanu, L. Intracellular Transport Routes for MHC I and Their Relevance for Antigen Cross-Presentation. Front. Immunol. 2015, 6, 335. [Google Scholar] [CrossRef]
- Mahmutefendić, H.; Zagorac, G.B.; Grabušić, K.; Karleuša, L.; Maćešić, S.; Momburg, F.; Lučin, P. Late Endosomal Recycling of Open MHC-I Conformers. J. Cell. Physiol. 2016, 232, 872–887. [Google Scholar] [CrossRef]
- Lynch, S.; Santos, S.G.; Campbell, E.C.; Nimmo, A.; Botting, C.; Prescott, A.; Antoniou, A.N.; Powis, S.J. Novel MHC Class I Structures on Exosomes. J. Immunol. 2009, 183, 1884–1891. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, A.; Matko, J.; Rahman, N.A.; Barisas, B.G.; Edidin, M. Self-association of class I major histocompatibility com-plex molecules in liposome and cell surface membranes. Biochemistry 1991, 31, 7182–7289. [Google Scholar] [CrossRef]
- Capps, G.G.; Robinson, B.E.; Lewis, K.D.; Zúñiga, M.C. In vivo dimeric association of class I MHC heavy chains. Possible relationship to class I MHC heavy chain-beta 2-microglobulin dissociation. J. Immunol. 1993, 151, 159–169. [Google Scholar]
- Matko, J.; Bushkin, Y.; Wei, T.; Edidin, M. Clustering of class I HLA molecules on the surfaces of activated and trans-formed human cells. J. Immunol. 1994, 152, 3353–3360. [Google Scholar] [PubMed]
- Bodnár, A.; Bacsó, Z.; Jenei, A.; Jovin, T.M.; Edidin, M.; Damjanovich, S.; Matkó, J. Class I HLA oligomerization at the surface of B cells is controlled by exogenous beta(2)-microglobulin: Implications in activation of cytotoxic T lymphocytes. Int. Immunol. 2003, 15, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamosi, G.; Bodnar, A.; Vereb, G.; Jenei, A.; Goldman, C.K.; Langowski, J.; Tóth, K.; Matyus, L.; Szollosi, J.; Waldmann, T.A.; et al. IL-2 and IL-15 receptor alpha-subunits are coexpressed in a supramolecular receptor cluster in lipid rafts of T cells. Proc. Natl. Acad. Sci. USA 2004, 101, 11082–11087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, R.L.; O’Callaghan, C.A.; McMichael, A.J.; Bowness, P. Cutting edge: HLA-B27 can form a novel β2-microglobulin-free heavy chain homodimer structure. J. Immunol. 1999, 162, 5045–5048. [Google Scholar] [PubMed]
- Raine, T.; Brown, D.; Bowness, P.; Gaston, J.S.H.; Moffett, A.; Trowsdale, J.; Allen, R. Consistent patterns of expression of HLA class I free heavy chains in healthy individuals and raised expression in spondyloarthropathy patients point to physiological and pathological roles. Rheumatology 2006, 45, 1338–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruda, R.; Achdout, H.; Stern-Ginossar, N.; Gazit, R.; Betser-Cohen, G.; Manaster, I.; Katz, G.; Gonen-Gross, T.; Tirosh, B.; Mandelboim, O. Intracellular cysteine residues in the tail of MHC class I proteins are crucial for extracellular recognition by leuko-cyte Ig-like receptor 1. J. Immunol. 2007, 179, 3655–3661. [Google Scholar] [CrossRef] [Green Version]
- Baía, D.; Pou-Sánchez, J.; Jones, D.; Mandelboim, O.; Trowsdale, J.; Muntasell, A.; López-Botet, M. Interaction of the LILRB1 inhibitory receptor with HLA class Ia dimers. Eur. J. Immunol. 2016, 46, 1681–1690. [Google Scholar] [CrossRef]
- Boyson, J.E.; Erskine, R.; Whitman, M.; Chiu, M.; Lau, J.M.; Koopman, L.A.; Valter, M.M.; Angelisova, P.; Horejsí, V.; Strominger, J.L. Disulfide bond-mediated dimerization of HLA-G on the cell surface. Proc. Natl. Acad. Sci. USA 2002, 99, 16180–16185. [Google Scholar] [CrossRef] [Green Version]
- Goodridge, J.P.; Burian, A.; Lee, N.; Geraghty, D.E. HLA-F Complex without Peptide Binds to MHC Class I Protein in the Open Conformer Form. J. Immunol. 2010, 184, 6199–6208. [Google Scholar] [CrossRef]
- Cabrita, M.; Pereira, C.-F.; Rodrigues, P.; Cardoso, E.M.; Arosa, F.A. Altered expression of CD1d molecules and lipid accumulation in the human hepatoma cell line HepG2 after iron loading. FEBS J. 2004, 272, 152–165. [Google Scholar] [CrossRef]
- Kollnberger, S. The Role of HLA-Class I Heavy-Chain Interactions with Killer-Cell Immunoglobulin-Like Receptors in Immune Regulation. Crit. Rev. Immunol. 2016, 36, 269–282. [Google Scholar] [CrossRef]
- Hudson, L.E.; Allen, R.L. Leukocyte Ig-Like Receptors—A Model for MHC Class I Disease Associations. Front. Immunol. 2016, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Gonen-Gross, T.; Achdout, H.; Arnon, T.I.; Gazit, R.; Stern, N.; Hořejší, V.; Goldman-Wohl, D.; Yagel, S.; Mandelboim, O. The CD85J/Leukocyte Inhibitory Receptor-1 Distinguishes between Conformed and β2-Microglobulin-Free HLA-G Molecules. J. Immunol. 2005, 175, 4866–4874. [Google Scholar] [CrossRef] [Green Version]
- Shiroishi, M.; Kuroki, K.; Ose, T.; Rasubala, L.; Shiratori, I.; Arase, H.; Tsumoto, K.; Kumagai, I.; Kohda, D.; Maenaka, K. Efficient Leukocyte Ig-like Receptor Signaling and Crystal Structure of Disulfide-linked HLA-G Dimer. J. Biol. Chem. 2006, 281, 10439–10447. [Google Scholar] [CrossRef] [Green Version]
- Goodridge, J.P.; Burian, A.; Lee, N.; Geraghty, D.E. HLA-F and MHC Class I Open Conformers Are Ligands for NK Cell Ig-like Receptors. J. Immunol. 2013, 191, 3553–3562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burian, A.; Wang, K.L.; Finton, K.A.K.; Lee, N.; Ishitani, A.; Strong, R.K.; Geraghty, D.E. HLA-F and MHC-I Open Conformers Bind Natural Killer Cell Ig-Like Receptor KIR3DS1. PLoS ONE 2016, 11, e0163297. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Hölzemer, A.; Martrus, G.; Chung, A.; Pacheco, Y.; Simoneau, C.R.; Rucevic, M.; Lamothe-Molina, P.A.; Pertel, T.; Kim, T.-E.; et al. Open conformers of HLA-F are high-affinity ligands of the activating NK-cell receptor KIR3DS1. Nat. Immunol. 2016, 17, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, N.; Sayed, A.; Jeppesen, H.B.; Persson, G.; Weisdorf, I.; Funck, T.; Hviid, T.V.F. Characterization of HLA-G Regulation and HLA Expression in Breast Cancer and Malignant Melanoma Cell Lines upon IFN-γ Stimulation and Inhibition of DNA Methylation. Int. J. Mol. Sci. 2020, 21, 4307. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Cloutier, M.; Santharam, M.A.; Ramanathan, S.; Ilangumaran, S. The MHC Class-I Transactivator NLRC5: Implications to Cancer Immunology and Potential Applications to Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 1964. [Google Scholar] [CrossRef] [PubMed]
- Marozzi, A.; Meneveri, R.; Bunone, G.; De Santis, C.; Lopalco, L.; Beretta, A.; Agresti, A.; Siccardi, A.G.; Della Valle, G.; Ginelli, E. Expression of beta2m-Free HLA Class I Heavy Chains in Neuroblastoma Cell Lines. Scand. J. Immunol. 1993, 37, 661–667. [Google Scholar] [CrossRef]
- Giacomini, P.; Beretta, A.; Nicotra, M.; Ciccarelli, G.; Martayan, A.; Cerboni, C.; Lopalco, L.; Bini, D.; Delfino, L.; Ferrara, G.; et al. HLA-C heavy chains free of beta2-microglobulin: Distribution in normal tissues and neoplastic lesions of non-lymphoid origin and interferon-gamma responsiveness. Tissue Antigens 1997, 50, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, S.; Schenowitz, C.; Agaugué, S.; Favier, B.; Riteau, B.; Rouzier, R.; Carosella, E.D.; Rouas-Freiss, N.; Menier, C. HLA-G1 and HLA-G5 active dimers are present in malignant cells and effusions: The influence of the tumor microenvironment. Eur. J. Immunol. 2012, 42, 1599–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccone, E.; Pende, D.; Nanni, L.; Di Donato, C.; Viale, O.; Beretta, A.; Vitale, M.; Sivori, S.; Moretta, A.; Moretta, L. General role of HLA class I molecules in the protection of target cells from lysis by natural killer cells: Evidence that the free heavy chains of class I molecules are not sufficient to mediate the protective effect. Int. Immunol. 1995, 7, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Stuber, G.; Andrée, S.; Franksson, L.; Klein, E.; Beretta, A.; Siccardi, A.G.; Kärre, K. Reduced expression of major histocompatibility complex class I free heavy chains and enhanced sensitivity to natural killer cells after incubation of human lymphoid lines with beta2-microglobulin. Eur. J. Immunol. 1993, 23, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Edidin, M. Class I MHC molecules as probes of membrane patchiness: From biophysical measurements to modulation of immune responses. Immunol. Res. 2010, 47, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, J.; Altman, J.D.; Krishnakumar, S.; Raghavan, M. Empty conformers of HLA-B preferentially bind CD8 and regulate CD8+ T cell function. eLife 2018, 7, e36341. [Google Scholar] [CrossRef]
- Chvatchko, Y.; Van Obberghen, E.; Kiger, N.; Fehlmann, M. Immunoprecipitation of insulin receptors by antibodies against Class 1 antigens of the murine H-2 major histocompatibility complex. FEBS Lett. 1983, 163, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Fehlmann, M.; Peyron, J.F.; Samson, M.; Van Obberghen, E.; Brandenburg, D.; Brossette, N. Molecular association between major histocompatibility complex class I antigens and insulin receptors in mouse liver membranes. Proc. Natl. Acad. Sci. USA 1985, 82, 8634–8637. [Google Scholar] [CrossRef] [Green Version]
- Due, C.; Simonsen, M.; Olsson, L. The major histocompatibility complex class I heavy chain as a structural subunit of the human cell membrane insulin receptor: Implications for the range of biological functions of histocompatibility antigens. Proc. Natl. Acad. Sci. USA 1986, 83, 6007–6011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, M.; Cousin, J.L.; Fehlmann, M. Cross-linking of insulin receptors to MHC antigens in human B lymphocytes: Evi-dence for selective molecular interactions. J. Immunol. 1986, 137, 2293–2298. [Google Scholar]
- Phillips, M.L.; Moule, M.L.; Delovitch, T.L.; Yip, C.C. Class I histocompatibility antigens and insulin receptors: Evidence for interactions. Proc. Natl. Acad. Sci. USA 1986, 83, 3474–3478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittur, D.; Shimizu, Y.; DeMars, R.; Edidin, M. Insulin binding to human B lymphoblasts is a function of HLA haplotype. Proc. Natl. Acad. Sci. USA 1987, 84, 1351–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, A.B.; Schlessinger, J.; Edidin, M. Interaction between major histocompatibility complex antigens and epidermal growth factor receptors on human cells. J. Cell Biol. 1984, 98, 725–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solano, Á.R.; Sánchez, M.L.; Sardañons, M.L.; Dada, L.; Podestá, E.J. Luteinizing Hormone Triggers a Molecular Association between Its Receptor and the Major Histocompatibility Complex Class I Antigen to Produce Cell Activation. Endocrinology 1988, 122, 2080–2083. [Google Scholar] [CrossRef]
- Solano, A.R.; Cremaschi, G.; Sánchez, M.L.; Borda, E.; Sterin-Borda, L.; Podestá, E.J. Molecular and biological interaction be-tween major histocompatibility complex class I antigens and luteinizing hormone receptors or beta-adrenergic receptors trig-gers cellular response in mice. Proc. Natl. Acad. Sci. USA 1988, 85, 5087–5091. [Google Scholar] [CrossRef] [Green Version]
- Cremaschi, G.; Borda, E.; Sales, M.; Genaro, A.; Sterin-Borda, L. Major histocompatibility complex modulation of beta-adrenoceptor function. Biochem. Pharmacol. 1990, 39, 1861–1868. [Google Scholar] [CrossRef]
- Mommaas, A.M.; Wijsman, M.C.; Verduijn, W.; Vermeer, B.J.; Claas, F.M.J. Internalization of MHC class I molecules is a prerequisite for endocytosis of endorphin by lymphocytes. Clin. Exp. Immunol. 2008, 84, 170–174. [Google Scholar] [CrossRef]
- Sharon, M.; Gnarra, J.R.; Baniyash, M.; Leonard, W.J. Possible association between IL-2 receptors and class I HLA molecules on T cells. J. Immunol. 1988, 141, 3512–3515. [Google Scholar]
- Harel-Bellan, A.; Krief, P.; Rimsky, L.; Farrar, W.L.; Mishal, Z. Flow cytometry resonance energy transfer suggests an associ-ation between low-affinity interleukin 2 binding sites and HLA class I molecules. Biochem J. 1990, 268, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Mocsár, G.; Volkó, J.; Rönnlund, D.; Widengren, J.; Nagy, P.; Szöllősi, J.; Tóth, K.; Goldman, C.K.; Damjanovich, S.; Waldmann, T.A.; et al. MHC I Expression Regulates Co-clustering and Mobility of Interleukin-2 and -15 Receptors in T Cells. Biophys. J. 2016, 111, 100–112. [Google Scholar] [CrossRef]
- Mátyus, L.; Bene, L.; Heiligen, H.; Rausch, J.; Damjanovich, S. Distinct association of transferrin receptor with HLA class I molecules on HUT-102B and JY cells. Immunol. Lett. 1995, 44, 203–208. [Google Scholar] [CrossRef]
- Bushkin, Y.; Demaria, S.; Le, J.M.; Schwab, R. Physical association between the CD8 and HLA class I molecules on the sur-face of activated human T lymphocytes. Proc. Natl. Acad. Sci. USA 1988, 85, 3985–3989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auphan, N.; Boyer, C.; Andre, P.; Bongrand, P.; Schmitt-Verhulst, A.-M. Biochemical and functional association between CD8 and H-2 at the surface of a T cell clone. Mol. Immunol. 1991, 28, 827–837. [Google Scholar] [CrossRef]
- Lagaudriere-Gesbert, C.; Lebel-Binay, S.; Wiertz, E.; Ploegh, H.L.; Fradelizi, D.; Conjeaud, H. The tetraspanin protein CD82 associates with both free HLA class I heavy chain and heterodimeric beta 2-microglobulin complexes. J. Immunol. 1997, 158, 2790–2797. [Google Scholar] [PubMed]
- Held, W.; Mariuzza, R.A. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat. Rev. Immunol. 2008, 8, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Doucey, M.-A.; Scarpellino, L.; Zimmer, J.; Guillaume, P.; Luescher, I.F.; Bron, C.; Held, W. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat. Immunol. 2004, 5, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Nakamura, A.; Maeda, T.; Sakamoto, Y.; Takai, T. Cis binding between inhibitory receptors and MHC class I can regulate mast cell activation. J. Exp. Med. 2007, 204, 907–920. [Google Scholar] [CrossRef]
- Mori, Y.; Tsuji, S.; Inui, M.; Sakamoto, Y.; Endo, S.; Ito, Y.; Fujimura, S.; Koga, T.; Nakamura, A.; Takayanagi, H.; et al. Inhibitory Immunoglobulin-Like Receptors LILRB and PIR-B Negatively Regulate Osteoclast Development. J. Immunol. 2008, 181, 4742–4751. [Google Scholar] [CrossRef] [Green Version]
- Reiland, J.; Edidin, M. Chemical cross-linking detects association of insulin receptors with four different class I human leukocyte antigen molecules on cell surfaces. Diabetes 1993, 42, 619–625. [Google Scholar] [CrossRef]
- Zhang, X.; Rozengurt, E.; Reed, E.F. HLA Class I Molecules Partner with Integrin beta4 to Stimulate Endothelial Cell Proliferation and Migration. Sci. Signal. 2010, 3, ra85. [Google Scholar] [CrossRef] [Green Version]
- Donner, D.B.; Yonkers, K. Hormone-induced conformational changes in the hepatic insulin receptor. J. Biol. Chem. 1983, 258, 9413–9418. [Google Scholar] [CrossRef]
- Liegler, T.; Szollosi, J.; Hyun, W.; Goodenow, R.S. Proximity measurements between H-2 antigens and the insulin receptor by fluorescence energy transfer: Evidence that a close association does not influence insulin binding. Proc. Natl. Acad. Sci. USA 1991, 88, 6755–6759. [Google Scholar] [CrossRef] [Green Version]
- Berhanu, P.; Saunders, D.J.; Brandenburg, D. Adipocyte insulin receptor. Generation of a cryptic domain of the alpha-subunit during internalization of hormone-receptor complexes. Biochem. J. 1987, 242, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florke, R.-R.; Schnaith, K.; Passlack, W.; Wichert, M.; Kuehn, L.; Fabry, M.; Federwisch, M.; Reinauer, H. Hormone-triggered conformational changes within the insulin-receptor ectodomain: Requirement for transmembrane anchors. Biochem. J. 2001, 360, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Baron, V.; Kaliman, P.; Gautier, N.; Van Obberghen, E. The insulin receptor activation process involves localized confor-mational changes. J. Biol. Chem. 1992, 267, 23290–23294. [Google Scholar] [CrossRef]
- Lee, J.; Pilch, P.F.; Shoelson, S.E.; Scarlata, S.F. Conformational Changes of the Insulin Receptor upon Insulin Binding and Activation as Monitored by Fluorescence Spectroscopy. Biochemistry 1997, 36, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. Structural Dynamics of Insulin Receptor and Transmembrane Signaling. Biochemistry 2015, 54, 5523–5532. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, T.; Schäfer, I.B.; Poojari, C.; Brankatschk, B.; Vattulainen, I.; Strauss, M.; Coskun, Ü. Cryo-EM structure of the complete and ligand-saturated insulin receptor ectodomain. J. Cell Biol. 2020, 219, e201907210. [Google Scholar] [CrossRef] [Green Version]
- Fishman, D.; Elhyany, S.; Segal, S. Non-immune functions of MHC class I glycoproteins in normal and malignant cells. Folia Biol. (Praha) 2004, 50, 35–42. [Google Scholar]
- Wang, Z.; Margulies, L.; Hicklin, D.J.; Ferrone, S. Molecular and functional phenotypes of melanoma cells with abnormalities in HLA Class I antigen expression. Tissue Antigens 1996, 47, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Arienti, F.; Parmiani, G.; Ferrone, S. Induction and functional characterization of beta2-microglobulin (beta2-mu)-free HLA class I heavy chains expressed by beta2-mu-deficient human FO-1 melanoma cells. Eur. J. Immunol. 1998, 28, 2817–2826. [Google Scholar] [CrossRef]
- Ramnath, N.; Tan, D.; Li, Q.; Hylander, B.L.; Bogner, P.; Ryes, L.; Ferrone, S. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunol. Immunother. 2005, 55, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Paco, L.; Romero, I.; Berruguilla, E.; Stefansky, J.; Collado, A.; Algarra, I.; Garrido, F.; Garcia-Lora, A.M. MHC class I molecules act as tumor suppressor genes regulating the cell cycle gene expression, invasion and intrinsic tumorigenicity of melanoma cells. Carcinogenesis 2012, 33, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagsted, J.; Reaven, G.M.; Hansen, T.; Goldstein, A.; Olsson, L. Regulation of insulin receptor functions by a peptide derived from a major histocompatibility complex class I antigen. Cell 1990, 62, 297–307. [Google Scholar] [CrossRef]
- Stagsted, J.; Baase, W.A.; Goldstein, A.; Olsson, L. A preformed, ordered structure of a 25-residue peptide derived from a major histocompatibility complex class I antigen is required to affect insulin receptor function. J. Biol. Chem. 1991, 266, 12844–12847. [Google Scholar] [CrossRef]
- Stagsted, J.; Olsson, L.; Holman, G.D.; Cushman, S.W.; Satoh, S. Inhibition of internalization of glucose transporters and IGF-II receptors. Mechanism of action of MHC class I-derived peptides which augment the insulin response in rat adipose cells. J. Biol. Chem. 1993, 268, 22809–22813. [Google Scholar] [CrossRef]
- Naranda, T.; Goldstein, A.; Olsson, L. A peptide derived from an extracellular domain selectively inhibits receptor internalization: Target sequences on insulin and insulin-like growth factor 1 receptors. Proc. Natl. Acad. Sci. USA 1997, 94, 11692–11697. [Google Scholar] [CrossRef] [Green Version]
- Stagsted, J. Journey beyond immunology. Regulation of receptor internalization by major histocompatibility complex class I (MHC-I) and effect of peptides derived from MHC-I. APMIS. Suppl. 1998, 85, 1–40. [Google Scholar] [CrossRef]
- Peppicelli, S.; Ruzzolini, J.; Andreucci, E.; Bianchini, F.; Kontos, F.; Yamada, T.; Ferrone, S.; Calorini, L. Potential Role of HLA Class I Antigens in the Glycolytic Metabolism and Motility of Melanoma Cells. Cancers 2019, 11, 1249. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.-L.; Chen, C.-M.; Cline, H.T. Insulin Receptor Signaling Regulates Synapse Number, Dendritic Plasticity, and Circuit Function In Vivo. Neuron 2008, 58, 708–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon-Salazar, T.J.; Fourgeaud, L.; Tyler, C.M.; Poole, J.R.; Park, J.J.; Boulanger, L.M. MHC class I limits hippocampal synapse density by inhibiting neuronal insulin receptor signaling. J. Neurosci. 2014, 34, 11844–11856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rood, J.J.; van Leeuwen, A.; van Santen, M. Anti HL-A2 inhibitor in normal human serum. Nature 1970, 226, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Charlton, R.K.; Zmijewski, C.M. Soluble HL-A7 Antigen: Localization in the beta-Lipoprotein Fraction of Human Serum. Science 1970, 170, 636–637. [Google Scholar] [CrossRef]
- Allison, J.P.; Pellegrino, M.A.; Ferrone, S.; Callahan, G.N.; Reisfeld, R.A. Biologic and chemical characterization of HLA anti-gens in human serum. J. Immunol. 1977, 118, 1004–1009. [Google Scholar] [PubMed]
- Krangel, M.S. Secretion of HLA-A and -B antigens via an alternative RNA splicing pathway. J. Exp. Med. 1986, 163, 1173–1190. [Google Scholar] [CrossRef]
- Haga, J.A.; She, J.X.; Kao, K.J. Biochemical characterization of 39-kDa class I histocompatibility antigen in plasma. A secretable membrane protein derived from transmembrane domain deletion. J. Biol. Chem. 1991, 266, 3695–3701. [Google Scholar] [CrossRef]
- Dobbe, L.M.E.; Stam, N.J.; Neefjes, J.; Giphart, M.J. Biochemical complexity of serum HLA class I molecules. Immunogenetics 1988, 27, 203–210. [Google Scholar] [CrossRef]
- Tabayoyong, W.B.; Zavazava, N. Soluble HLA revisited. Leuk. Res. 2007, 31, 121–125. [Google Scholar] [CrossRef]
- DeMaria, S.; Schwab, R.; Gottesman, S.R.; Bushkin, Y. Soluble beta 2-microglobulin-free class I heavy chains are released from the surface of activated and leukemia cells by a metalloprotease. J. Biol. Chem. 1994, 269, 6689–6694. [Google Scholar] [CrossRef]
- Dong, Y.; Lieskovská, J.; Kedrin, D.; Porcelli, S.; Mandelboim, O.; Bushkin, Y. Soluble nonclassical HLA generated by the metalloproteinase pathway. Hum. Immunol. 2003, 64, 802–810. [Google Scholar] [CrossRef]
- Pickl, W.F.; Majdic, O.; Faé, I.; Reuschel, R.; Holter, W.; Knapp, W. The soluble pool of b2-microglobulin free HLA class I al-pha-chains. Qualitative and quantitative characterization. J. Immunol. 1993, 151, 2613–2622. [Google Scholar]
- Puppo, F.; Bignardi, D.; Contini, P.; Hamby, C.; Brenci, S.; Lanza, L.; Ghio, M.; Scudeletti, M.; Indiveri, F.; Ferrone, S. Beta2-micro-free HLA class I heavy chain levels in sera of healthy individuals. Lack of association with Beta2-micro-associated HLA class I heavy chain levels and HLA phenotype. Tissue Antigens 1999, 53, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Doxiadis, I.; Westhoff, U.; Grosse-Wilde, H. Quantification of soluble HLA class I gene products by an enzyme linked immunosorbent assay. Blut 1989, 59, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Perosa, F.; Prete, M.; Luccarelli, G.; Favoino, B.; Ferrone, S.; Dammacco, F. Serum levels of beta-2-microglobulin-free heavy chain of HLA class I antigen in healthy individuals: Relationship to their class I allotype. Hum. Immunol. 1999, 60, 1058–1066. [Google Scholar] [CrossRef]
- Buelow, R.; Burlingham, W.J.; Clayberger, C. Immunomodulation by soluble HLA CLASS I. Transplantation 1995, 59, 649–654. [Google Scholar] [CrossRef]
- Zavazava, N. Soluble HLA class I molecules: Biological significance and clinical implications. Mol. Med. Today 1998, 4, 116–121. [Google Scholar] [CrossRef]
- Bakela, K.; Athanassakis, I. Soluble major histocompatibility complex molecules in immune regulation: Highlighting class II antigens. Immunology 2018, 153, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Cermeño, J.C.; Casado, C.; Villar, L.M.; Ferreira, A.; Varela, J.M.; Dominguez, M.; Bootello, A.; Najera, R.; Gonzalez-Porque, P. Soluble class 1 antigens (sHLA) in CSF and serum of patients with HIV infection. Acta Neurol. Scand. 1990, 82, 14–16. [Google Scholar] [CrossRef]
- Puppo, F.; Brenci, S.; Lanza, L.; Bosco, O.; Imro, M.A.; Scudeletti, M.; Indiveri, F.; Ferrone, S. Increased level of serum HLA class I antigens in HIV infection correlation with disease progression. Hum. Immunol. 1994, 40, 259–266. [Google Scholar] [CrossRef]
- Adamashvili, I.M.; McDonald, J.C.; Fraser, P.A.; Milford, E.L.; Pressly, T.A.; Gelder, F.B. Soluble class I HLA antigens in patients with rheumatoid arthritis and their families. J. Rheumatol. 1995, 22, 1025–1031. [Google Scholar]
- Filaci, G.; Contini, P.; Brenci, S.; Gazzola, P.; Lanza, L.; Scudeletti, M.; Indiveri, F.; Mancardi, G.L.; Puppo, F. Soluble HLA Class I and Class II Molecule Levels in Serum and Cerebrospinal Fluid of Multiple Sclerosis Patients. Hum. Immunol. 1997, 54, 54–62. [Google Scholar] [CrossRef]
- Brescianl, A.; Pirozzi, G.; Spera, M.; Lombardi, M.; Ambrosone, L.; Migliaresi, S.; Ferrone, S.; Manzo, C. Increased level of serum HLA class I antigens in patients with systemic lupus in patients with systemic lupus erythematosus. Correlation with disease activity. Tissue Antigens 1998, 52, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Nocito, M.; Montalbán, C.; González-Porque, P.; Villar, L.M. Increased soluble serum HLA class I antigens in patients with lymphoma. Hum. Immunol. 1997, 58, 106–111. [Google Scholar] [CrossRef]
- Shimura, T.; Tsutsumi, S.; Hosouchi, Y.; Kojima, T.; Kon, Y.; Yonezu, M.; Kuwano, H. Clinical significance of soluble form of HLA class I molecule in Japanese patients with pancreatic cancer. Hum. Immunol. 2001, 62, 615–619. [Google Scholar] [CrossRef]
- Bangia, N.; Ferrone, S. Antigen Presentation Machinery (APM) Modulation and Soluble HLA Molecules in the Tumor Microenvironment: Do They Provide Tumor Cells with Escape Mechanisms from Recognition by Cytotoxic T Lymphocytes? Immunol. Investig. 2006, 35, 485–503. [Google Scholar] [CrossRef]
- Albitar, M.; Johnson, M.; Do, K.A.; Day, A.; Jilani, I.; Pierce, S.; Estey, E.; Kantarjian, H.; Keating, M.; Verstovsek, S.; et al. Levels of soluble HLA-I and beta2M in patients with acute myeloid leukemia and advanced myelodysplastic syn-drome: Association with clinical behavior and outcome of induction therapy. Leukemia 2007, 21, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Zavazava, N.; Böttcher, H.; Ruchholtz, W.M. Soluble MHC class I antigens (sHLA) and anti-HLA antibodies in heart and kidney allograft recipients. Tissue Antigens 1993, 42, 20–26. [Google Scholar] [CrossRef]
- Mathew, J.M.; Shenoy, S.; Phelan, D.; Lowell, J.; Howard, T.; Mohanakumar, T. Biochemical and immunological evaluation of donor-specific soluble hla in the circulation of liver transplant recipients. Transplantation 1996, 62, 217–223. [Google Scholar] [CrossRef]
- Ghio, M.; Contini, P.; Mazzei, C.; Brenci, S.; Barberis, G.; Filaci, G.; Indiveri, F.; Puppo, F. Soluble HLA class I, HLA class II, and Fas ligand in blood components: A possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood 1999, 93, 1770–1777. [Google Scholar] [CrossRef]
- Ghio, M.; Contini, P.; Ubezio, G.; Mazzei, C.; Puppo, F.; Indiveri, F. Immunomodulatory effects of blood transfusions: The synergic role of soluble HLA Class I free heavy-chain molecules detectable in blood components. Transfusion 2008, 48, 1591–1597. [Google Scholar] [CrossRef]
- Le Morvan, C.; Cogne, M.; Drouet, M. An elevation in the concentration of HLA class I molecules in human blood due to ageing. Mechan Ageing Devel. 2001, 122, 335–340. [Google Scholar] [CrossRef]
- Brieva, J.A.; Villar, L.M.; Leoro, G.; Alvarez-Cermeño, J.C.; Roldan, E.; Gonzalez-Porqué, P. Soluble HLA class I antigen secretion by normal lymphocytes: Relationship with cell activation and effect of interferon-gamma. Clin. Exp. Immunol. 2008, 82, 390–395. [Google Scholar] [CrossRef]
- Aulitzky, W.E.; Grosse-Wilde, H.; Westhoff, U.; Tilg, H.; Gastl, G.; Herold, M.; Huber, C.; Aulitzky, W. Enhanced serum levels of soluble HLA class I molecules are induced by treatment with recombinant interferon-gamma (IFN-gamma). Clin. Exp. Immunol. 1991, 86, 236–239. [Google Scholar] [CrossRef]
- Le, J.; Hua, J.-C. Production of soluble HLA-class-I molecules by IFN-gamma-induced colon-adenocarcinoma cells. Int. J. Cancer 1995, 60, 576–581. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Le, J. Alternative Splicing of HLA Class I Transcripts Induced by IFN-gamma and TNF in Fibroblasts: Release of Soluble HLA Class I Heavy Chain and an Associate Protein. Cell. Immunol. 1995, 162, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Contini, P.; Carosio, R.; Arvigo, M.; Ghio, M.; Oddone, D.; Dondero, A.; Zocchi, M.R.; Puppo, F.; Indiveri, F.; et al. Soluble HLA class I molecules induce natural killer cell apoptosis through the engagement of CD8: Evidence for a neg-ative regulation exerted by CD94/NKG2A complex and KIR2D. Blood 2002, 99, 1706–1714. [Google Scholar] [CrossRef] [Green Version]
- Spaggiari, G.M.; Contini, P.; Dondero, A.; Carosio, R.; Puppo, F.; Indiveri, F.; Zocchi, M.R.; Poggi, A. Soluble HLA class I in-duces NK cell apoptosis upon the engagement of killer activating HLA class I receptors through FasL/Fas interaction. Blood 2002, 100, 4098–4107. [Google Scholar] [CrossRef] [PubMed]
- Contini, P.; Ghio, M.; Poggi, A.; Filaci, G.; Induveri, F.; Ferrone, S.; Puppo, F. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur. J. Immunol. 2003, 33, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Puppo, F.; Contini, P.; Ghio, M.; Brenci, S.; Scudeletti, M.; Filaci, G.; Ferrone, S.; Indiveri, F. Soluble human MHC class I mole-cules induce soluble Fas ligand secretion and trigger apoptosis in activated CD8+Fas(CD95)+ T lymphocytes. Int. Immunol. 2000, 12, 195–203. [Google Scholar] [CrossRef]
- Contini, P.; Ghio, M.; Merlo, A.; Poggi, A.; Indiveri, F.; Puppo, F. Apoptosis of antigen-specific T lymphocytes upon the en-gagement of CD8 by Soluble HLA Class I Molecules is FasLigand/Fas mediated: Evidence for the involvement of p56lck, cal-cium calmodulin kinase II, and calcium-independent protein kinase C signaling pathways and for NF-κB and NF-AT nuclear translocation. J. Immunol. 2005, 175, 7244–7254. [Google Scholar]
- Ghio, M.; Contini, P.; Negrini, S.; Boero, S.; Musso, A.; Poggi, A. Soluble HLA-I-mediated secretion of TGF-beta1 by human NK cells and consequent down-regulation of anti-tumor cytolytic activity. Eur. J. Immunol. 2009, 39, 3459–3468. [Google Scholar] [CrossRef]
- Campoli, M.; Ferrone, S. Tumor escape mechanisms: Potential role of soluble HLA antigens and NK cells activating ligands. Tissue Antigens 2008, 72, 321–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Guo, Y.; Yan, Z.; Zhang, J.; Bushkin, Y.; Liang, P. Soluble MHC I and Soluble MIC Molecules: Potential Therapeutic Targets for Cancer. Int. Rev. Immunol. 2011, 30, 35–43. [Google Scholar] [CrossRef]
- Ghio, M.; Contini, P.; Negrini, S.; Mazzei, C.; Zocchi, M.R.; Poggi, A. Down regulation of human natural killer cell-mediated cytolysis induced by blood transfusion: Role of transforming growth factor-β1, soluble Fas ligand, and soluble Class I human leukocyte antigen. Transfusion 2011, 51, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Ghio, M.; Contini, P. A possible role of soluble HLA-I molecule in the immunomodulatory effects of therapeutic apheresis. Blood Transfus. 2014, 12, s167–s169. [Google Scholar]
- Krensky, A.M.; Clayberger, C. Structure of HLA Molecules and Immunosuppressive Effects of HLA Derived Peptides. Int. Rev. Immunol. 1996, 13, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Kaveri, S.; Vassilev, T.; Hurez, V.; Lengagne, R.; Lefranc, C.; Cot, S.; Pouletty, P.; Glotz, D.; Kazatchkine, M.D. Antibodies to a conserved region of HLA class I molecules, capable of modulating CD8 T cell-mediated function, are present in pooled nor-mal immunoglobulin for therapeutic use. J. Clin. Investig. 1996, 97, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Ephrem, A.; Misra, N.; Hassan, G.; Dasgupta, S.; Delignat, S.; Duong Van Huyen, J.P.; Chamat, S.; Prost, F.; Lacroix-Desmazes, S.; Ka-very, S.V.; et al. Immunomodulation of autoimmune and inflammatory diseases with intravenous immu-noglobulin. Clin. Exp. Med. 2005, 5, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, E.W. Intravenous Immune Globulin in Autoimmune and Inflammatory Diseases. N. Engl. J. Med. 2012, 367, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.; Terasaki, P.I.; Pham, T.; Jucaud, V.; Kawakita, S. Suppression of blastogenesis and proliferation of activated CD4+T cells: Intravenous immunoglobulin (IVIg)versusnovel anti-human leucocyte antigen (HLA)-E monoclonal antibodies mimicking HLA-I reactivity of IVIg. Clin. Exp. Immunol. 2014, 178, 154–177. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H. HLA Class Ia and Ib Polyreactive Anti-HLA-E IgG2a Monoclonal Antibodies (TFL-006 and TFL-007) Suppress Anti-HLA IgG Production by CD19+ B Cells and Proliferation of CD4+ T Cells While Upregulating Tregs. J. Immunol. Res. 2017, 2017, 3475926. [Google Scholar] [CrossRef] [Green Version]
- Ravindranath, M.; Hilali, F.; Filippone, E. Therapeutic Potential of HLA-I Polyreactive mAbs Mimicking the HLA-I Polyreactivity and Immunoregulatory Functions of IVIg. Vaccines 2021, 9, 680. [Google Scholar] [CrossRef]
- Cai, J.; Terasaki, P.I.; Anderson, N.; Lachmann, N.; Schönemann, C. Intact HLA not beta2m-free heavy chain-specific HLA class I antibodies are predictive of graft failure. Transplantation 2009, 88, 226–230. [Google Scholar] [CrossRef]
- Visentin, J.; Bachelet, T.; Aubert, O.; Del Bello, A.; Martinez, C.; Jambon, F.; Guidicelli, L.; Ralazamahaleo, M.; Bouthemy, C.; Cargou, M.; et al. Reassessment of the clinical impact of preformed donor-specific anti-HLA-Cw antibodies in kidney transplantation. Am. J. Transplant. 2019, 20, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Muntjewerff, E.M.; Meesters, L.D.; Bogaart, G.V.D.; Revelo, N.H. Reverse Signaling by MHC-I Molecules in Immune and Non-Immune Cell Types. Front. Immunol. 2020, 11, 605958. [Google Scholar] [CrossRef]
- Lebedeva, T.; Anikeeva, N.; Kalams, S.A.; Walker, B.D.; Gaidarov, I.; Keen, J.H.; Sykulev, Y. Major histocompatibility complex class I–intercellular adhesion molecule-1 association on the surface of target cells: Implications for antigen presentation to cytotoxic T lymphocytes. Immunology 2004, 113, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, X.; Bao, Y.; Zhu, X.; Han, C.; Zhang, P.; Zhang, X.; Li, W.; Cao, X. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps–SHP-2 pathway. Nat. Immunol. 2012, 13, 551–559. [Google Scholar] [CrossRef]
- Xia, S.; Tao, Y.; Cui, L.; Yu, Y.; Xu, S. MHC Class I Molecules Exacerbate Viral Infection by Disrupting Type I Interferon Signaling. J. Immunol. Res. 2019, 2019, 5370706. [Google Scholar] [CrossRef]
- Corriveau, R.A.; Huh, G.S.; Shatz, C.J. Regulation of Class I MHC Gene Expression in the Developing and Mature CNS by Neural Activity. Neuron 1998, 21, 505–520. [Google Scholar] [CrossRef] [Green Version]
- Huh, G.S.; Boulanger, L.M.; Du, H.; Riquelme, P.A.; Brotz, T.M.; Shatz, C.J. Functional Requirement for Class I MHC in CNS Development and Plasticity. Science 2000, 290, 2155–2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulanger, L.M.; Shatz, C.J. Immune signalling in neural development, synaptic plasticity and disease. Nat. Rev. Neurosci. 2004, 5, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Shatz, C.J. MHC Class I: An Unexpected Role in Neuronal Plasticity. Neuron 2009, 64, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulanger, L.M. Immune Proteins in Brain Development and Synaptic Plasticity. Neuron 2009, 64, 93–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmer, B.M.; McAllister, A.K. Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 2012, 35, 660–670. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Yu, H.; He, Y.; Shen, Y.; Zhang, Y.; Liu, J.; Fu, B.; Lv, D.; Miao, F.; Zhang, J. Developmental expression and localization of MHC class I molecules in the human central nervous system. Exp. Brain Res. 2015, 233, 2733–2743. [Google Scholar] [CrossRef]
- Washburn, L.R.; Zekzer, D.; Eitan, S.; Lu, Y.; Dang, H.; Middleton, B.; Evans, C.J.; Tian, J.; Kaufman, D.L. A Potential Role for Shed Soluble Major Histocompatibility Class I Molecules as Modulators of Neurite Outgrowth. PLoS ONE 2011, 6, e18439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilousova, T.; Dang, H.; Xu, W.; Gustafson, S.; Jin, Y.; Wickramasinghe, L.; Won, T.; Bobarnac, G.; Middleton, B.; Tian, J.; et al. Major histocompatibility complex class I molecules modulate embryonic neuritogenesis and neuronal polarization. J. Neuroimmunol. 2012, 247, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Atwal, J.K.; Pinkston-Gosse, J.; Syken, J.; Stawicki, S.; Wu, Y.; Shatz, C.; Tessier-Lavigne, M. PirB is a Functional Receptor for Myelin Inhibitors of Axonal Regeneration. Science 2008, 322, 967–970. [Google Scholar] [CrossRef]
- Kim, T.; Vidal, G.; Djurisic, M.; William, C.; Birnbaum, M.; Garcia, K.C.; Hyman, B.T.; Shatz, C.J. Human LilrB2 Is a β-Amyloid Receptor and Its Murine Homolog PirB Regulates Synaptic Plasticity in an Alzheimer’s Model. Science 2013, 341, 1399–1404. [Google Scholar] [CrossRef] [Green Version]
- Frietze, K.K.; Pappy, A.L., 2nd; Melson, J.W.; O’Driscoll, E.E.; Tyler, C.M.; Perlman, D.H.; Boulanger, L.M. Cryptic protein-protein interaction motifs in the cytoplasmic domain of MHCI proteins. BMC Immunol. 2016, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Sheng, M. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 2004, 5, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Good, M.C.; Zalatan, J.G.; Lim, W.A. Scaffold proteins: Hubs for controlling the flow of cellular information. Science 2011, 332, 680–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenzweig, S.A. The Continuing Evolution of Insulin-Like Growth Factor Signaling. F1000Research 2020, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.R.; Sweeten, T.L.; Cutler, A.; Bedke, B.J.; Fillmore, M.; Stubbs, E.G.; Odell, D. The association and linkage of the HLA-A2 class I allele with autism. Hum. Immunol. 2006, 67, 346–351. [Google Scholar] [CrossRef]
- Needleman, L.A.; McAllister, A.K. The major histocompatibility complex and autism spectrum disorder. Dev. Neurobiol. 2012, 72, 1288–1301. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat. Rev. Neurosci. 2015, 16, 469–486. [Google Scholar] [CrossRef] [Green Version]
- McAllister, A.K. Major histocompatibility complex I in brain development and schizophrenia. Biol. Psychiatry 2014, 75, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Cebrián, C.; Loike, J.D.; Sulzer, D. Neuronal MHC-I expression and its implications in synaptic function, axonal regeneration and Parkinson’s and other brain diseases. Front. Neuroanat. 2014, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Esgalhado, A.J.; Reste-Ferreira, D.; Albino, S.E.; Sousa, A.; Amaral, A.P.; Martinho, A.; Oliveira, I.T.; Verde, I.; Lourenço, O.; Fonseca, A.M.; et al. CD45RA, CD8β, and IFNγ Are Potential Immune Biomarkers of Human Cognitive Function. Front. Immunol. 2020, 11, 592656. [Google Scholar] [CrossRef] [PubMed]
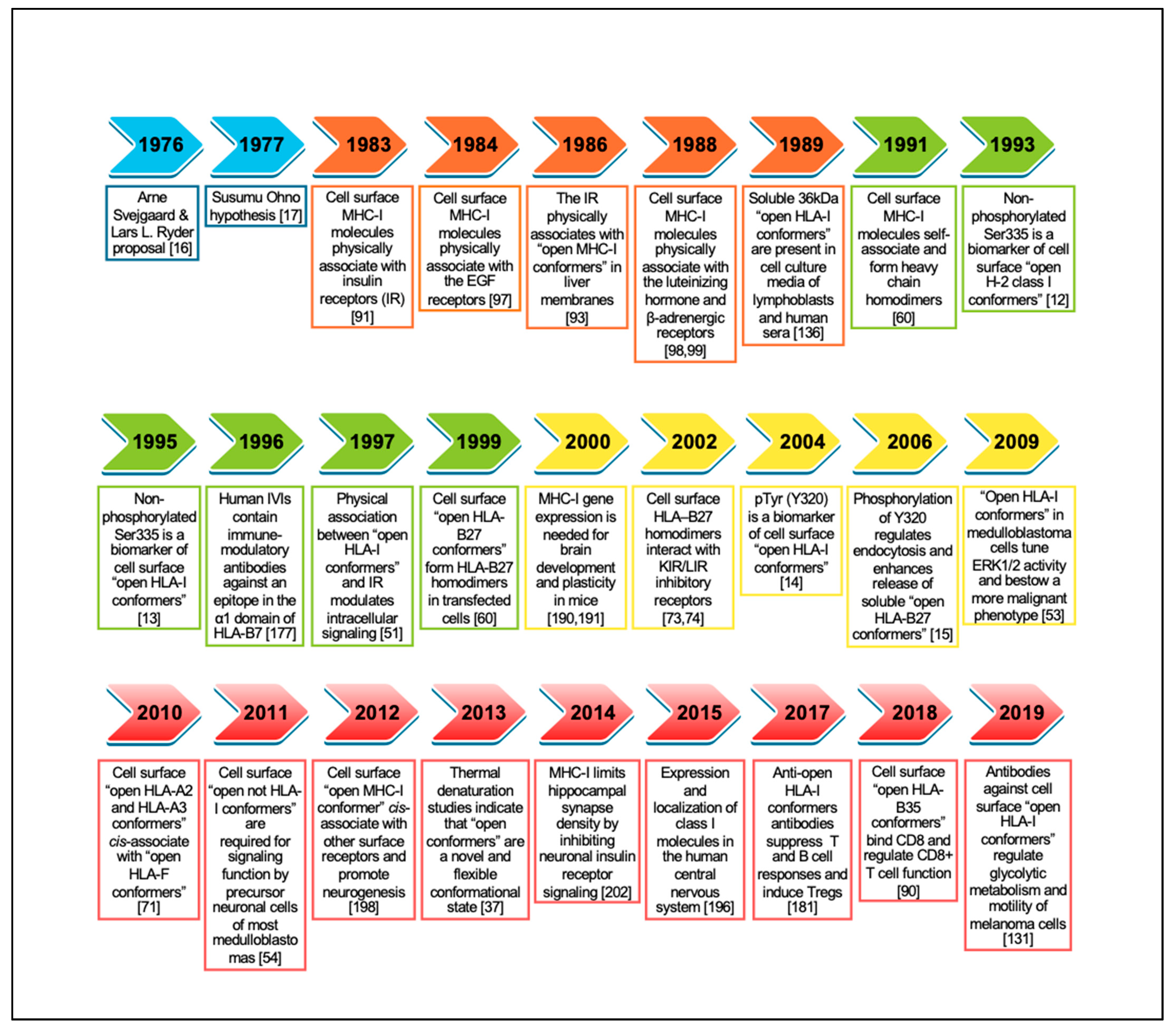

| Year | Main Findings | Involved Alleles | Ref. |
|---|---|---|---|
| 1983 | Physical association between H-2 class I molecules and insulin receptors in mouse liver membranes | H2-Kb, H-2Db | [90] |
| 1984 | Physical association between HLA-I molecules and epidermal growth factor (EGF) receptors in cancer cells and fibroblasts | Not determined | [96] |
| 1988 | Physical association between HLA-I molecules and CD8 receptors on activated normal human T cells | Not determined | [105] |
| 1988 | Binding of luteinizing hormone to its receptor triggers an association with H-2 class I molecules in Leydig cells | H-2Dd, H-2Dk, H-2Kd, H-2Kk | [97,98] |
| 1988/1990 | Physical association between HLA-I molecules and the IL-2R α and β chains in normal and transformed T cells | Not determined | [101,102] |
| 1990 | Functional interaction between H-2 class I molecules and β-adrenoceptors in cardiac membrane preparations | H-2Dk, H-2Kk | [99] |
| 1991 | Interactions between HLA-I molecules and γ-endorphin receptors on activated T cells and transformed B cells | Not determined | [100] |
| 1991 | Physical association between H-2 class I molecules and CD8αβ receptors on T cells | H-2Kk | [106] |
| 1993 | Physical association association of insulin receptors with four different class I human leukocyte antigen molecules on cell surfaces | HLA-A1, HLA-A2, HLA-B5, and HLA-B8 | [112] |
| 1995 | Physical association between HLA-I molecules and the transferrin receptor in B lymphoblastoid cell lines | Not determined | [104] |
| 1997 | Physical association between HLA-I molecules and the tetraspanin protein CD82 in human B cell lines | HLA-A2, HLA-A23, HLA-B5, HLA-B8, HLA-B13 | [107] |
| 1998 | Physical interaction between H-2 class I molecules and the N-terminal domains of the CD8α and CD8β receptors | H-2Ld | [48] |
| 2004 | Clusters containing HLA-I molecules and α, β, and ɣc chains of the IL-2/IL-15 receptors in a leukemia T cell line | Not determined | [64] |
| 2004 | Physical association between H-2 class I molecules and Ly49 receptors on NK cell transfectants | H-2Dd, H-2Dk | [109] |
| 2004 | Physical association between HLA-I molecules and CD8αβ-Lck in normal human T cells activated in vitro | Not determined | [14] |
| 2006 | Physical interaction between HLA-B27 molecules lacking Tyr320 and transferrin receptors in a thymoma cell line | HLA-B27 | [15] |
| 2007 | Physical association between HLA-I molecules and LILRB2, and its mouse ortholog PirB, in mast cells | Not determined | [110] |
| 2008 | Physical association between HLA-I molecules and LILRB receptors, and their mouse ortholog PirB, in osteoclasts | Not determined | [111] |
| 2010 | Physical association between HLA-I molecules and the β4 integrin in transfected endothelial cells | HLA-A2, HLA-B56 | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arosa, F.A.; Esgalhado, A.J.; Reste-Ferreira, D.; Cardoso, E.M. Open MHC Class I Conformers: A Look through the Looking Glass. Int. J. Mol. Sci. 2021, 22, 9738. https://doi.org/10.3390/ijms22189738
Arosa FA, Esgalhado AJ, Reste-Ferreira D, Cardoso EM. Open MHC Class I Conformers: A Look through the Looking Glass. International Journal of Molecular Sciences. 2021; 22(18):9738. https://doi.org/10.3390/ijms22189738
Chicago/Turabian StyleArosa, Fernando A., André J. Esgalhado, Débora Reste-Ferreira, and Elsa M. Cardoso. 2021. "Open MHC Class I Conformers: A Look through the Looking Glass" International Journal of Molecular Sciences 22, no. 18: 9738. https://doi.org/10.3390/ijms22189738
APA StyleArosa, F. A., Esgalhado, A. J., Reste-Ferreira, D., & Cardoso, E. M. (2021). Open MHC Class I Conformers: A Look through the Looking Glass. International Journal of Molecular Sciences, 22(18), 9738. https://doi.org/10.3390/ijms22189738








