Prenatal and Postnatal Methyl-Modulator Intervention Corrects the Stress-Induced Glucocorticoid Response in Low-Birthweight Rats
Abstract
1. Introduction
2. Results
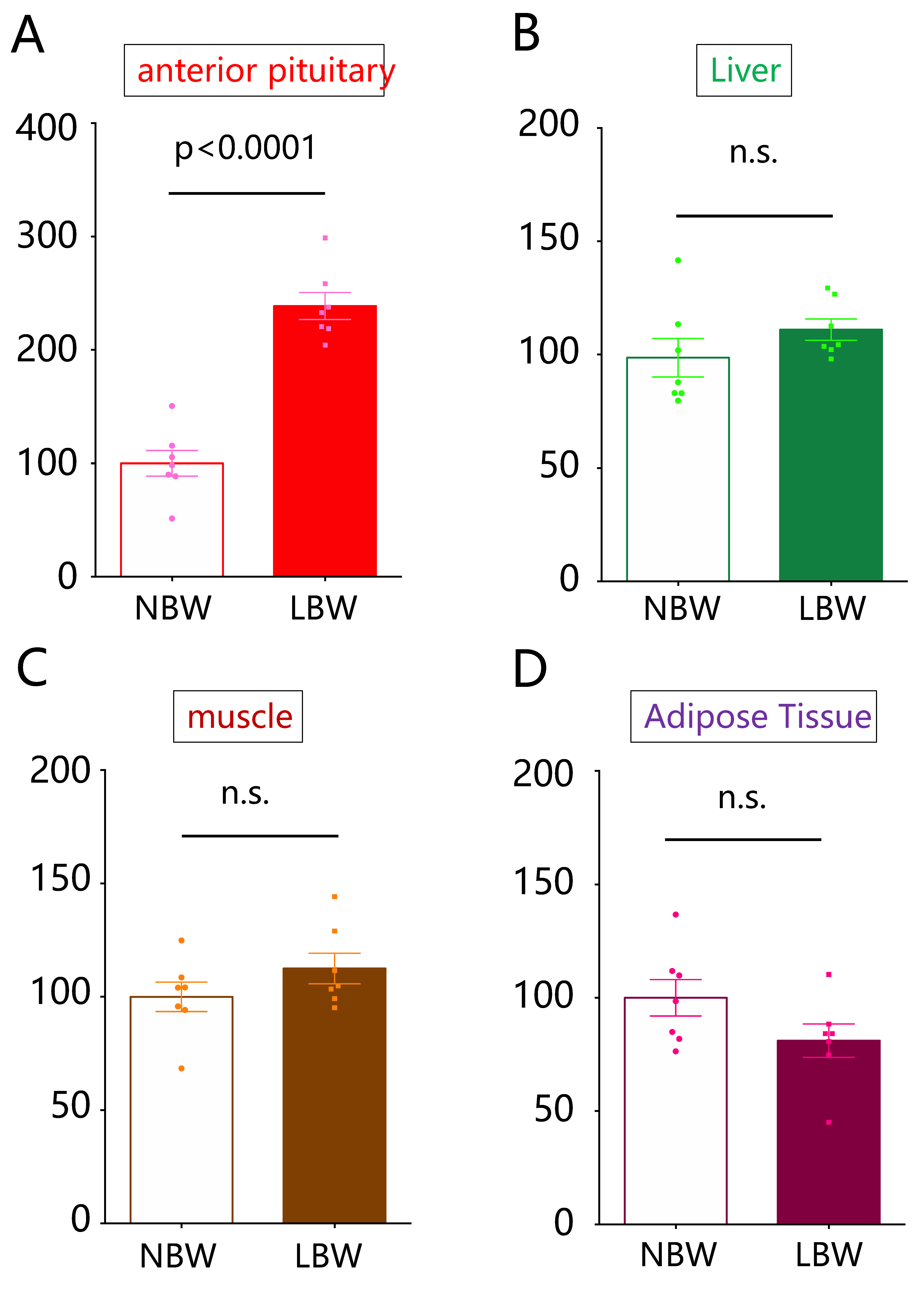
2.1. Comparison of Gas5 lncRNA Expression Levels
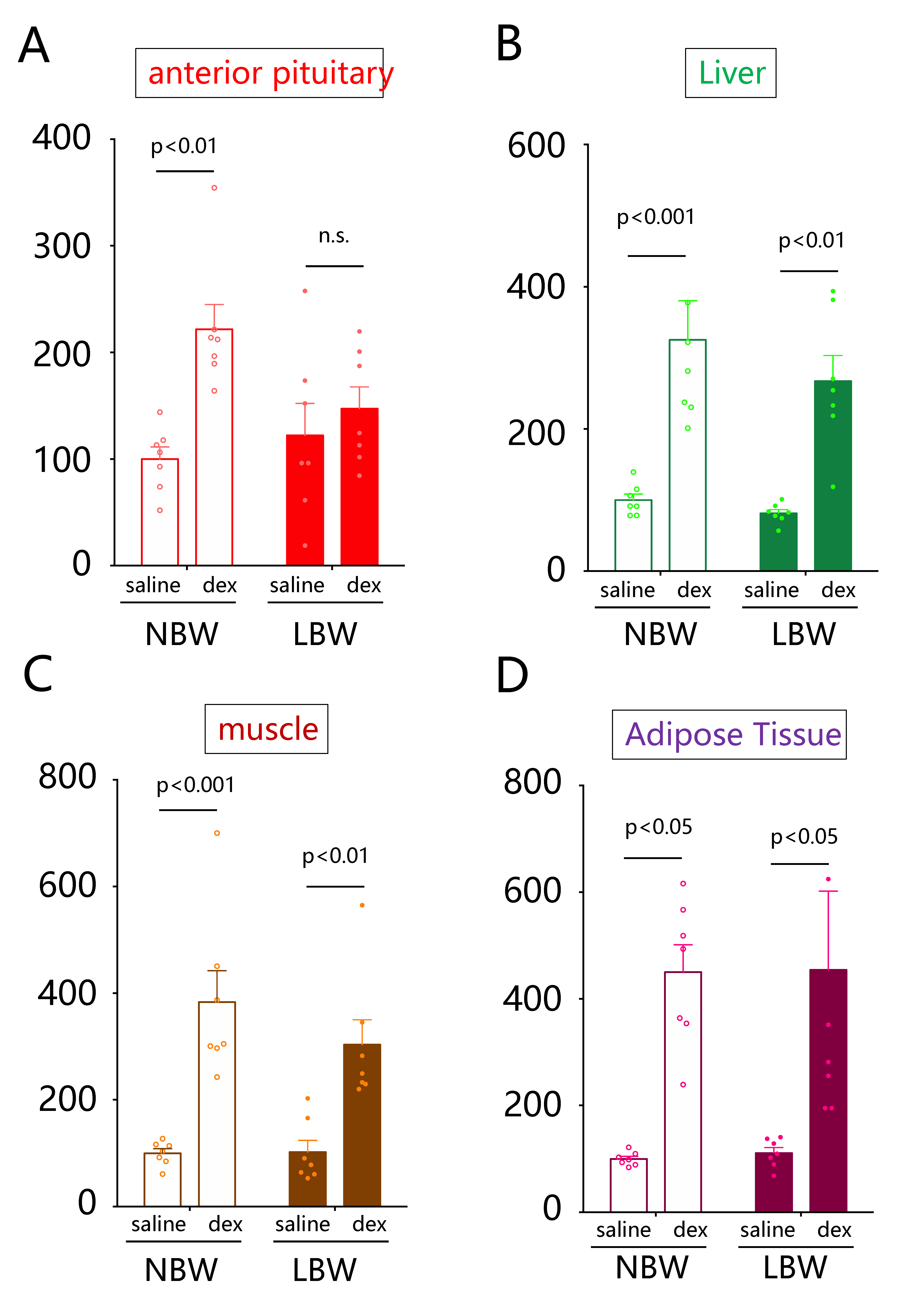
2.2. Comparison of Fkbp5 Expression Levels in Response to Dexamethasone
2.3. Normalization of Gas5 lncRNA Expression in the Pituitary by Methyl Modulator Nutritional Intervention
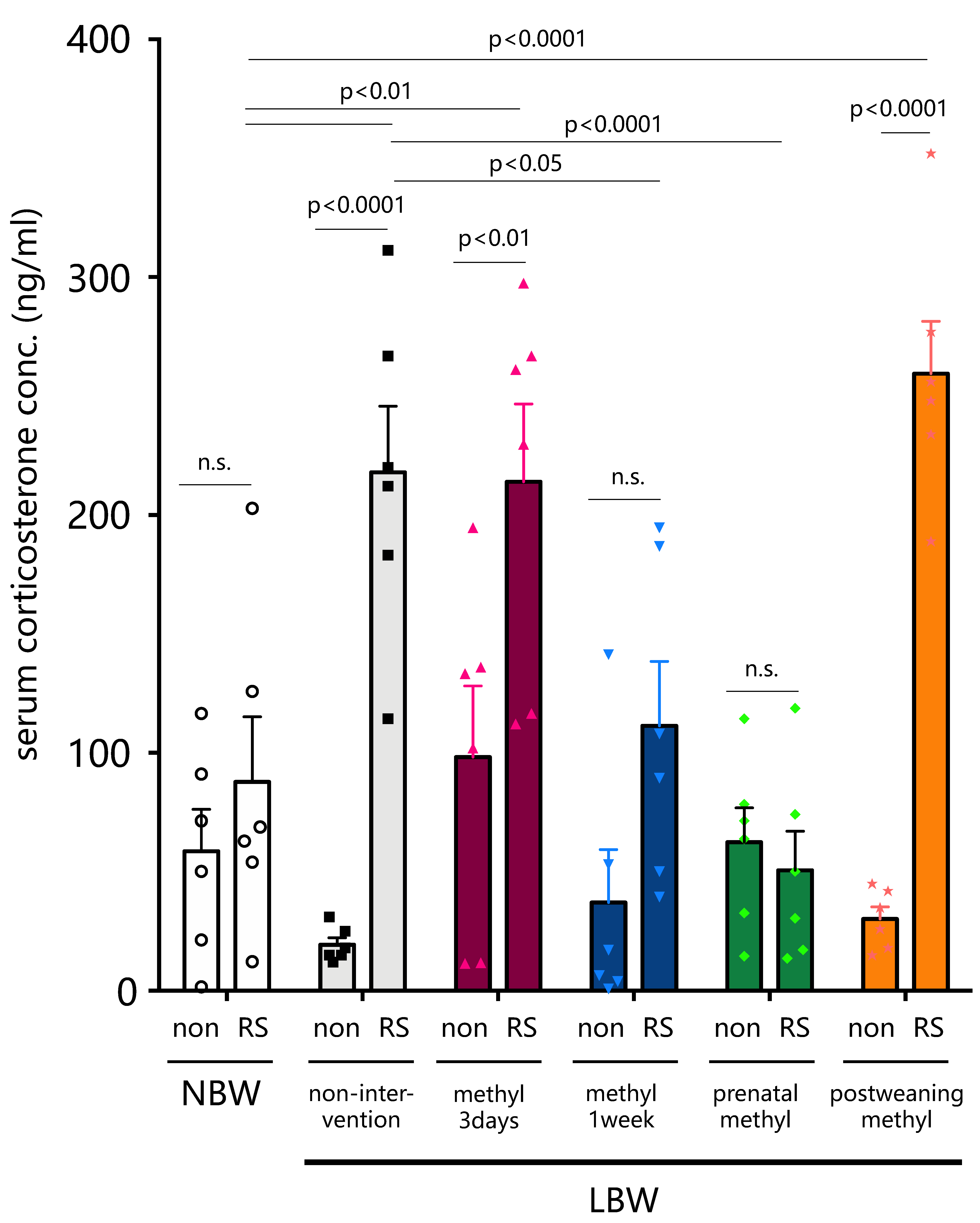
2.4. Effect of Methyl Modulator Nutritional Intervention on Stress-Induced Corticosterone Levels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Dexamethasone Administration
4.3. Methyl Modulator Supplementation
4.4. Restraint Stress Exposure
4.5. RNA Extraction and Real-Time RT-PCR
4.6. Measurement of Blood Corticosterone Levels
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roseboom, T.J.; van der Meulen, J.H.; Osmond, C.; Barker, D.J.; Ravelli, A.C.; Schroeder-Tanka, J.M.; van Montfransb, G.A.; Michelsc, R.P.J.; Blekerd, O.P. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 2000, 84, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Schulz, L.C. The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl. Acad. Sci. USA 2010, 107, 16757–16758. [Google Scholar] [CrossRef]
- Phillips, A.C.; Roseboom, T.J.; Carroll, D.; de Rooij, S.R. Cardiovascular and cortisol reactions to acute psychological stress and adiposity: Cross-sectional and prospective associations in the Dutch Famine Birth Cohort Study. Psychosom. Med. 2012, 74, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Susser, E.; St Clair, D. Prenatal famine and adult mental illness: Interpreting concordant and discordant results from the Dutch and Chinese Famines. Soc. Sci. Med. 2013, 97, 325–330. [Google Scholar] [CrossRef]
- Sudo, A.; Miki, K. Dissociation of catecholamine and corticosterone responses to different types of stress in rats. Ind. Health 1993, 31, 101–111. [Google Scholar] [CrossRef]
- McDonald, L.T.; Lopez, M.F.; Helke, K.L.; McCrackin, M.A.; Cray, J.J., Jr.; Becker, H.C.; LaRue, A.C. Early Blood Profile of C57BL/6 Mice Exposed to Chronic Unpredictable Stress. Front. Psychiatry 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Sarjan, H.N.; Yajurvedi, H.N. Chronic stress induced duration dependent alterations in immune system and their reversibility in rats. Immunol. Lett. 2018, 197, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wu, S.P.; Hu, Y.; Smith, D.E.; Wiley, J.W.; Hong, S. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterol. Motil. 2013, 25, e127–e139. [Google Scholar] [CrossRef]
- Nemoto, T.; Iwasaki-Sekino, A.; Yamauchi, N.; Shibasaki, T. Role of urocortin 2 secreted by the pituitary in the stress-induced suppression of luteinizing hormone secretion in rats. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E567–E575. [Google Scholar] [CrossRef] [PubMed]
- Armario, A.; Castellanos, J.M.; Balasch, J. Dissociation between corticosterone and growth hormone adaptation to chronic stress in the rat. Horm. Metab. Res. 1984, 16, 142–145. [Google Scholar] [CrossRef]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Muthuraju, S.; Wan Mohammad, W.M. Mood and memory function in ovariectomised rats exposed to social instability stress. Biomed. Res. Int. 2013, 2013, 493643. [Google Scholar] [CrossRef]
- Sadowski, R.N.; Jackson, G.R.; Wieczorek, L.; Gold, P.E. Effects of stress, corticosterone, and epinephrine administration on learning in place and response tasks. Behav. Brain Res. 2009, 205, 19–25. [Google Scholar] [CrossRef][Green Version]
- Jia, M.; Smerin, S.E.; Zhang, L.; Xing, G.; Li, X.; Benedek, D.; Ursano, R.; Li, H. Corticosterone mitigates the stress response in an animal model of PTSD. J. Psychiatr. Res. 2015, 60, 29–39. [Google Scholar] [CrossRef]
- Nemoto, T.; Kakinuma, Y.; Shibasaki, T. Impaired miR449a-induced downregulation of Crhr1 expression in low-birth-weight rats. J. Endocrinol. 2015, 224, 195–203. [Google Scholar] [CrossRef]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 2010, 3, ra8. [Google Scholar] [CrossRef]
- Nemoto, T.; Kakinuma, Y. Involvement of Noncoding RNAs in Stress-Related Neuropsychiatric Diseases Caused by DOHaD Theory: NcRNAs and DOHaD-Induced Neuropsychiatric Diseases. Adv. Exp. Med. Biol. 2018, 1012, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, T.; Mano, A.; Shibasaki, T. miR-449a contributes to glucocorticoid-induced CRF-R1 downregulation in the pituitary during stress. Mol. Endocrinol. 2013, 27, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, N.; Urayama, K.Y.; Yoshii, K.; Subramanian, S.V.; Yokoya, S. Ecological analysis of secular trends in low birth weight births and adult height in Japan. J. Epidemiol. Community Health 2017, 71, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef]
- Kageyama, K.; Iwasaki, Y.; Watanuki, Y.; Niioka, K.; Daimon, M. Differential Effects of Fkbp4 and Fkbp5 on Regulation of the Proopiomelanocortin Gene in Murine AtT-20 Corticotroph Cells. Int. J. Mol. Sci. 2021, 22, 5724. [Google Scholar] [CrossRef]
- Kalimi, M. Glucocorticoid receptors: From development to aging. A review. Mech. Ageing Dev. 1984, 24, 129–138. [Google Scholar] [CrossRef]
- Muller, M.; Renkawitz, R. The glucocorticoid receptor. Biochim. Biophys. Acta. 1991, 1088, 171–182. [Google Scholar] [CrossRef]
- Rose, A.J.; Herzig, S. Metabolic control through glucocorticoid hormones: An update. Mol. Cell Endocrinol. 2013, 380, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Smoak, K.A.; Cidlowski, J.A. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech. Ageing Dev. 2004, 125, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Doane, L.D.; Mineka, S.; Zinbarg, R.E.; Craske, M.; Griffith, J.W.; Adam, E.K. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Dev. Psychopathol. 2013, 25, 629–642. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Yuan, H.; Huang, X.W.; Xiang, T.; Dai, S. Up-regulated lncRNA GAS5 promotes chemosensitivity and apoptosis of triple-negative breast cancer cells. Cell Cycle 2019, 18, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yue, S.; Jiang, Y.; Mao, Y.; Zhao, Z.; Liu, X.; Zhang, X.; Pei, D.; Li, Y. LncRNA GAS5 is upregulated in polycystic ovary syndrome and regulates cell apoptosis and the expression of IL-6. J. Ovarian Res. 2020, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Cong, C.; Tian, J.; Gao, T.; Zhou, C.; Wang, Y.; Cui, X.; Zhu, L. lncRNA GAS5 Is Upregulated in Osteoporosis and Downregulates miR-21 to Promote Apoptosis of Osteoclasts. Clin. Interv. Aging. 2020, 15, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xie, Z.; Lei, X.; Gan, R. Long non-coding RNA GAS5 in human cancer. Oncol. Lett. 2020, 20, 2587–2594. [Google Scholar] [CrossRef]
- Wu, Y.; Rong, W.; Jiang, Q.; Wang, R.; Huang, H. Downregulation of lncRNA GAS5 Alleviates Hippocampal Neuronal Damage in Mice with Depression-Like Behaviors Via Modulation of MicroRNA-26a/EGR1 Axis. J. Stroke Cerebrovasc. Dis. 2021, 30, 105550. [Google Scholar] [CrossRef]
- Schiene-Fischer, C.; Yu, C. Receptor accessory folding helper enzymes: The functional role of peptidyl prolyl cis/trans isomerases. FEBS Lett. 2001, 495, 1–6. [Google Scholar] [CrossRef]
- Wochnik, G.M.; Ruegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.H.; Ning, Y.M.; Sanchez, E.R. A new first step in activation of steroid receptors: Hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem. 2002, 277, 4597–4600. [Google Scholar] [CrossRef]
- Jaaskelainen, T.; Makkonen, H.; Palvimo, J.J. Steroid up-regulation of FKBP51 and its role in hormone signaling. Curr. Opin. Pharmacol. 2011, 11, 326–331. [Google Scholar] [CrossRef]
- Vermeer, H.; Hendriks-Stegeman, B.I.; van der Burg, B.; van Buul-Offers, S.C.; Jansen, M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: A potential marker for glucocorticoid sensitivity, potency, and bioavailability. J. Clin. Endocrinol. Metab. 2003, 88, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Arloth, J.; Putz, B.; Weber, P.; Klengel, T.; Mehta, D.; Gonik, M.; Rex-Haffner, M.; Rubel, J.; Uhr, M.; et al. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology 2012, 37, 1455–1464. [Google Scholar] [CrossRef]
- Scharf, S.H.; Liebl, C.; Binder, E.B.; Schmidt, M.V.; Muller, M.B. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS ONE 2011, 6, e16883. [Google Scholar] [CrossRef]
- Saunderson, E.A.; Spiers, H.; Mifsud, K.R.; Gutierrez-Mecinas, M.; Trollope, A.F.; Shaikh, A.; Mill, J.; Reul, J.M. Stress-induced gene expression and behavior are controlled by DNA methylation and methyl donor availability in the dentate gyrus. Proc. Natl. Acad. Sci. USA 2016, 113, 4830–4835. [Google Scholar] [CrossRef]
- Nemoto, T.; Mano, A.; Shibasaki, T. Increased expression of miR-325-3p by urocortin 2 and its involvement in stress-induced suppression of LH secretion in rat pituitary. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E781–E787. [Google Scholar] [CrossRef]
- Nemoto, T.; Kakinuma, Y. Fetal malnutrition-induced catch up failure is caused by elevated levels of miR-322 in rats. Sci. Rep. 2020, 10, 1339. [Google Scholar] [CrossRef]
- Cordero, P.; Gomez-Uriz, A.M.; Campion, J.; Milagro, F.I.; Martinez, J.A. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2013, 8, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemoto, T.; Kakinuma, Y. Prenatal and Postnatal Methyl-Modulator Intervention Corrects the Stress-Induced Glucocorticoid Response in Low-Birthweight Rats. Int. J. Mol. Sci. 2021, 22, 9767. https://doi.org/10.3390/ijms22189767
Nemoto T, Kakinuma Y. Prenatal and Postnatal Methyl-Modulator Intervention Corrects the Stress-Induced Glucocorticoid Response in Low-Birthweight Rats. International Journal of Molecular Sciences. 2021; 22(18):9767. https://doi.org/10.3390/ijms22189767
Chicago/Turabian StyleNemoto, Takahiro, and Yoshihiko Kakinuma. 2021. "Prenatal and Postnatal Methyl-Modulator Intervention Corrects the Stress-Induced Glucocorticoid Response in Low-Birthweight Rats" International Journal of Molecular Sciences 22, no. 18: 9767. https://doi.org/10.3390/ijms22189767
APA StyleNemoto, T., & Kakinuma, Y. (2021). Prenatal and Postnatal Methyl-Modulator Intervention Corrects the Stress-Induced Glucocorticoid Response in Low-Birthweight Rats. International Journal of Molecular Sciences, 22(18), 9767. https://doi.org/10.3390/ijms22189767





