A Body of Circumstantial Evidence for the Irreversible Ectonucleotidase Inhibitory Action of FSCPX, an Agent Known as a Selective Irreversible A1 Adenosine Receptor Antagonist So Far
Abstract
:1. Introduction
2. Results
2.1. CD39 and CD73 Inhibitor Assays
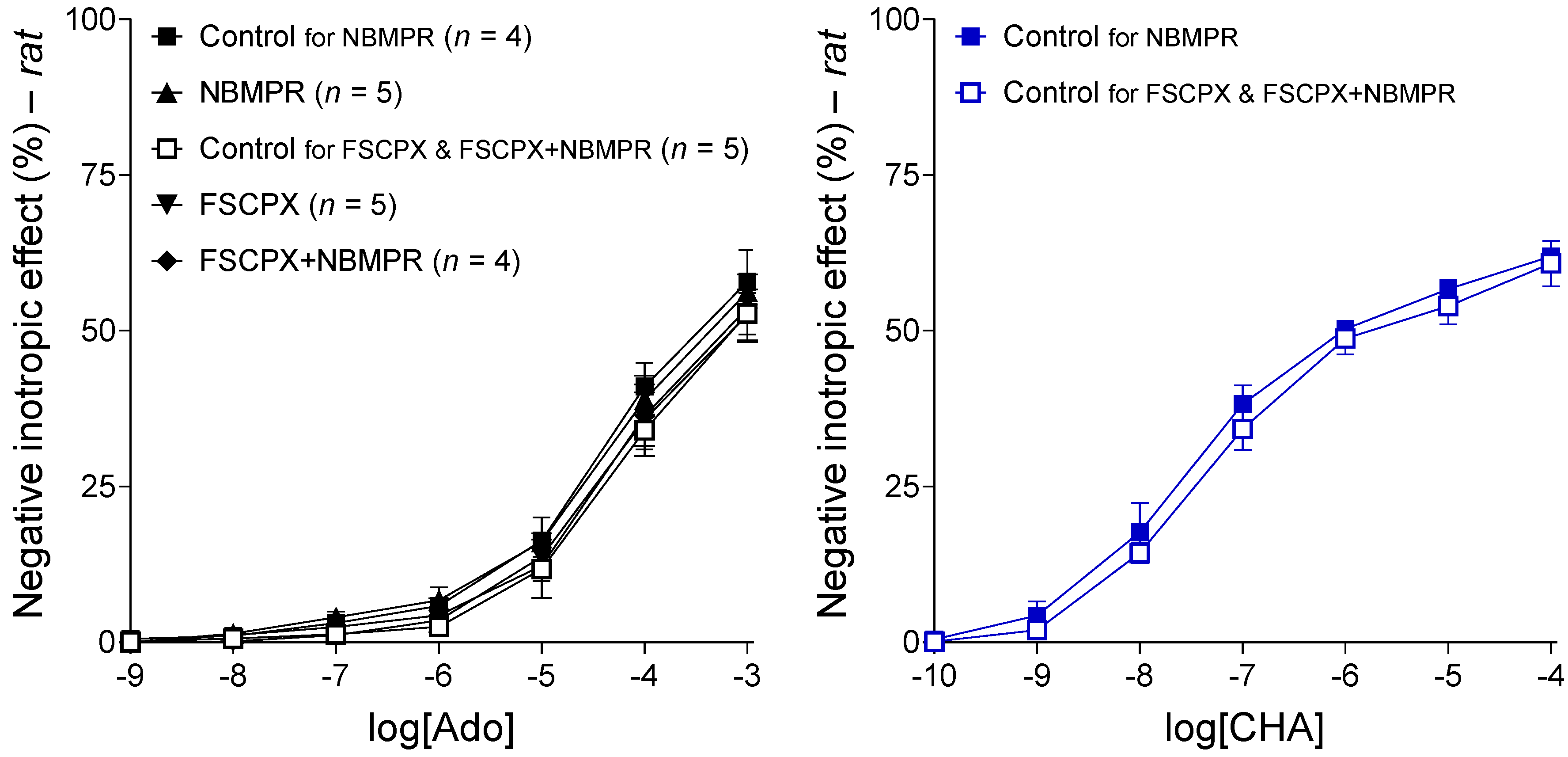
2.2. Interaction of FSCPX with NBMPR in the Rat Left Atrium
2.2.1. Response to Adenosine before Any In Vitro Treatment
2.2.2. Response to CHA in the Control Groups
2.2.3. Response to CHA after Different In Vitro Treatments
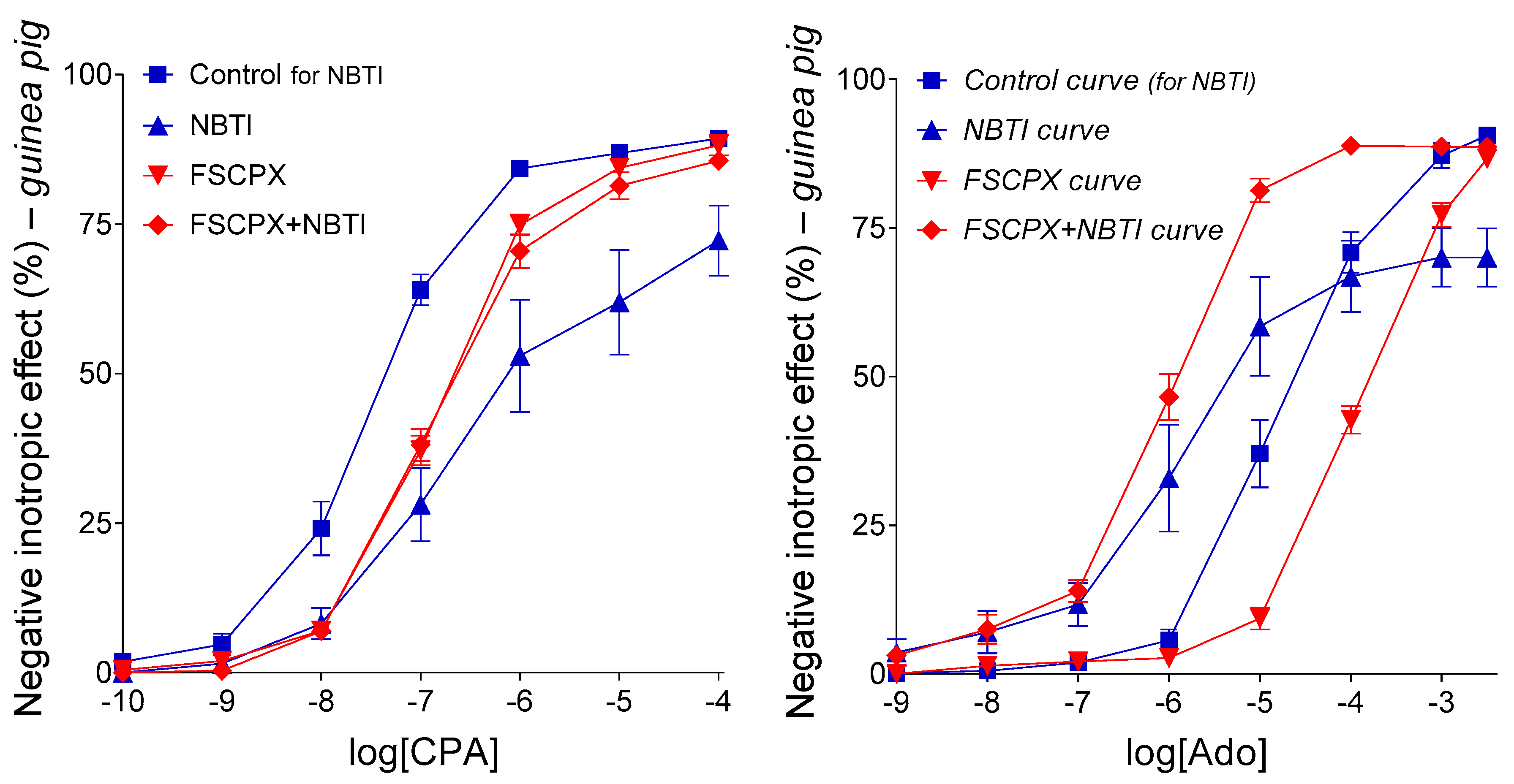
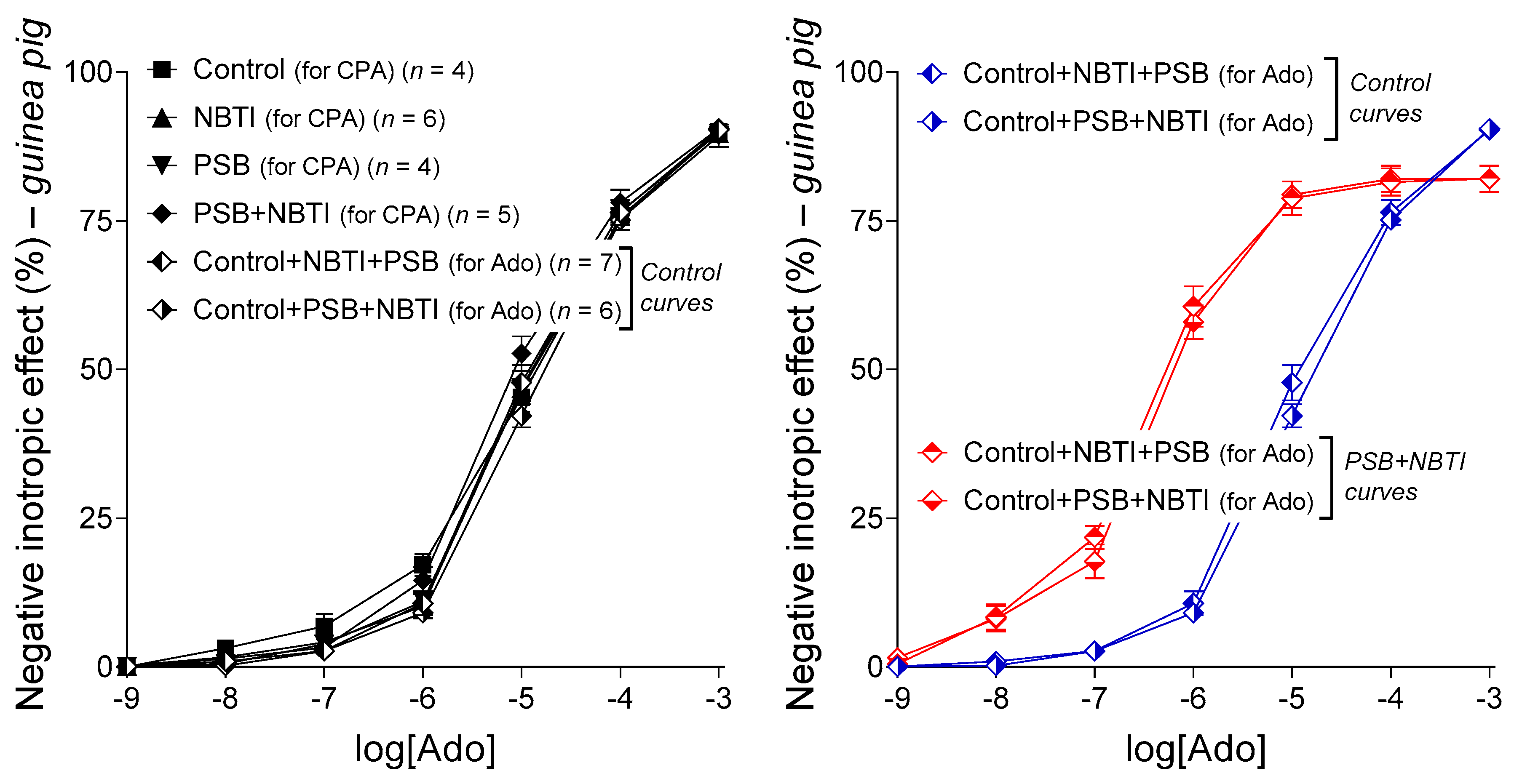
2.3. Interaction of PSB-12379 with NBTI in the Guinea Pig Left Atrium
2.3.1. Response to Adenosine in All Groups
2.3.2. Response to CPA in the Groups Labeled with “for CPA”
2.3.3. Response to Adenosine in the Groups Labeled with “for Ado”
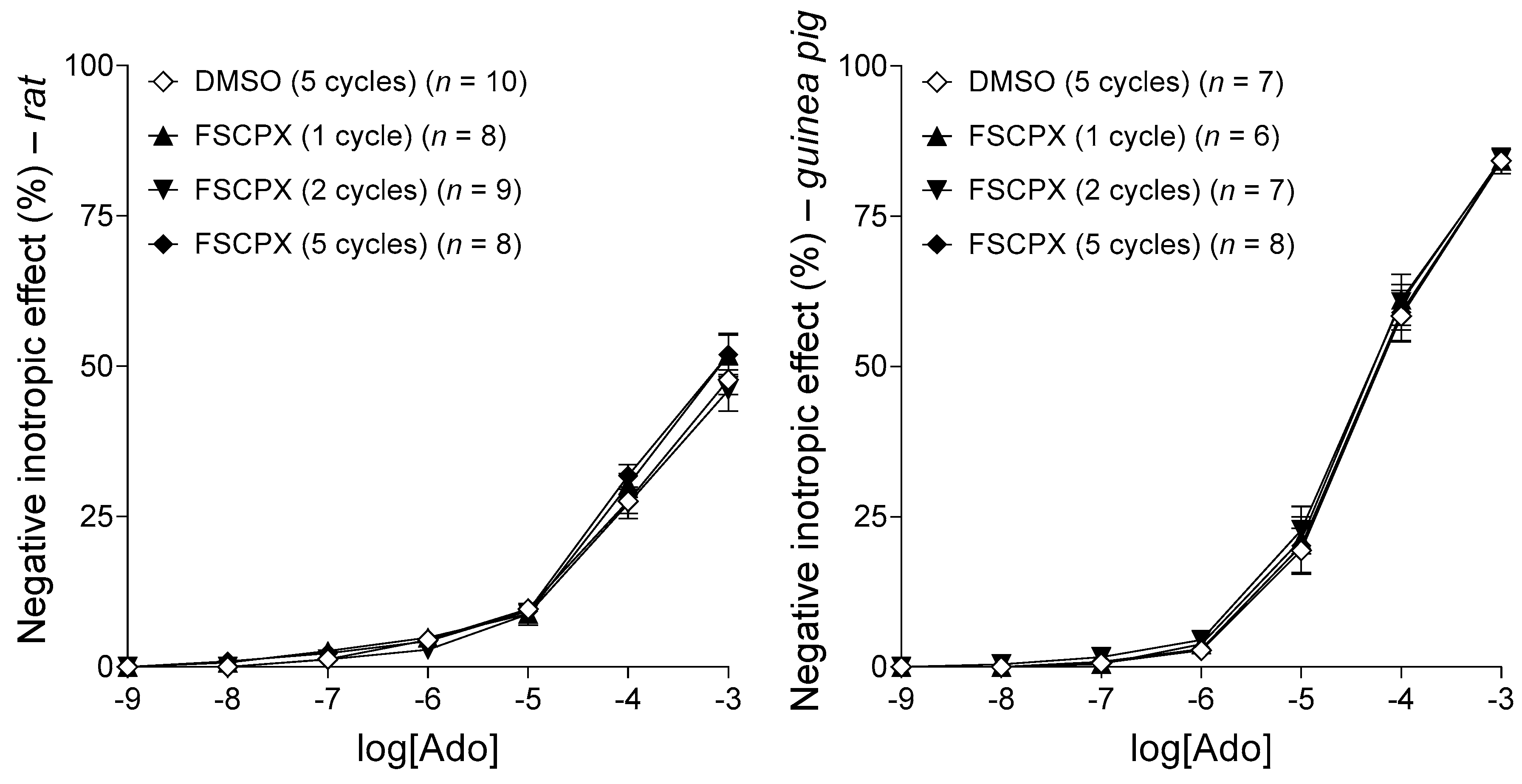
2.4. The Influence of Different Administration Regimens on the Effect of FSCPX in Rat and Guinea Pig Left Atria
2.4.1. Response to Adenosine before Any In Vitro Treatment
2.4.2. Response to CPA after an In Vitro Pretreatment with 10 μM FSCPX Using Different Administration Regimens
3. Discussion
4. Materials and Methods
4.1. In Vitro Enzyme Inhibitor Assays
4.1.1. Materials
4.1.2. Protocol
4.2. Ex Vivo Functional Assays
4.2.1. Materials
4.2.2. Animals and Groups
4.2.3. Protocols
4.2.4. Characterization of the E/c Curves
4.2.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 27 June 2021).
- Williams, T.M.; Bengtson, P.; Steller, D.L.; Croll, D.A.; Davis, R.W. The Healthy Heart: Lessons from Nature’s Elite Athletes. Physiology 2015, 30, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G.; Pelleg, A. Cardiac purinergic signalling in health and disease. Purinergic Signal. 2015, 11, 1–46. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Procopio, M.C.; Lauro, R.; Nasso, C.; Carerj, S.; Squadrito, F.; Bitto, A.; Di Bella, G.; Micari, A.; Irrera, N.; Costa, F. Role of Adenosine and Purinergic Receptors in Myocardial Infarction: Focus on Different Signal Transduction Pathways. Biomedicines 2021, 9, 204. [Google Scholar] [CrossRef]
- Guieu, R.; Brignole, M.; Deharo, J.C.; Deharo, P.; Mottola, G.; Groppelli, A.; Paganelli, F.; Ruf, J. Adenosine Receptor Reserve and Long-Term Potentiation: Unconventional Adaptive Mechanisms in Cardiovascular Diseases? Int. J. Mol. Sci. 2021, 22, 7584. [Google Scholar] [CrossRef] [PubMed]
- Alnouri, M.W.; Jepards, S.; Casari, A.; Schiedel, A.C.; Hinz, S.; Müller, C.E. Selectivity is species-dependent: Characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal. 2015, 11, 389–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; IJzerman, A.P. Processing of adenosine receptor agonists in rat and human whole blood. Biochem. Pharmacol. 1998, 56, 1625–1632. [Google Scholar] [CrossRef]
- Gesztelyi, R.; Kiss, Z.; Wachal, Z.; Juhasz, B.; Bombicz, M.; Csepanyi, E.; Pak, K.; Zsuga, J.; Papp, C.; Galajda, Z.; et al. The surmountable effect of FSCPX, an irreversible A(1) adenosine receptor antagonist, on the negative inotropic action of A(1) adenosine receptor full agonists in isolated guinea pig left atria. Arch. Pharm. Res. 2013, 36, 293–305. [Google Scholar] [CrossRef]
- Belardinelli, L.; Shryock, J.C.; Song, Y.; Wang, D.; Srinivas, M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995, 9, 359–365. [Google Scholar] [CrossRef]
- Headrick, J.P.; Ashton, K.J.; Rose’meyer, R.B.; Peart, J.N. Cardiovascular adenosine receptors: Expression, actions and interactions. Pharmacol. Ther. 2013, 140, 92–111. [Google Scholar] [CrossRef]
- Thorn, J.A.; Jarvis, S.M. Adenosine transporters. Gen. Pharmacol. 1996, 27, 613–620. [Google Scholar] [CrossRef]
- Dekanski, D.; Piperski, V.; Tasić, J.; Marković, I.D.; Jokanović, M.; Stukalov, P.; Mitrović, D.M. Transport of endogenous nucleosides in guinea pig heart. Can. J. Physiol. Pharmacol. 2004, 82, 1061–1067. [Google Scholar] [CrossRef]
- Boswell-Casteel, R.C.; Hays, F.A. Equilibrative nucleoside transporters-a review. Nucleosides Nucleotides Nucleic Acids 2017, 36, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Deussen, A.; Stappert, M.; Schäfer, S.; Kelm, M. Quantification of extracellular and intracellular adenosine production: Understanding the transmembranous concentration gradient. Circulation 1999, 99, 2041–2047. [Google Scholar] [CrossRef]
- Deussen, A.; Weichsel, J.; Pexa, A. Features of adenosine metabolism of mouse heart. Purinergic Signal. 2006, 2, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Erdei, T.; Szabo, A.M.; Lampe, N.; Szabo, K.; Kiss, R.; Zsuga, J.; Papp, C.; Pinter, A.; Szentmiklosi, A.J.; Szilvassy, Z.; et al. FSCPX, a Chemical Widely Used as an Irreversible A₁ Adenosine Receptor Antagonist, Modifies the Effect of NBTI, a Nucleoside Transport Inhibitor, by Reducing the Interstitial Adenosine Level in the Guinea Pig Atrium. Molecules 2018, 23, 2186. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, A.; Beukers, M.W.; van der Graaf, P.H.; Lang, H.; van Muijlwijk-Koezen, J.; de Groote, M.; Menge, W.; Schwabe, U.; IJzerman, A.P. Modulation of agonist responses at the A(1) adenosine receptor by an irreversible antagonist, receptor-G protein uncoupling and by the G protein activation state. Biochem. Pharmacol. 2002, 64, 1251–1265. [Google Scholar] [CrossRef]
- Scammells, P.J.; Baker, S.P.; Belardinelli, L.; Olsson, R.A. Substituted 1,3-dipropylxanthines as irreversible antagonists of A1 adenosine receptors. J. Med. Chem. 1994, 37, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Shryock, J.C.; Scammells, P.J.; Ruble, J.; Baker, S.P.; Belardinelli, L. A novel irreversible antagonist of the A1-adenosine receptor. Mol. Pharmacol. 1996, 50, 196–205. [Google Scholar]
- Kiss, Z.; Pak, K.; Zsuga, J.; Juhasz, B.; Varga, B.; Szentmiklosi, A.J.; Haines, D.D.; Tosaki, A.; Gesztelyi, R. The guinea pig atrial A1 adenosine receptor reserve for the direct negative inotropic effect of adenosine. Gen. Physiol. Biophys. 2013, 32, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Pak, K.; Papp, C.; Galajda, Z.; Szerafin, T.; Varga, B.; Juhasz, B.; Haines, D.; Szentmiklosi, A.J.; Tosaki, A.; Gesztelyi, R. Approximation of A1 adenosine receptor reserve appertaining to the direct negative inotropic effect of adenosine in hyperthyroid guinea pig left atria. Gen. Physiol. Biophys. 2014, 33, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Gesztelyi, R.; Zsuga, J.; Juhász, B.; Dér, P.; Vecsernyés, M.; Szentmiklósi, A.J. Concentration estimation via curve fitting: Quantification of negative inotropic agents by using a simple mathematical method in guinea pig atria. Bull. Math. Biol. 2004, 66, 1439–1453. [Google Scholar] [CrossRef]
- Grenczer, M.; Pinter, A.; Zsuga, J.; Kemeny-Beke, A.; Juhasz, B.; Szodoray, P.; Tosaki, A.; Gesztelyi, R. The influence of affinity, efficacy, and slope factor on the estimates obtained by the receptorial responsiveness method (RRM): A computer simulation study. Can. J. Physiol. Pharmacol. 2010, 88, 1061–1073. [Google Scholar] [CrossRef]
- Zsuga, J.; Erdei, T.; Szabó, K.; Lampe, N.; Papp, C.; Pinter, A.; Szentmiklosi, A.J.; Juhasz, B.; Szilvássy, Z.; Gesztelyi, R. Methodical Challenges and a Possible Resolution in the Assessment of Receptor Reserve for Adenosine, an Agonist with Short Half-Life. Molecules 2017, 22, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, A.M.; Erdei, T.; Viczjan, G.; Kiss, R.; Zsuga, J.; Papp, C.; Pinter, A.; Juhasz, B.; Szilvassy, Z.; Gesztelyi, R. An Advanced In Silico Modelling of the Interaction between FSCPX, an Irreversible A1 Adenosine Receptor Antagonist, and NBTI, a Nucleoside Transport Inhibitor, in the Guinea Pig Atrium. Molecules 2019, 24, 2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 362, 299–309. [Google Scholar] [CrossRef]
- Müller, C.E.; Iqbal, J.; Baqi, Y.; Zimmermann, H.; Röllich, A.; Stephan, H. Polyoxometalates–a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 5943–5947. [Google Scholar] [CrossRef]
- Pimenta-Dos-Reis, G.; Torres, E.J.L.; Quintana, P.G.; Vidal, L.O.; Dos Santos, B.A.F.; Lin, C.S.; Heise, N.; Persechini, P.M.; Schachter, J. POM-1 inhibits P2 receptors and exhibits anti-inflammatory effects in macrophages. Purinergic Signal. 2017, 13, 611–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattarai, S.; Freundlieb, M.; Pippel, J.; Meyer, A.; Abdelrahman, A.; Fiene, A.; Lee, S.Y.; Zimmermann, H.; Yegutkin, G.G.; Sträter, N.; et al. α,β-Methylene-ADP (AOPCP) Derivatives and Analogues: Development of Potent and Selective ecto-5’-Nucleotidase (CD73) Inhibitors. J. Med. Chem. 2015, 58, 6248–6263. [Google Scholar] [CrossRef]
- Schmies, C.C.; Rolshoven, G.; Idris, R.M.; Losenkova, K.; Renn, C.; Schäkel, L.; Al-Hroub, H.; Wang, Y.; Garofano, F.; Schmidt-Wolf, I.G.H.; et al. Fluorescent Probes for Ecto-5’-nucleotidase (CD73). ACS Med. Chem. Lett. 2020, 11, 2253–2260. [Google Scholar] [CrossRef]
- Karsai, D.; Zsuga, J.; Juhasz, B.; Der, P.; Szentmiklosi, A.J.; Tosaki, A.; Gesztelyi, R. Effect of nucleoside transport blockade on the interstitial adenosine level characterized by a novel method in guinea pig atria. J. Cardiovasc. Pharmacol. 2006, 47, 103–109. [Google Scholar] [CrossRef]
- Karsai, D.; Gesztelyi, R.; Zsuga, J.; Jakab, A.; Szendrei, L.; Juhasz, B.; Bak, I.; Szabo, G.; Lekli, I.; Vecsernyes, M.; et al. Influence of hyperthyroidism on the effect of adenosine transport blockade assessed by a novel method in guinea pig atria. Cell Biochem. Biophys. 2007, 47, 45–52. [Google Scholar] [CrossRef]
- Van Muijlwijk-Koezen, J.E.; Timmerman, H.; van der Sluis, R.P.; van de Stolpe, A.C.; Menge, W.M.; Beukers, M.W.; van der Graaf, P.H.; de Groote, M.; IJzerman, A.P. Synthesis and use of FSCPX, an irreversible adenosine A1 antagonist, as a ‘receptor knock-down’ tool. Bioorg. Med. Chem. Lett. 2001, 11, 815–818. [Google Scholar] [CrossRef]
- Von der Leyen, H.; Schmitz, W.; Scholz, H.; Scholz, J.; Lohse, M.J.; Schwabe, U. Effects of 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), a highly selective adenosine receptor antagonist, on force of contraction in guinea-pig atrial and ventricular cardiac preparations. Naunyn Schmiedebergs Arch. Pharmacol. 1989, 340, 204–209. [Google Scholar] [CrossRef]
- Van Rossum, J.M. General Introduction. In Kinetics of Drug Action, 1st ed.; van Rossum, J.M., Ed.; Springer: Heidelberg, Germany, 1977. [Google Scholar] [CrossRef]
- Pak, K.; Zsuga, J.; Kepes, Z.; Erdei, T.; Varga, B.; Juhasz, B.; Szentmiklosi, A.J.; Gesztelyi, R. The effect of adenosine deaminase inhibition on the A1 adenosinergic and M2 muscarinergic control of contractility in eu- and hyperthyroid guinea pig atria. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 853–868. [Google Scholar] [CrossRef] [Green Version]
- Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Varga, B.; Juhasz, B.; Tosaki, A. The Hill equation and the origin of quantitative pharmacology. Arch. Hist. Exact Sci. 2012, 66, 427–438. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression. A Practical Guide to Curve Fitting; GraphPad Software Inc.: San Diego, CA, USA, 2004. [Google Scholar]
- Monaghan, N. Law of Evidence; Cambridge University Press: Cambridge, UK, 2015; p. 6. ISBN 9781107020337. [Google Scholar]




| Ectonucleotidases | |||
| ecto-nucleoside 5′-triphosphate diphosphohydrolase family (E-NTPDases) a.k.a. ecto-apyrases | |||
| group E-NTPDase1–4 | |||
| E-NTPDase1 a.k.a. lymphocyte surface protein CD39 a.k.a. ecto-apyrase CD39 | |||
| E-NTPDase2 a.k.a. ecto-ATPase CD39L1 | |||
| E-NTPDase3 a.k.a. HB6 | |||
| E-NTPDase4: UDPase and LALP70 | |||
| group E-NTPDase5,6 | |||
| E-NTPDase5 a.k.a. CD39L4 | |||
| E-NTPDase6 (?) | |||
| ectonucleotide pyrophosphatase/phosphodiesterase family (E-NPP family) a.k.a. ecto-phosphodiesterase/pyrophosphatase family a.k.a. PC-1 family a.k.a. phosphodiesterase/nucleotide pyrophosphatase (PDNP) family | |||
| murine plasma cell differentiation antigen NPP1 (PC-1) | |||
| murine plasma cell differentiation antigen NPP2 (PD-Iα and autotaxin) | |||
| murine plasma cell differentiation antigen NPP3 (PD-Iβ a.k.a. B10 a.k.a. gp130RB13-6) | |||
| alkaline phosphatases a.k.a. non-specific ecto-phosphomonoesterases | |||
| lymphocyte surface protein CD73 a.k.a. ecto-5′-nucleotidase | |||
| DMSO (5 Cycles) | FSCPX (1 Cycle) | FSCPX (2 Cycles) | FSCPX (5 Cycles) | ||
|---|---|---|---|---|---|
| R-i | Emax (%) | 47.65 | 54.15 | 54.53 | 57.1 |
| (45.08 to 50.57) | (47.92 to 64.04) | (48.41 to 65.63) | (49.92 to 73.19) | ||
| logEC50 | −6.956 | −5.558 | −5.17 | −5.123 | |
| (−7.105 to −6.788) | (−5.809 to −5.209) | (−5.391 to −4.812) | (−5.346 to −4.689) | ||
| n | 0.797 | 0.739 | 0.68 | 0.815 | |
| (0.632 to 1.046) | (0.547 to 1.006) | (0.533 to 0.852) | (0.567 to 1.136) | ||
| r2 | 0.9474 | 0.9285 | 0.9526 | 0.9261 | |
| R-g | Emax (%) | 49.99 | |||
| (47.96 to 52.23) | |||||
| logEC50 | −6.879 | −5.693 | −5.311 | −5.313 | |
| (−7.019 to −6.73) | (−5.836 to −5.548) | (−5.45 to −5.173) | (−5.446 to −5.181) | ||
| n | 0.814 | ||||
| (0.719 to 0.926) | |||||
| r2 | 0.9431 | 0.9261 | 0.9505 | 0.9161 | |
| GP-i | Emax (%) | 93.41 | 93.92 | 95.78 | 96.2 |
| (89.19 to 97.92) | (89.71 to 98.72) | (90.73 to 101.9) | (91.4 to 101.9) | ||
| logEC50 | −7.319 | −6.224 | −6.028 | −5.886 | |
| (−7.457 to −7.18) | (−6.326 to −6.113) | (−6.148 to −5.891) | (−5.994 to −5.762) | ||
| n | 0.819 | 0.852 | 0.745 | 0.745 | |
| (0.666 to 1.02) | (0.711 to 1.026) | (0.626 to 0.894) | (0.636 to 0.878) | ||
| r2 | 0.9618 | 0.9829 | 0.9789 | 0.9812 | |
| GP-g | Emax (%) | 94.35 | |||
| (92.15 to 96.67) | |||||
| logEC50 | −7.303 | −6.218 | −6.052 | −5.917 | |
| (−7.398 to −7.209) | (−6.316 to −6.119) | (−6.146 to −5.958) | (−6.005 to −5.828) | ||
| n | 0.794 | ||||
| (0.727 to 0.869) | |||||
| r2 | 0.9616 | 0.9826 | 0.9787 | 0.981 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viczjan, G.; Erdei, T.; Ovari, I.; Lampe, N.; Szekeres, R.; Bombicz, M.; Takacs, B.; Szilagyi, A.; Zsuga, J.; Szilvassy, Z.; et al. A Body of Circumstantial Evidence for the Irreversible Ectonucleotidase Inhibitory Action of FSCPX, an Agent Known as a Selective Irreversible A1 Adenosine Receptor Antagonist So Far. Int. J. Mol. Sci. 2021, 22, 9831. https://doi.org/10.3390/ijms22189831
Viczjan G, Erdei T, Ovari I, Lampe N, Szekeres R, Bombicz M, Takacs B, Szilagyi A, Zsuga J, Szilvassy Z, et al. A Body of Circumstantial Evidence for the Irreversible Ectonucleotidase Inhibitory Action of FSCPX, an Agent Known as a Selective Irreversible A1 Adenosine Receptor Antagonist So Far. International Journal of Molecular Sciences. 2021; 22(18):9831. https://doi.org/10.3390/ijms22189831
Chicago/Turabian StyleViczjan, Gabor, Tamas Erdei, Ignac Ovari, Nora Lampe, Reka Szekeres, Mariann Bombicz, Barbara Takacs, Anna Szilagyi, Judit Zsuga, Zoltan Szilvassy, and et al. 2021. "A Body of Circumstantial Evidence for the Irreversible Ectonucleotidase Inhibitory Action of FSCPX, an Agent Known as a Selective Irreversible A1 Adenosine Receptor Antagonist So Far" International Journal of Molecular Sciences 22, no. 18: 9831. https://doi.org/10.3390/ijms22189831








