Nitrosative Stress and Human Disease: Therapeutic Potential of Denitrosylation
Abstract
:1. Introduction
1.1. Posttranslational Modification
1.2. Nitric Oxide Modification
1.3. S-Nitrosylation
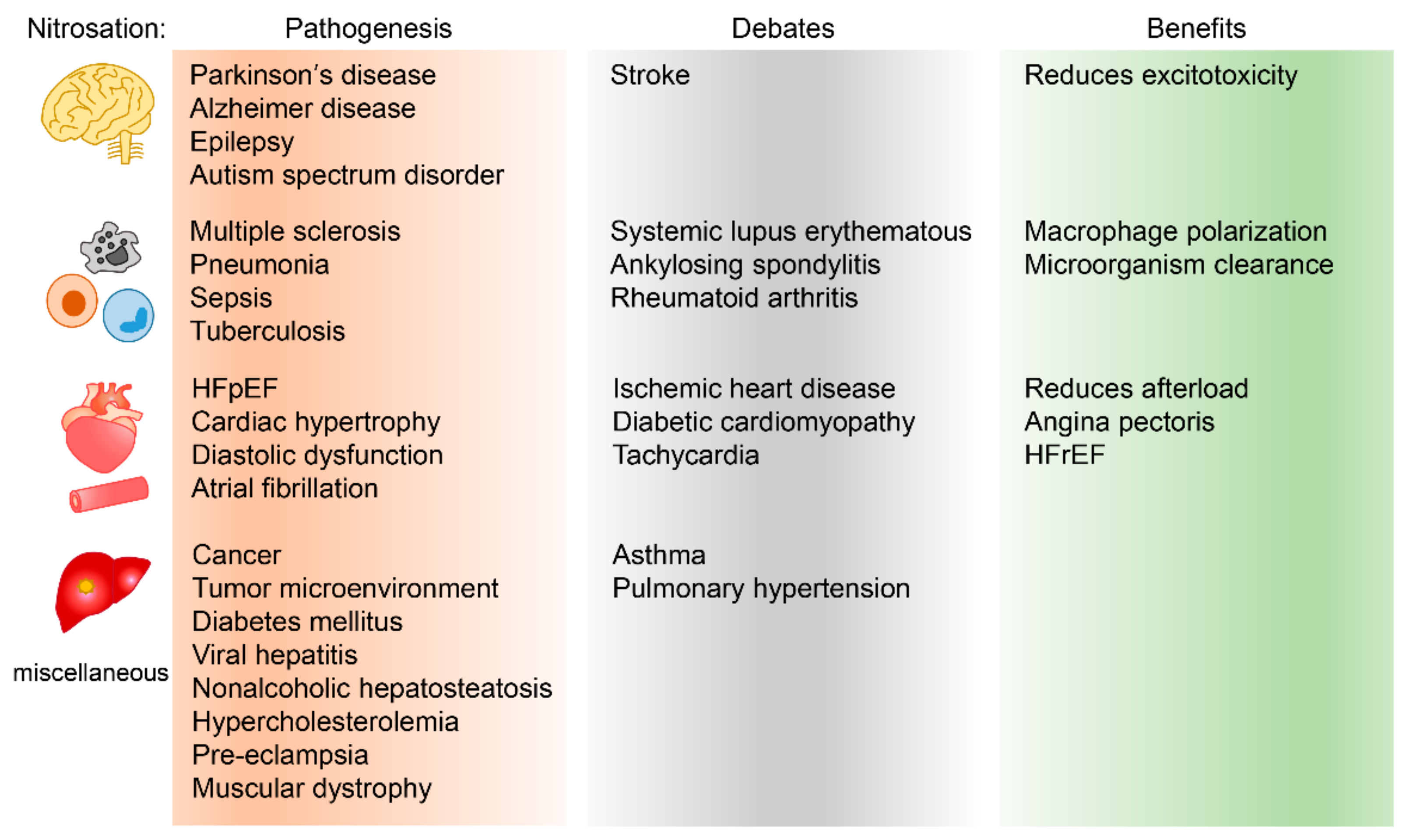
2. Human Disease and Nitrosation
2.1. Central Nervous System
2.1.1. Neurogenesis and S-Nitrosylation
2.1.2. Neurodegenerative Diseases and S-Nitrosylation
Alzheimer’s Disease
Parkinson’s Disease
2.1.3. Epilepsy and S-Nitrosylation
2.1.4. Autism Spectrum Disorder and S-Nitrosylation
2.2. The Immune System and Its Associated Disease
2.2.1. T Cells
2.2.2. B Cells
2.2.3. Macrophages
2.2.4. Autoimmune Diseases
Multiple Sclerosis
Rheumatoid Arthritis
2.2.5. Septic Shock
2.2.6. Miscellaneous
2.3. The Cardiovascular System
2.3.1. Cardiac Ion Channels and Ion Homeostasis Proteins
L-Type Calcium Channel
Sarco/Endoplasmic Reticulum Ca2+-ATPase
2.3.2. Arrhythmia
2.3.3. Dilated Cardiomyopathy
2.3.4. Ischemic Heart Disease
2.3.5. Heart Failure with Preserved Ejection Fraction
2.3.6. Aortic Dissection
2.4. Tumor Microenvironment
2.5. Miscellaneous Diseases
2.5.1. Diabetes Mellitus
2.5.2. Chronic Hepatitis and Hepatocellular Carcinoma
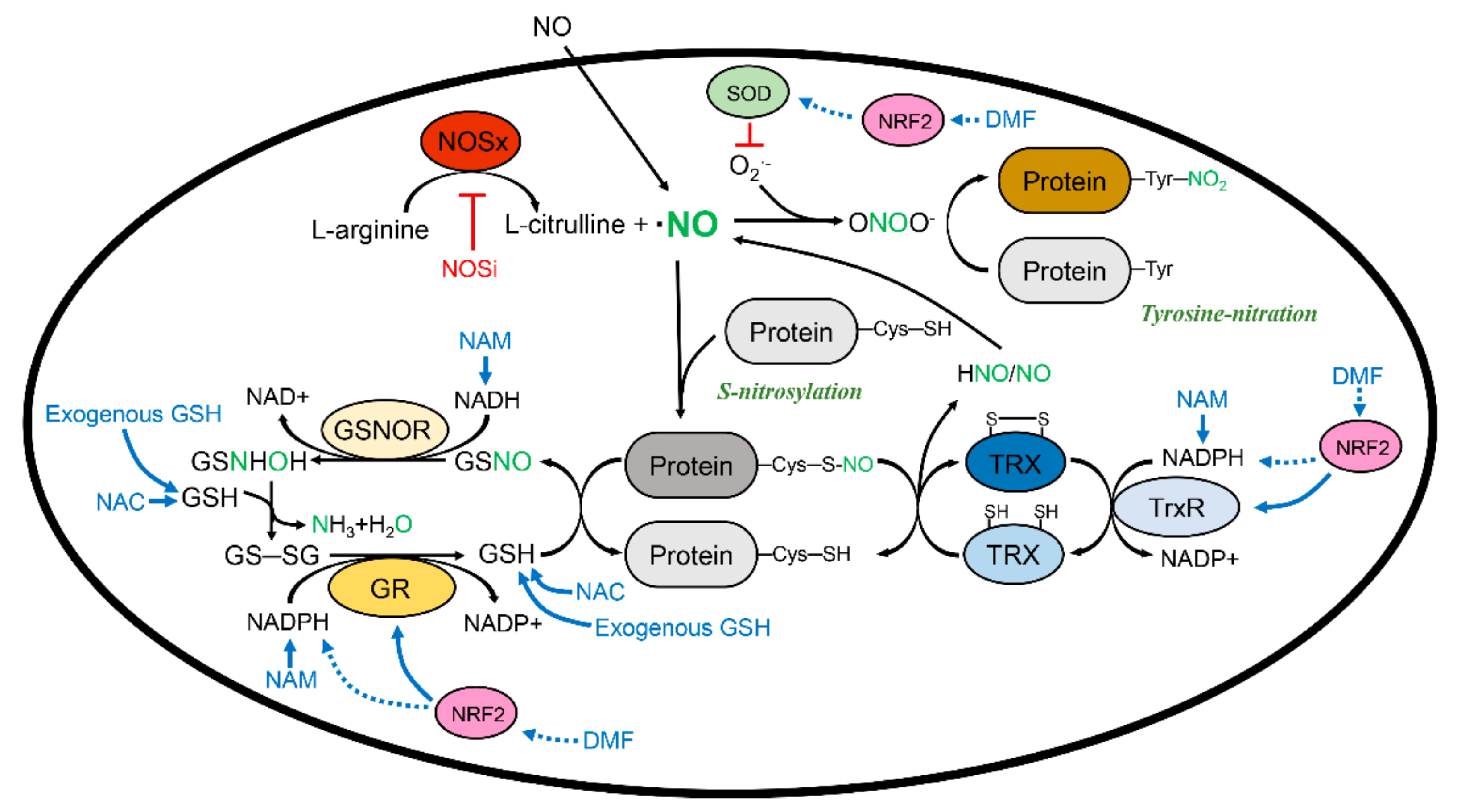
3. Reducing Protein Nitrosation and Its Therapeutic Potential
3.1. Glutathione and S-Nitrosoglutathione System
3.2. Thioredoxin and the Thioredoxin Reductase System
3.3. Clinical Importance of Denitrosylation
3.3.1. Therapeutic Potential of Detritylation: Nitric Oxide Synthase Inhibitor
3.3.2. Therapeutic Potential of Denitrosylation: Complement of Glutathione
3.3.3. Therapeutic Potential of Denitrosylation: Thioredoxin and Thioredoxin Reductase Inducer
3.3.4. Indirect Denitrosylase
Nicotinamide Adenine Dinucleotide and Nicotinamide Adenine Dinucleotide Phosphate
Antioxidants
Nuclear Factor Erythroid 2-Related Factor 2 and Its Inducers
4. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aβ25–35 | amyloid β-peptide 25–35 |
| ASD | autism spectrum disorder |
| AF | atrial fibrillation |
| BDNF | brain-derived neurotrophic factor |
| BH4 | tetrahydrobiopterin |
| cAMP | cyclic adenosine monophosphate |
| CREB | cAMP response element-binding protein |
| DMF | dimethyl fumarate |
| Drp1 | dynamin-related protein 1 |
| eNOS (NOS3) | endothelial NOS |
| FLS | fibroblast-like synoviocytes |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GR | glutathione reductase |
| GRIK2 | glutamate ionotropic receptor kainate type subunit 2 |
| GSH | glutathione (γ-l-glutamyl-l-cysteinyl-glycine) |
| GSNHOH | N-hydroxysulfinamide |
| GSNO | S-nitrosoglutathione |
| GSNOR | S-nitrosoglutathione reductase |
| GSSG | glutathione disulfide |
| H2O2 | hydrogen peroxide |
| H3K9 | histone 3 lysine 9 |
| HDAC2 | histone deacetylase 2 |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| HIF1α | hypoxia inducible factor-1α |
| HNO | nitroxyl |
| I/R | ischemia/reperfusion |
| ICa,L | L-type calcium current |
| IK1 | inward rectifier |
| IL | interleukin |
| INa | Na+ inward currents |
| iNOS (NOS2) | inducible NOS |
| IRE1α | inositol-requiring protein 1α |
| KO | knockout |
| L-NAME | N(ω)-nitro-L-arginine methyl ester |
| L-NMMA | N(G)-monomethyl L-arginine |
| LPS | lipopolysaccharide |
| LTCC | L-type calcium channels |
| M1 | immuno-stimulatory macrophage |
| M2 | immune-suppressive macrophage |
| MEF | myocyte enhancer factor |
| Keap1 | Kelch-like ECH-associated protein 1 |
| MS | multiple sclerosis |
| NADH | nicotinamide adenine phosphate |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NAM | nicotinamide |
| NF-κB | nuclear factor Kappa B |
| NMDA | N-methyl-D-aspartate |
| NO | nitric oxide |
| nNOS (NOS1) | neuronal NOS |
| NOS | nitric oxide synthase |
| NOSi | NOS inhibitor |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| O2 | oxygen |
| O2·− | superoxide radical |
| PDI | protein-disulfide isomerase |
| PSD-95 | postsynaptic density protein-95 |
| RA | rheumatoid arthritis |
| RNS | reactive nitrogen species |
| RORγt | retinoic acid receptor-related orphan nuclear receptor gamma |
| ROS | reactive oxygen species |
| RyR | ryanodine receptor |
| SERCA | sarco/endoplasmic reticulum Ca2+-ATPase |
| sGC | soluble guanylyl cyclase |
| SIAH1 | siah E3 ubiquitin protein ligase 1 |
| SNAP | S-nitroso-N-acetylpenicillamine |
| SNO-GAPDH | S-nitrosylated glyceraldehyde 3-phosphate dehydrogenase |
| SNO-PDI | S-nitrosylated protein-disulfide isomerase |
| SOD | superoxide dismutase |
| SO2H | sulfinic acid |
| SR | sarcoplasmic reticulum |
| SS | disulfide |
| TAM | tumor-associated macrophage |
| TLX | tailless |
| TNFα | tumor necrosis factor-α |
| TRIM72 | tripartite motif-containing protein 72 |
| TRX | thioredoxin |
| TrxR | thioredoxin reductase |
| VEGF | vascular endothelial growth factor |
| WT | wild-type |
| XBP1 | X-box binding protein |
References
- Eom, G.H.; Kook, H. Posttranslational modifications of histone deacetylases: Implications for cardiovascular diseases. Pharmacol. Ther. 2014, 143, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-Y.; Liu, Y.-C.; Chen, N.-Y.; Tsai, C.-F.; Wang, Y.-T.; Chen, Y.-J.; Hsu, T.-L.; Yang, P.-C.; Wong, C.-H. Effect of sialylation on EGFR phosphorylation and resistance to tyrosine kinase inhibition. Proc. Natl. Acad. Sci. USA 2015, 112, 6955–6960. [Google Scholar] [CrossRef] [Green Version]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2014, 148, 114–131. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Zhang, Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 2005, 6, 838–849. [Google Scholar] [CrossRef]
- Wu, Q.; Schapira, M.; Arrowsmith, C.H.; Barsyte-Lovejoy, D. Protein arginine methylation: From enigmatic functions to therapeutic targeting. Nat. Rev. Drug Discov. 2021, 20, 509–530. [Google Scholar] [CrossRef]
- Poleshko, A.; Shah, P.P.; Gupta, M.; Babu, A.; Morley, M.P.; Manderfield, L.J.; Ifkovits, J.L.; Calderon, D.; Aghajanian, H.; Sierra-Pagan, J.; et al. Genome-Nuclear Lamina Interactions Regulate Cardiac Stem Cell Lineage Restriction. Cell 2017, 171, 573–587.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poleshko, A.; Smith, C.L.; Nguyen, S.C.; Sivaramakrishnan, P.; Wong, K.G.; Murray, J.I.; Lakadamyali, M.; Joyce, E.F.; Jain, R.; Epstein, J.A. H3K9me2 orchestrates inheritance of spatial positioning of peripheral heterochromatin through mitosis. eLife 2019, 8, e49278. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rosa, H.; Schneider, R.; Bannister, A.; Sherriff, J.; Bernstein, B.E.; Emre, T.; Schreiber, S.L.; Mellor, J.; Kouzarides, T. Active genes are tri-methylated at K4 of histone H3. Nature 2002, 419, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric Oxide Donors: Chemical Activities and Biological Applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Cai, C.-Y.; Chen, C.; Zhou, Y.; Han, Z.; Qin, C.; Cao, B.; Tao, Y.; Bian, X.-L.; Lin, Y.-H.; Chang, L.; et al. PSD-95-nNOS Coupling Regulates Contextual Fear Extinction in the Dorsal CA3. Sci. Rep. 2018, 8, 12775. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2011, 33, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premont, R.T.; Reynolds, J.D.; Zhang, R.; Stamler, J.S. Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology. Circ. Res. 2020, 126, 129–158. [Google Scholar] [CrossRef]
- Stamler, J.S.; Jia, L.; Eu, J.P.; McMahon, T.J.; Demchenko, I.T.; Bonaventura, J.; Gernert, K.; Piantadosi, C.A. Blood Flow Regulation by S-Nitrosohemoglobin in the Physiological Oxygen Gradient. Science 1997, 276, 2034–2037. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthi, S.; Jessop, C.E.; Bulleid, N.J. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 2006, 7, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Zuccarelli, R.; Coelho, A.C.; Peres, L.E.; Freschi, L. Shedding light on NO homeostasis: Light as a key regulator of glutathione and nitric oxide metabolisms during seedling deetiolation. Nitric Oxide 2017, 68, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Seidler, M.; Ückert, S.; Waldkirch, E.; Stief, C.G.; Oelke, M.; Tsikas, D.; Sohn, M.; Jonas, U. In Vitro Effects of a Novel Class of Nitric Oxide (NO) Donating Compounds on Isolated Human Erectile Tissue. Eur. Urol. 2002, 42, 523–528. [Google Scholar] [CrossRef]
- Ballou, D.P.; Zhao, Y.; Brandish, P.E.; Marletta, M.A. Revisiting the kinetics of nitric oxide (NO) binding to soluble guanylate cyclase: The simple NO-binding model is incorrect. Proc. Natl. Acad. Sci. USA 2002, 99, 12097–12101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: A new target for the development of specific therapeutic agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef]
- Heckman, P.; Blokland, A.; Bollen, E.; Prickaerts, J. Phosphodiesterase inhibition and modulation of corticostriatal and hippocampal circuits: Clinical overview and translational considerations. Neurosci. Biobehav. Rev. 2018, 87, 233–254. [Google Scholar] [CrossRef]
- Archer, S.L.; Michelakis, E.D. Phosphodiesterase Type 5 Inhibitors for Pulmonary Arterial Hypertension. N. Engl. J. Med. 2009, 361, 1864–1871. [Google Scholar] [CrossRef] [Green Version]
- Ribaudo, G.; Ongaro, A.; Zagotto, G.; Memo, M.; Gianoncelli, A. Therapeutic Potential of Phosphodiesterase Inhibitors against Neurodegeneration: The Perspective of the Medicinal Chemist. ACS Chem. Neurosci. 2020, 11, 1726–1739. [Google Scholar] [CrossRef]
- Leroy, J.; Fischmeister, R. Inhibit a Phosphodiesterase to Treat Heart Failure? Circulation 2018, 138, 2003–2006. [Google Scholar] [CrossRef]
- Zuo, H.; Cattani-Cavalieri, I.; Musheshe, N.; Nikolaev, V.O.; Schmidt, M. Phosphodiesterases as therapeutic targets for respiratory diseases. Pharmacol. Ther. 2019, 197, 225–242. [Google Scholar] [CrossRef]
- Komatsu, K.; Lee, J.-Y.; Miyata, M.; Lim, J.H.; Jono, H.; Koga, T.; Xu, H.; Yan, C.; Kai, H.; Li, J.-D. Inhibition of PDE4B suppresses inflammation by increasing expression of the deubiquitinase CYLD. Nat. Commun. 2013, 4, 1684. [Google Scholar] [CrossRef] [Green Version]
- Savai, R.; Pullamsetti, S.S.; Banat, G.-A.; Weissmann, N.; Ghofrani, H.A.; Grimminger, F.; Schermuly, R.T. Targeting cancer with phosphodiesterase inhibitors. Expert Opin. Investig. Drugs 2009, 19, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.H.; Palmer, D.; Tilley, D.; Dunkerley, H.A.; Netherton, S.J.; Raymond, D.R.; Elbatarny, H.S.; Jimmo, S.L. Cyclic Nucleotide Phosphodiesterase Activity, Expression, and Targeting in Cells of the Cardiovascular System. Mol. Pharmacol. 2003, 64, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Mumby, M.; Beavo, J. Purification and characterization of a cyclic GMP-stimulated cyclic nucleotide phosphodiesterase from bovine tissues. J. Biol. Chem. 1982, 257, 1973–1979. [Google Scholar] [CrossRef]
- Aizawa, T.; Wei, H.; Miano, J.M.; Abe, J.-I.; Berk, B.C.; Yan, C. Role of Phosphodiesterase 3 in NO/cGMP-Mediated Antiinflammatory Effects in Vascular Smooth Muscle Cells. Circ. Res. 2003, 93, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, A.M.S.; Ancillotti, M.T.C.; Rangel, L.P.; Fontenele, M.; Figueiredo-Freitas, C.; Possidonio, A.C.; Soares, C.P.; Sorenson, M.M.; Mermelstein, C.; Nogueira, L. Balance between S-nitrosylation and denitrosylation modulates myoblast proliferation independently of soluble guanylyl cyclase activation. Am. J. Physiol. Physiol. 2017, 313, C11–C26. [Google Scholar] [CrossRef] [Green Version]
- Vitturi, D.; Minarrieta, L.; Salvatore, S.; Postlethwait, E.M.; Fazzari, M.; Ferrer-Sueta, G.; Lancaster, J.R.; Freeman, B.A.; Schopfer, F.J. Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride. Nat. Chem. Biol. 2015, 11, 504–510. [Google Scholar] [CrossRef]
- Sabadashka, M.; Nagalievska, M.; Sybirna, N. Tyrosine nitration as a key event of signal transduction that regulates functional state of the cell. Cell Biol. Int. 2020, 45, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Lipton, S.A. Emerging Role of Protein-Protein Transnitrosylation in Cell Signaling Pathways. Antioxid. Redox Signal. 2013, 18, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Jaffrey, S.R.; Snyder, S.H. The Biotin Switch Method for the Detection of S-Nitrosylated Proteins. Sci. Signal. 2001, 2001, pl1. [Google Scholar] [CrossRef]
- Wolhuter, K.; Whitwell, H.; Switzer, C.H.; Burgoyne, J.; Timms, J.; Eaton, P. Evidence against Stable Protein S-Nitrosylation as a Widespread Mechanism of Post-translational Regulation. Mol. Cell 2018, 69, 438–450.e5. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Jung, J.-Y.; Kim, H.-J.; Ham, M.R.; Lee, T.R.; Shin, D.W. S-nitrosylation of fatty acid synthase regulates its activity through dimerization. J. Lipid Res. 2016, 57, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.-Q.; Ye, J.-S.; Zong, Y.-Y.; Sun, C.-C.; Liu, D.-H.; Wu, Y.-P.; Song, T.; Zhang, G.-Y. S-Nitrosylation of Mixed Lineage Kinase 3 Contributes to Its Activation after Cerebral Ischemia. J. Biol. Chem. 2012, 287, 2364–2377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnelle, D.; Stamler, J. NO+, NO, and NO− Donation by S-Nitrosothiols: Implications for Regulation of Physiological Functions by S-Nitrosylation and Acceleration of Disulfide Formation. Arch. Biochem. Biophys. 1995, 318, 279–285. [Google Scholar] [CrossRef]
- Won, S.; Incontro, S.; Nicoll, R.A.; Roche, K.W. PSD-95 stabilizes NMDA receptors by inducing the degradation of STEP61. Proc. Natl. Acad. Sci. USA 2016, 113, E4736–E4744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.-B.; Tenneti, L.; Le, D.A.; Ortiz, J.; Bai, G.; Chen, H.-S.V.; Lipton, S.A. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat. Neurosci. 2000, 3, 15–21. [Google Scholar] [CrossRef]
- Moreno-López, B.; Romero-Grimaldi, C.; Noval, J.A.; Murillo-Carretero, M.; Matarredona, E.R.; Estrada, C. Nitric Oxide Is a Physiological Inhibitor of Neurogenesis in the Adult Mouse Subventricular Zone and Olfactory Bulb. J. Neurosci. 2004, 24, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, S.-I.; Nakamura, T.; Cieplak, P.; Chan, S.F.; Kalashnikova, E.; Liao, L.; Saleem, S.; Han, X.; Clemente, A.; Nutter, A.; et al. S-Nitrosylation-Mediated Redox Transcriptional Switch Modulates Neurogenesis and Neuronal Cell Death. Cell Rep. 2014, 8, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Nott, A.; Watson, P.M.; Robinson, J.D.; Crepaldi, L.; Riccio, A. S-nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 2008, 455, 411–415. [Google Scholar] [CrossRef]
- Delcuve, G.P.; Khan, D.H.; Davie, J.R. Roles of histone deacetylases in epigenetic regulation: Emerging paradigms from studies with inhibitors. Clin. Epigenet. 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nott, A.; Nitarska, J.; Veenvliet, J.V.; Schacke, S.; Derijck, A.A.H.A.; Sirko, P.; Muchardt, C.; Pasterkamp, J.; Smidt, M.; Riccio, A. S-nitrosylation of HDAC2 regulates the expression of the chromatin-remodeling factor Brm during radial neuron migration. Proc. Natl. Acad. Sci. USA 2013, 110, 3113–3118. [Google Scholar] [CrossRef] [Green Version]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Cros, E.T. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, E.; Griffin, W.; Cunningham, C. Astrocytes Are Primed by Chronic Neurodegeneration to Produce Exaggerated Chemokine and Cell Infiltration Responses to Acute Stimulation with the Cytokines IL-1β and TNF-α. J. Neurosci. 2015, 35, 8411–8422. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, M.; Sen, N.; Hara, M.R.; Juluri, K.R.; Nguyen, J.V.K.; Snowman, A.M.; Law, L.; Hester, L.D.; Snyder, S.H. GAPDH mediates nitrosylation of nuclear proteins. Nature 2010, 12, 1094–1100. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.R.; Agrawal, N.; Kim, S.F.; Cascio, M.B.; Fujimuro, M.; Ozeki, Y.; Takahashi, M.; Cheah, J.H.; Tankou, S.K.; Hester, L.D.; et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nature 2005, 7, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.; Beal, M.F. Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Pharmacol. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Twig, G.; Shirihai, O.S. The Interplay Between Mitochondrial Dynamics and Mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.-H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-Nitrosylation of Drp1 Mediates β-Amyloid-Related Mitochondrial Fission and Neuronal Injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uehara, T.; Nakamura, T.; Yao, D.; Shi, Z.-Q.; Gu, Z.; Ma, Y.; Masliah, E.; Nomura, Y.; Lipton, S.A. S-Nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 2006, 441, 513–517. [Google Scholar] [CrossRef]
- Moon, J.U.; Cho, K.-O. Current Pharmacologic Strategies for Treatment of Intractable Epilepsy in Children. Int. Neurourol. J. 2021, 25, S8–S18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Lu, R.; Dong, G.; Chen, X.; Yun, W.; Zhou, X. The role of S-nitrosylation of kainate-type of ionotropic glutamate receptor 2 in epilepsy induced by kainic acid. J. Neurochem. 2017, 144, 255–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amal, H.; Barak, B.; Bhat, V.; Gong, G.; Joughin, B.A.; Wang, X.; Wishnok, J.S.; Feng, G.; Tannenbaum, S.R. Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol. Psychiatry 2018, 25, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-S.; Rai, P.R.; Chu, H.W.; Cool, C.; Chan, E.D. Analysis of Nitric Oxide Synthase and Nitrotyrosine Expression in Human Pulmonary Tuberculosis. Am. J. Respir. Crit. Care Med. 2002, 166, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connelly, L.; Jacobs, A.T.; Palacios-Callender, M.; Moncada, S.; Hobbs, A. Macrophage Endothelial Nitric-oxide Synthase Autoregulates Cellular Activation and Pro-inflammatory Protein Expression. J. Biol. Chem. 2003, 278, 26480–26487. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, R.; Lu, G.; Shen, Y.; Peng, L.; Zhu, C.; Cui, M.; Wang, W.; Arnaboldi, P.; Tang, M.; et al. T cell–derived inducible nitric oxide synthase switches off TH17 cell differentiation. J. Exp. Med. 2013, 210, 1447–1462. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.; Zhu, C.; Li, F.; Hegazi, R.; He, K.; Babyatsky, M.; Bauer, A.J.; Plevy, S.E. Inhibition of Interleukin-12 p40 Transcription and NF-κB Activation by Nitric Oxide in Murine Macrophages and Dendritic Cells. J. Biol. Chem. 2004, 279, 10776–10783. [Google Scholar] [CrossRef] [Green Version]
- Klein, L.; Kyewski, B.; Allen, P.; Hogquist, K. Positive and negative selection of the T cell repertoire: What thymocytes see (and don’t see). Nat. Rev. Immunol. 2014, 14, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Saini, A.S.; Shenoy, G.; Rath, S.; Bal, V.; George, A. Inducible nitric oxide synthase is a major intermediate in signaling pathways for the survival of plasma cells. Nat. Immunol. 2014, 15, 275–282. [Google Scholar] [CrossRef]
- Epelman, S.; LaVine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Gan, S.; Zhu, Q.; Dai, D.; Li, N.; Wang, H.; Chen, X.; Hou, D.; Wang, Y.; Pan, Q.; et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat. Commun. 2019, 10, 4353. [Google Scholar] [CrossRef] [Green Version]
- Hemmer, B.; Cepok, S.; Zhou, D.; Sommer, N. Multiple Sclerosis—A Coordinated Immune Attack Across the Blood Brain Barrier. Curr. Neurovasc. Res. 2004, 1, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Rejdak, K.; Eikelenboom, M.J.; Petzold, A.; Thompson, E.J.; Stelmasiak, Z.; Lazeron, R.H.; Barkhof, F.; Polman, C.H.; Uitdehaag, B.M.; Giovannoni, G. CSF nitric oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology 2004, 63, 1439–1445. [Google Scholar] [CrossRef]
- Huber, L.C.; Distler, O.; Tarner, I.; Gay, R.E.; Gay, S.; Pap, T. Synovial fibroblasts: Key players in rheumatoid arthritis. Rheumatology 2006, 45, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Raghav, S.K.; Das, H.R. S-Nitrosylation of mannose binding lectin regulates its functional activities and the formation of autoantibody in rheumatoid arthritis. Nitric Oxide 2008, 18, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-C.; Pan, K.-T.; Chang, G.-F.; Hsu, C.-H.; Khoo, K.-H.; Hung, C.-H.; Jiang, Y.-J.; Ho, F.-M.; Meng, T.-C. Nitrite-Mediated S -Nitrosylation of Caspase-3 Prevents Hypoxia-Induced Endothelial Barrier Dysfunction. Circ. Res. 2011, 109, 1375–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Török, N.J.; Higuchi, H.; Bronk, S.; Gores, G.J. Nitric oxide inhibits apoptosis downstream of cytochrome C release by nitrosylating caspase 9. Cancer Res. 2002, 62, 1648–1653. [Google Scholar]
- Benedet, P.O.; Menegatti, A.C.; Gonçalves, M.C.; Terenzi, H.; Assreuy, J. The therapeutic value of protein (de)nitrosylation in experimental septic shock. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Osuru, H.P.; Paila, U.; Ikeda, K.; Zuo, Z.; Thiele, R.H. Anesthesia-Sepsis-Associated Alterations in Liver Gene Expression Profiles and Mitochondrial Oxidative Phosphorylation Complexes. Front. Med. 2020, 7, 969. [Google Scholar] [CrossRef] [PubMed]
- Hacquard-Bouder, C.; Ittah, M.; Breban, M. Animal models of HLA-B27-associated diseases: New outcomes. Jt. Bone Spine 2006, 73, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.L.; Gilkeson, G. Mouse models of lupus: What they tell us and what they don’t. Lupus Sci. Med. 2018, 5, e000199. [Google Scholar] [CrossRef] [PubMed]
- Massion, P.; Feron, O.; Dessy, C.; Balligand, J.-L. Nitric Oxide and Cardiac Function. Circ. Res. 2003, 93, 388–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canton, M.; Menazza, S.; Sheeran, F.L.; de Laureto, P.P.; Di Lisa, F.; Pepe, S. Oxidation of Myofibrillar Proteins in Human Heart Failure. J. Am. Coll. Cardiol. 2011, 57, 300–309. [Google Scholar] [CrossRef]
- Menazza, S.; Aponte, A.; Sun, J.; Gucek, M.; Steenbergen, C.; Murphy, E. Molecular Signature of Nitroso–Redox Balance in Idiopathic Dilated Cardiomyopathies. J. Am. Heart Assoc. 2015, 4, e002251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.; Kim, M.; Lee, H.; Kang, G.; Bedi, K.; Margulies, K.B.; Jain, R.; Nam, K.-I.; Kook, H.; Eom, G.H. S-Nitrosylation of Histone Deacetylase 2 by Neuronal Nitric Oxide Synthase as a Mechanism of Diastolic Dysfunction. Circulation 2021, 143, 1912–1925. [Google Scholar] [CrossRef]
- Carnes, C.; Janssen, P.M.L.; Ruehr, M.L.; Nakayama, H.; Nakayama, T.; Haase, H.; Bauer, J.A.; Chung, M.K.; Fearon, I.M.; Gillinov, A.M.; et al. Atrial Glutathione Content, Calcium Current, and Contractility. J. Biol. Chem. 2007, 282, 28063–28073. [Google Scholar] [CrossRef] [Green Version]
- Burger, D.E.; Lu, X.; Lei, M.; Xiang, F.-L.; Hammoud, L.; Jiang, M.; Wang, H.; Jones, D.L.; Sims, S.M.; Feng, Q. Neuronal Nitric Oxide Synthase Protects Against Myocardial Infarction-Induced Ventricular Arrhythmia and Mortality in Mice. Circulation 2009, 120, 1345–1354. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, D.R.; Beigi, F.; Treuer, A.V.; Hare, J.M. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 20612–20617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Viatchenko-Karpinski, S.; Sun, J.; Gyorke, I.; Benkusky, N.A.; Kohr, M.J.; Valdivia, H.H.; Murphy, E.; Györke, S.; Ziolo, M.T. Regulation of myocyte contraction via neuronal nitric oxide synthase: Role of ryanodine receptorS-nitrosylation. J. Physiol. 2010, 588, 2905–2917. [Google Scholar] [CrossRef]
- Beigi, F.; Gonzalez, D.; Minhas, K.M.; Sun, Q.-A.; Foster, M.W.; Khan, S.A.; Treuer, A.V.; Dulce, R.A.; Harrison, R.W.; Saraiva, R.; et al. Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc. Natl. Acad. Sci. USA 2012, 109, 4314–4319. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Eu, J.P.; Meissner, G.; Stamler, J.S. Activation of the Cardiac Calcium Release Channel (Ryanodine Receptor) by Poly-S-Nitrosylation. Science 1998, 279, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Vielma, A.Z.; León, L.; Fernandez, I.; Gonzalez, D.; Boric, M.P. Nitric Oxide Synthase 1 Modulates Basal and β-Adrenergic-Stimulated Contractility by Rapid and Reversible Redox-Dependent S-Nitrosylation of the Heart. PLoS ONE 2016, 11, e0160813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rullman, E.; Andersson, D.C.; Melin, M.; Reiken, S.; Mancini, N.M.; Marks, A.R.; Lund, L.H.; Gustafsson, T. Modifications of skeletal muscle ryanodine receptor type 1 and exercise intolerance in heart failure. J. Heart Lung Transplant. 2013, 32, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Picht, E.; Ginsburg, K.S.; Bers, D.M.; Steenbergen, C.; Murphy, E. Hypercontractile Female Hearts Exhibit Increased S -Nitrosylation of the L-Type Ca 2+ Channel α1 Subunit and Reduced Ischemia/Reperfusion Injury. Circ. Res. 2006, 98, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Rozmaritsa, N.; Christ, T.; Van Wagoner, D.R.; Haase, H.; Stasch, J.-P.; Matschke, K.; Ravens, U. Attenuated response of L-type calcium current to nitric oxide in atrial fibrillation. Cardiovasc. Res. 2013, 101, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Bencsik, P.; Kupai, K.; Giricz, Z.; Görbe, A.; Huliák, I.; Fürst, S.; Dux, L.; Csont, T.; Jancsó, G.; Ferdinandy, P. Cardiac capsaicin-sensitive sensory nerves regulate myocardial relaxation via S -nitrosylation of SERCA: Role of peroxynitrite. Br. J. Pharmacol. 2008, 153, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Fallica, J.; Casin, K.; Murphy, E.; Steenbergen, C.; Kohr, M.J. Characterization of the sex-dependent myocardial S-nitrosothiol proteome. Am. J. Physiol. Circ. Physiol. 2016, 310, H505–H515. [Google Scholar] [CrossRef] [Green Version]
- Dallas, M.L.; Yang, Z.; Boyle, J.P.; Boycott, H.E.; Scragg, J.L.; Milligan, C.J.; Elies, J.; Duke, A.; Thireau, J.; Reboul, C.; et al. Carbon Monoxide Induces Cardiac Arrhythmia via Induction of the Late Na+ Current. Am. J. Respir. Crit. Care Med. 2012, 186, 648–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Valdivia, C.R.; Vaidyanathan, R.; Balijepalli, R.C.; Ackerman, M.J.; Makielski, J.C. Caveolin-3 suppresses late sodium current by inhibiting nNOS-dependent S-nitrosylation of SCN5A. J. Mol. Cell. Cardiol. 2013, 61, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, R.; Caballero, R.; Barana, A.; Amorós, I.; Calvo, E.; Lopez, J.A.; Klein, H.; Vaquero, M.; Osuna, L.; Atienza, F.; et al. Nitric Oxide Increases Cardiac IK1 by Nitrosylation of Cysteine 76 of Kir2.1 Channels. Circ. Res. 2009, 105, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Heusch, P.; Aker, S.; Boengler, K.; Deindl, E.; van de Sand, A.; Klein, K.; Rassaf, T.; Konietzka, I.; Sewell, A.; Menazza, S.; et al. Increased inducible nitric oxide synthase and arginase II expression in heart failure: No net nitrite/nitrate production and protein S-nitrosylation. Am. J. Physiol. Circ. Physiol. 2010, 299, H446–H453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paslawska, U.; Kiczak, L.; Bania, J.; Paslawski, R.; Janiszewski, A.; Dzięgiel, P.; Zacharski, M.; Tomaszek, A.; Michlik, K.; Information, P.E.K.F.C. Inducible NO synthase is constitutively expressed in porcine myocardium and its level decreases along with tachycardia-induced heart failure. Cardiovasc. Pathol. 2016, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Otani, H.; Shimazu, T.; Yoshioka, K.; Fujita, M.; Katano, T.; Ito, S.; Iwasaka, T. Reversal of inducible nitric oxide synthase uncoupling unmasks tolerance to ischemia/reperfusion injury in the diabetic rat heart. J. Mol. Cell. Cardiol. 2010, 50, 534–544. [Google Scholar] [CrossRef]
- Puthanveetil, P.; Zhang, D.; Wang, Y.; Wang, F.; Wan, A.; Abrahani, A.; Rodrigues, B. Diabetes triggers a PARP1 mediated death pathway in the heart through participation of FoxO1. J. Mol. Cell. Cardiol. 2012, 53, 677–686. [Google Scholar] [CrossRef]
- Kleindienst, A.; Battault, S.; Belaidi, E.; Tanguy, S.; Rosselin, M.; Boulghobra, D.; Meyer, G.; Gayrard, S.; Walther, G.; Geny, B.; et al. Exercise does not activate the β3 adrenergic receptor–eNOS pathway, but reduces inducible NOS expression to protect the heart of obese diabetic mice. Basic Res. Cardiol. 2016, 111, 40. [Google Scholar] [CrossRef]
- Sun, J.; Kohr, M.J.; Nguyen, T.; Aponte, A.M.; Connelly, P.S.; Esfahani, S.G.; Gucek, M.; Daniels, M.P.; Steenbergen, C.; Murphy, E. Disruption of Caveolae Blocks Ischemic Preconditioning-Mediated S-Nitrosylation of Mitochondrial Proteins. Antioxid. Redox Signal. 2012, 16, 45–56. [Google Scholar] [CrossRef]
- Lima, B.; Lam, G.K.W.; Xie, L.; Diesen, D.L.; Villamizar, N.; Nienaber, J.; Messina, E.; Bowles, D.; Kontos, C.D.; Hare, J.M.; et al. Endogenous S-nitrosothiols protect against myocardial injury. Proc. Natl. Acad. Sci. USA 2009, 106, 6297–6302. [Google Scholar] [CrossRef] [Green Version]
- Fillmore, N.; Casin, K.; Sinha, P.; Sun, J.; Ma, H.; Boylston, J.; Noguchi, A.; Liu, C.; Wang, N.; Zhou, G.; et al. A knock-in mutation at cysteine 144 of TRIM72 is cardioprotective and reduces myocardial TRIM72 release. J. Mol. Cell. Cardiol. 2019, 136, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kohr, M.J.; Evangelista, A.M.; Ferlito, M.; Steenbergen, C.; Murphy, E. S-nitrosylation of TRIM72 at cysteine 144 is critical for protection against oxidation-induced protein degradation and cell death. J. Mol. Cell. Cardiol. 2014, 69, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiattarella, G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Pan, L.; Lin, Z.; Tang, X.; Tian, J.; Zheng, Q.; Jing, J.; Xie, L.; Chen, H.; Lu, Q.; Wang, H.; et al. S-Nitrosylation of Plastin-3 Exacerbates Thoracic Aortic Dissection Formation via Endothelial Barrier Dysfunction. Arter. Thromb. Vasc. Biol. 2020, 40, 175–188. [Google Scholar] [CrossRef]
- Switzer, C.H.; Cheng, R.Y.-S.; Ridnour, L.A.; A Glynn, S.; Ambs, S.; Wink, D.A. Ets-1 is a transcriptional mediator of oncogenic nitric oxide signaling in estrogen receptor-negative breast cancer. Breast Cancer Res. 2012, 14, R125. [Google Scholar] [CrossRef] [Green Version]
- Yasinska, I.; Sumbayev, V.V. S-nitrosation of Cys-800 of HIF-1α protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett. 2003, 549, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [Green Version]
- Parker, K.H.; Beury, D.W.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv. Cancer Res. 2015, 128, 95–139. [Google Scholar] [CrossRef] [Green Version]
- Molon, B.; Ugel, S.; Del Pozzo, F.; Soldani, C.; Zilio, S.; Avella, D.; De Palma, A.; Mauri, P.; Monegal, A.; Rescigno, M.; et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011, 208, 1949–1962. [Google Scholar] [CrossRef]
- Zhou, Z.; Mahdi, A.; Tratsiakovich, Y.; Zahorán, S.; Kövamees, O.; Nordin, F.; Gonzalez, A.E.U.; Alvarsson, M.; Östenson, C.-G.; Andersson, D.C.; et al. Erythrocytes from Patients with Type 2 Diabetes Induce Endothelial Dysfunction via Arginase I. J. Am. Coll. Cardiol. 2018, 72, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Beckman, J.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Zhang, X.; Cai, X.; Wang, K.; Chen, Y.; Deng, Y. Puerarin Attenuated Early Diabetic Kidney Injury through Down-Regulation of Matrix Metalloproteinase 9 in Streptozotocin-Induced Diabetic Rats. PLoS ONE 2014, 9, 5690. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.-J.; Park, Y.-H.; Kim, S.-U.; Moon, H.-B.; Park, D.S.; Han, Y.-H.; Lee, C.-H.; Lee, D.-S.; Song, I.-S.; Lee, D.H.; et al. Hepatitis B Virus X Protein Regulates Hepatic Glucose Homeostasis via Activation of Inducible Nitric Oxide Synthase. J. Biol. Chem. 2011, 286, 29872–29881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.-H.; Wei, W.; Hanes, M.A.; Liu, L. Hepatocarcinogenesis Driven by GSNOR Deficiency Is Prevented by iNOS Inhibition. Cancer Res. 2013, 73, 2897–2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Li, B.; Hanes, M.A.; Kakar, S.; Chen, X.; Liu, L. S-Nitrosylation from GSNOR Deficiency Impairs DNA Repair and Promotes Hepatocarcinogenesis. Sci. Transl. Med. 2010, 2, 19ra13. [Google Scholar] [CrossRef] [Green Version]
- Yeh, S.; Chen, P.; Shau, W.; Chen, Y.; Lee, P.; Chen, J.; Chen, D. Chromosomal allelic imbalance evolving from liver cirrhosis to hepatocellular carcinoma. Gastroenterology 2001, 121, 699–709. [Google Scholar] [CrossRef]
- Wang, H.; Li, F.; Feng, J.; Wang, J.; Liu, X. The effects of S-nitrosylation-induced PPARγ/SFRP5 pathway inhibition on the conversion of non-alcoholic fatty liver to non-alcoholic steatohepatitis. Ann. Transl. Med. 2021, 9, 684. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ganini, D.; Tokar, E.J.; Kumar, A.; Das, S.; Corbett, J.; Kadiiska, M.B.; Waalkes, M.P.; Diehl, A.M.; Mason, R.P. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J. Hepatol. 2012, 58, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Charbonneau, A.; Marette, A. Inducible Nitric Oxide Synthase Induction Underlies Lipid-Induced Hepatic Insulin Resistance in Mice: Potential Role of Tyrosine Nitration of Insulin Signaling Proteins. Diabetes 2010, 59, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar] [CrossRef]
- Broniowska, K.; Diers, A.R.; Hogg, N. S-Nitrosoglutathione. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3173–3181. [Google Scholar] [CrossRef] [Green Version]
- Adaya, I.D.S.; Gonsebatt, M.E.; Guevara, J. Thioredoxin System Regulation in the Central Nervous System: Experimental Models and Clinical Evidence. Oxidative Med. Cell. Longev. 2014, 2014, 590808. [Google Scholar] [CrossRef] [Green Version]
- Irvine, J.C.; Ritchie, R.; Favaloro, J.L.; Andrews, K.L.; Widdop, R.; Kemp-Harper, B.K. Nitroxyl (HNO): The Cinderella of the nitric oxide story. Trends Pharmacol. Sci. 2008, 29, 601–608. [Google Scholar] [CrossRef]
- Sabbah, H.N.; Tocchetti, C.G.; Wang, M.; Daya, S.; Gupta, R.C.; Tunin, R.S.; Mazhari, R.; Takimoto, E.; Paolocci, N.; Cowart, D.; et al. Nitroxyl (HNO). Circ. Heart Fail. 2013, 6, 1250–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garvey, E.P.; Oplinger, J.A.; Furfine, E.S.; Kiff, R.J.; Laszlo, F.; Whittle, B.J.R.; Knowles, R.G. 1400W Is a Slow, Tight Binding, and Highly Selective Inhibitor of Inducible Nitric-oxide Synthase in Vitro and in Vivo. J. Biol. Chem. 1997, 272, 4959–4963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, A.D.; Zhu, J.S.; Marshall, S.; Irsula, O.; Brechtel, G.; Keech, C. Insulin resistance after hypertension induced by the nitric oxide synthesis inhibitor L-NMMA in rats. Am. J. Physiol. Metab. 1995, 269, E709–E715. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Wu, R. Thioredoxin gene expression is transcriptionally up-regulated by retinol in monkey conducting airway epithelial cells. Biochem. Biophys. Res. Commun. 1992, 183, 170–175. [Google Scholar] [CrossRef]
- Maruyama, T.; Sachi, Y.; Furuke, K.; Kitaoka, Y.; Kanzaki, H.; Yoshimura, Y.; Yodoi, J. Induction of thioredoxin, a redox-active protein, by ovarian steroid hormones during growth and differentiation of endometrial stromal cells in vitro. Endocrinology 1999, 140, 365–372. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, N.; Tajima, H.; Furuke, K.; Ohira, A.; Honda, Y.; Yodoi, J. Induction of Human Thioredoxin in Cultured Human Retinal Pigment Epithelial Cells through Cyclic AMP-dependent Pathway; Involvement in the Cytoprotective Activity of Prostaglandin E1. Exp. Eye Res. 1997, 65, 645–652. [Google Scholar] [CrossRef]
- Tanito, M.; Kwon, Y.-W.; Kondo, N.; Bai, J.; Masutani, H.; Nakamura, H.; Fujii, J.; Ohira, A.; Yodoi, J. Cytoprotective Effects of Geranylgeranylacetone against Retinal Photooxidative Damage. J. Neurosci. 2005, 25, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Abdellatif, M.; Trummer-Herbst, V.; Koser, F.; Durand, S.; Adão, R.; Vasques-Nóvoa, F.; Freundt, J.K.; Voglhuber, J.; Pricolo, M.-R.; Kasa, M.; et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci. Transl. Med. 2021, 13, eabd7064. [Google Scholar] [CrossRef] [PubMed]
- Swamy, S.M.; Rajasekaran, N.S.; Thannickal, V.J. Nuclear Factor–Erythroid-2–Related Factor 2 in Aging and Lung Fibrosis. Am. J. Pathol. 2016, 186, 1712–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Yan, X.; Wintergerst, K.A.; Cai, L.; Keller, B.B.; Tan, Y. Nrf2: Redox and Metabolic Regulator of Stem Cell State and Function. Trends Mol. Med. 2019, 26, 185–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlström, K.E.; Ewing, E.; Granqvist, M.; Gyllenberg, A.; Aeinehband, S.; Enoksson, S.L.; Checa, A.; Badam, T.; Huang, J.; Gomez-Cabrero, D.; et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat. Commun. 2019, 10, 3081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019, 8, 2252–2267. [Google Scholar] [CrossRef]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [Green Version]
- Sykes, B.G.; Van Steyn, P.M.; Vignali, J.D.; Winalski, J.; Lozier, J.; Bell, W.E.; Turner, J.E. The Relationship between Estrogen and Nitric Oxide in the Prevention of Cardiac and Vascular Anomalies in the Developing Zebrafish (Danio Rerio). Brain Sci. 2016, 6, 51. [Google Scholar] [CrossRef]
- Southan, G.J.; Szabó, C.; Thiemermann, C. Isothioureas: Potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br. J. Pharmacol. 1995, 114, 510–516. [Google Scholar] [CrossRef]
- Sakamuri, S.S.V.P.; Sperling, J.A.; Evans, W.R.; Dholakia, M.H.; Albuck, A.L.; Sure, V.N.; Satou, R.; Mostany, R.; Katakam, P.V.G. Nitric oxide synthase inhibitors negatively regulate respiration in isolated rodent cardiac and brain mitochondria. Am. J. Physiol. Circ. Physiol. 2020, 318, H295–H300. [Google Scholar] [CrossRef]
- Leary, P.J.; Rajasekaran, S.; Morrison, R.R.; Tuomanen, E.I.; Chin, T.K.; Hofmann, P.A. A cardioprotective role for platelet-activating factor through NOS-dependent S-nitrosylation. Am. J. Physiol. Circ. Physiol. 2008, 294, H2775–H2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, S.; Lee, J.; Sehgal, P.B. Depletion of the ATPase NSF from Golgi membranes with hypo-S-nitrosylation of vasorelevant proteins in endothelial cells exposed to monocrotaline pyrrole. Am. J. Physiol. Circ. Physiol. 2008, 295, H1943–H1955. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, C.; Thimmulappa, R.; Singh, A.; Blake, D.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chorley, B.N.; Campbell, M.R.; Wang, X.; Karaca, M.; Sambandan, D.; Bangura, F.; Xue, P.; Pi, J.; Kleeberger, S.; Bell, D.A. Identification of novel NRF2-regulated genes by ChIP-Seq: Influence on retinoid X receptor alpha. Nucleic Acids Res. 2012, 40, 7416–7429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrell, M.D.; Jackson, J.P.; Augustine, L.M.; Fisher, C.D.; Slitt, A.L.; Maher, J.M.; Huang, W.; Moore, D.D.; Zhang, Y.; Klaassen, C.D.; et al. The Nrf2 Activator Oltipraz Also Activates the Constitutive Androstane Receptor. Drug Metab. Dispos. 2008, 36, 1716–1721. [Google Scholar] [CrossRef] [Green Version]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxidative Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hisamichi, M.; Kamijo-Ikemori, A.; Sugaya, T.; Hoshino, S.; Kimura, K.; Shibagaki, Y. Role of bardoxolone methyl, a nuclear factor erythroid 2-related factor 2 activator, in aldosterone- and salt-induced renal injury. Hypertens. Res. 2017, 41, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Category | Mechanism | Reference |
|---|---|---|
| Reduces NO Production | ||
| NOS inhibitor | ||
| Proadifen hydrochloride | nNOS/AchR inhibitor | [142] |
| N(ω)-propyl-L-arginine | nNOS selective inhibitor | [85] |
| S-methyl-L-thiocitrulline | nNOS selective inhibitor | [85] |
| 7-nitroindazole | nNOS selective inhibitor | [40] |
| 1400 W | iNOS selective inhibitor | [98] |
| (S)-methylisothiourea sulfate | iNOS selective inhibitor | [143] |
| N6-(1-iminoethyl)-l-lysine dihydrochloride | iNOS selective inhibitor | [59] |
| L-N5-(1-iminoethyl)ornithine | Nonselective NOS inhibitor | [144] |
| N(G)-monomethyl-L-arginine acetate | Nonselective NOS inhibitor | [92] |
| N-nitro-L-arginine methyl ester | Nonselective NOS inhibitor | [103] |
| N-nitro-L-arginine | Nonselective NOS inhibitor | [145] |
| 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxy-3-oxide | NO scavenger | [146] |
| Induces denitrosylation | ||
| GSH/GSNO system | ||
| Dietary GSH | GSH supplement | [147] |
| N-acetyl-L-cysteine | GSH precursor | [79] |
| Nrf2 | GR inducer | [148] |
| TRX/TrxR system | ||
| Retinol | TRX inducer | [129] |
| Estradiol | TRX inducer | [130] |
| Prostaglandin E1 | TRX inducer | [131] |
| Geranylgeranylacetone | TRX inducer | [132] |
| Nrf2 | TrxR inducer | [149] |
| Indirectly involved in denitrosation | ||
| Superoxide dismutase | ROS reducer | [92] |
| Niacin (Vitamin B3) | NAD(P)H precursor | [134] |
| Nicotinamide | NAD(P)H precursor | [134] |
| Dimethyl fumarate | Nrf2 inducer | [78] |
| Oltipraz | Nrf2 inducer | [150] |
| Sulforaphane | Nrf2 inducer | [151] |
| Bardoxolone-methyl | Nrf2 inducer | [152] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.; Eom, G.H.; Kang, G. Nitrosative Stress and Human Disease: Therapeutic Potential of Denitrosylation. Int. J. Mol. Sci. 2021, 22, 9794. https://doi.org/10.3390/ijms22189794
Yoon S, Eom GH, Kang G. Nitrosative Stress and Human Disease: Therapeutic Potential of Denitrosylation. International Journal of Molecular Sciences. 2021; 22(18):9794. https://doi.org/10.3390/ijms22189794
Chicago/Turabian StyleYoon, Somy, Gwang Hyeon Eom, and Gaeun Kang. 2021. "Nitrosative Stress and Human Disease: Therapeutic Potential of Denitrosylation" International Journal of Molecular Sciences 22, no. 18: 9794. https://doi.org/10.3390/ijms22189794
APA StyleYoon, S., Eom, G. H., & Kang, G. (2021). Nitrosative Stress and Human Disease: Therapeutic Potential of Denitrosylation. International Journal of Molecular Sciences, 22(18), 9794. https://doi.org/10.3390/ijms22189794






